Abstract
Studies on the interaction between crude oil, brine, and rock systems showed that the composition of water injected into the oil reservoir influences the amount of oil recovered from such a reservoir. Therefore, researchers are now emphasizing the use of SmartWater for enhanced oil recovery (EOR). In this research, the capability of activated clay to be used for tuning the chemistry of seawater for subsequent production of SmartWater was investigated. Filter cakes were formed using bentonite and its blends with raw clay and activated clay (which was produced in-house using locally obtained clay samples). The capability of the cakes to control the transport properties of permeating seawater was evaluated in terms of ion rejection. The average rejection for the raw clay cake for Na+, K+, Mg2+, and Ca2+ is 4.45, 49.64, 53.33, and 94.43%, respectively. The rejection results for the mixed-matrix cake containing the activated clay were 6.38, 51.34, 86.19, and 78.09 for Na+, K+, Mg2+, and Ca2+, respectively. It was observed that the selectivity of the filter cake for Mg2+ and Ca2+ was reversed due to the addition of the activated clay. Thus, activated clay possesses some potentials for SmartWater production for an EOR application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global energy consumption is expected to grow by about 1.2% yearly between the years 2010–2050 (Liu 2015). This value represents an increase of 11.2 billion tons as compared to the energy consumption in 2010. According to the World energy mix (2013), petroleum constitutes 78.4% of the total world energy consumption by source. To cater for the projected increase in energy demand, more wells are expected to be drilled worldwide and advanced oil recovery techniques are also expected to be developed to recover the left-over oil after secondary recovery techniques. Usually, the oil recovery rarely exceeds 35–40% of the original oil-in-place. This is when the best cases are considered. Therefore, there is growing research work on enhanced oil recovery techniques.
On a general note, hydrocarbon production stages are classified into three: primary, secondary, and tertiary (otherwise called enhanced oil recovery technique) (Adewole and Sultan 2014). The enhanced oil recovery (EOR) is an established method that is employed to prolong the life of an oil well and to obtain the maximum recoverable amount of oil from a reservoir (Al-Mutairi and Kokal 2011). Based on the recovery mechanism involved, EOR is further classified into thermal, chemical, miscible/immiscible gas, microbial, and water-based techniques (Nair et al. 2016).
One of the fluids that have been investigated for water-based techniques is the SmartWater. SmartWater is a type of fluid whose chemistry has been optimized in terms of salinity and ionic composition before being injected into a reservoir to realize incremental oil recoveries (Al-sofi and Yousef 2013). The injection of SmartWater into an oil–wet reservoir can result in a more water-wet rock surface leading to high oil recovery. The increment in oil recovery by SmartWater injection is mainly attributed to wettability modification in the presence of certain ions at high temperature (Gachuz-Muro and Sohrabi 2014). SmartWater can be designed by modifying the salinity and ionic composition of the injected fluid in such a way that the change in the equilibrium of the initial crude oil–brine–rock system will modify the initial wetting conditions of the reservoirs (Austad 2013). The SmartWater can be injected during early life cycles of reservoirs as well as in both secondary and tertiary EOR production phases (Mamonov et al. 2017; Austad 2013). The use of SmartWater can lead to a higher ultimate oil recovery with low added investment, especially when water-flooding infrastructure is already in place. Moreover, this technique has a low capital cost and expenses. It is an environmentally friendly EOR technique with no addition of chemicals. It is easily implementable in most oil reservoirs including carbonates and sandstones (Mamonov et al. 2017).
SmartWater-Flooding is one of the enhanced oil recovery techniques use in modifying the wettability of reservoirs to improve oil recovery. It is particularly suitable in sandstone and carbonate reservoirs (Gachuz-Muro and Sohrabi 2014). Carbonate reservoir where a considerable portion of the world’s hydrocarbon endowment is found is one of the most difficult targets for EOR applications (Strand et al. 2008; Mahmoud et al. 2017). Factors such as low permeability, low porosity, fracturing, high brine hardness, and other large-scale heterogeneity make the implementation of EOR a complicated issue in the carbonate reservoir (Manrique et al. 2007).
The technique of SmartWater-Flooding has evolved as an innovative method of enhancing the recovery of oil from carbonate and sandstone reservoirs by manipulating the ionic composition (or the salinity) of the injected water. Based on the accessibility of water, most of the sandstone oil reservoirs are produced using water flooding to maximize oil recovery. Several researches have pointed out the importance of the wetting condition of the porous medium for obtaining good displacement of the oil by the injected water. The initial wetting condition of an oil reservoir, which has been established during millions of years, may not be at the optimum for oil displacement by water. Thus, when injecting a SmartWater with different ion composition than the initial formation water, researchers have concluded that it is possible to modify the wetting condition of the reservoir in a favorable way which consequently has dramatic effects on oil recovery (especially in carbonate reservoirs) (Strand et al. 2006; Strand et al. 2008; Puntervold et al. 2009). Fathi and his team described the chemical mechanism explaining the details of why seawater acts as a smart fluid for EOR in high-temperature carbonate reservoirs (Fathi et al. 2012). Symbiotic interaction between the ions in seawater (Ca2+, Mg2+, and SO42−) and the carbonate surface increases the water wetness of the reservoirs. Consequently, the capillary force increases, and water imbibes spontaneously into the matrix and then displaces the oil. Moreover, other previously published literature revealed that advanced ion management of water has a very significant effect on oil recovery from carbonate and sandstone reservoirs (Gupta et al. 2011). The use of low salinity water increased the oil recovery to a significant level. Research work has been published on the effect of different cations, Na+, Ca2+, and Mg2+, on the oil recovery from carbonate cores. Proofs of the possibility to improve the performance of water flooding by chemically altering the brine/water composition have also been published (Strand et al. 2006). For example, it was reported that removing the calcium concentration from the flooding water will force the injected water to leach some of the calcium ions from the surface of the pores and cause instability of the exchanging cations. This will result in changing carbonate rock wettability to a more water-wet resulting in better oil recovery (Sarvestani et al. 2019; Gachuz-Muro and Sohrabi 2014). Although there are a quite number of publications on the use of SmartWater for EOR, there are very few research papers that talk about large-scale production of SmartWater where the chemistry of the initial seawater will be entirely different from the resulting SmartWater. To our knowledge, there is only one paper on this subject.
The most common practice of producing SmartWater is by dilution with fresh water (Strand et al. 2006). One of the major challenges with the dilution method is the need to use freshwater for the dilution. Also, it is nearly impossible to independently control the concentration of the individual element using this method. The resulting SmartWater has similar chemistry with the original seawater that was used for the preparation. Other methods such as membrane separation technology have also been reported (Nair et al. 2016). The problem with this method is that it is difficult to implement. A simpler method of producing SmartWater using activated clay is proposed and investigated in this work. The proposed method employed the cake filtration technique to tune the chemistry of seawater to produce SmartWater with entirely different chemistry from the original seawater for the EOR application.
The potential of raw and activated clay has been investigated by various authors (Abu-Eishah 2008; Al-Shahrani 2012; Komadel 2016). However, none of those authors have investigated the use of activated clay for SmartWater production. The material employed is locally available clay which can be activated using inexpensive methods and resources. The method has the potential to be used onsite preventing the use of trucks to transfer freshwater over a long distance. Therefore, the main objective of this work is to evaluate the potential of activated clay for the production of SmartWater from seawater. The activated clay used in this work was produced in-house using locally obtained clay.
Materials and methods
Materials
The main materials that were used are the raw clay that was obtained locally, commercial bentonite, and acid solution.
Sample collection and processing
Samples used in this work were obtained from the outcrop of clay deposits. Eight samples of the clay collected from various parts of the outcrop were analyzed. Analytical tests were performed to identify the various types of clays that are contained in each of the raw samples. Prior to activation, the clay samples were dried for 16 h at a temperature of 110 °C. The moisture removed due to the drying is ~ 12 wt%. The dried samples were then ground for various durations of time to determine the best grinding operating conditions. Ground samples were sieved, and particle size analysis was performed using sieve analysis.
Compositional analysis of raw clay
The compositional and morphological analyses of the raw clay were performed using X-ray powder diffraction (XRD), scanning electron microscope (SEM), and energy-dispersive spectroscopy (EDS).
Activation of clay
Clay samples were subjected to microwave treatment in order to extract some metal content out of the clay and create some binding sites. A variety of techniques are available for the extraction of metal content from solid samples. A technique that involves the partial dissolution of the samples was used in this work. This was chosen to limit the extraction to the solid surface and preserve the metals that are contained within silicate minerals (Chao and Sanzolone 1992). In this work, a microwave-assisted hot acid digestion method was used in combination with the procedure developed by the environmental protection agency (EPA) (EPA 1996). The method was slightly modified to suit the objective of the work. The method was executed using an automated/programmable microwave digester (MARS 6). The equipment was equipped with six sample vessels with 55 L cavity size.
One gram of the clay sample was placed in the microwave vessel and digested in 9 mL of concentrated nitric acid. The mixture was left for 15 min to allow any resulting gas (such as carbon dioxide) to escape. The sample and acid were placed in inert polymeric microwave vessels. The vessels were then sealed and heated in the microwave system. The microwave was operated at a ramp time of 20 min, hold time of 10 min, a temperature of 200 °C, and pressure of 800 psi. After cooling, the vessel contents were centrifuged for the residual (the activated clay which mainly siliceous) to settle out of the solution. Samples obtained afterward were washed several times with fresh water to remove the metallic ions. The activated clay was finally obtained by filtering the washed sample followed by air drying.
Particle size measurement
The particle size distribution of the samples was investigated by dynamic light scattering (DLS) in the water at room temperature using a Fritsch Analysette 22 MicroTec Plus Particle Size Analyzer. The measurement was done for three samples: commercial bentonite, raw clay, and the activated clay powder.
XRF analysis of elemental and oxide compositions
Elemental and oxide compositions of the samples were done using M4 Tornado µ-XRF (micro-X-ray fluorescence) by Bruker Nano GmbH, Germany. Since µ-XRF analysis is typically a spot analysis, it is difficult to obtain a perfect representative elemental composition of clay samples because no single small spot can be homogenous enough to represent the composition of the whole clay bulk. To forestall this, five carefully selected points of 10 µm diameter are picked on the samples’ surface to ensure the results obtained are relatively representative of the actual compositions. The average values of the five points and their respective standard deviations are reported. Both elemental atomic percentages and mass percentages of the oxides are determined and reported.
FTIR analysis
The FTIR analysis was performed using a fine powder of the samples. The analysis was carried out using Nicolet 6700 FTIR spectrometer by Thermo Fisher. The IR spectra were recorded for the MIR region (between 500 and 4000 cm−1). For each sample, 32 scans with a resolution of 4 cm−1 were recorded. Spectra processing was performed using the OMNIC software package.
Sample preparation for SmartWater production
The potential of clay for SmartWater production was tested using the cake filtration technique. Samples were prepared in the form of mud using 350 ml of water (seawater, and freshwater), and 22.5 g of a blend of commercial bentonite and the activated clay. The freshwater mud sample was used as a control sample. The transport properties of ions (Sodium, Calcium, Magnesium, and Potassium) through the formed cake membrane were measured using Metrohm 850 Professional Ion Chromatography (IC) equipped with MagIC Net software. The instrument was calibrated with 3-point standards prior to analysis. For the cation quantification, dilute HNO3 was used as the eluent solution. The eluent flows at 0.9 mL/min under pressure set at 7.83 MPa. Total dissolved solids were measured for both the feed and permeate using a Multi-Parameter Conductivity meter. The ability of the filter cake to tune the chemistry of seawater was calculated in terms of ion rejection.
Measurement of transport property
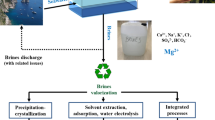
The transport property measurement method commonly employed in membrane filtration was used in this study. The filtration property is the most important variable for measuring the potential of membrane for filtration. In the practical sense, the SmartWater production is to be achieved via a cake filtration process. During the cake filtration process, suspended fluid particles are captured on the surface of a filter paper and accumulate as a filter cake. In this work, a dead-end approach was used. Therefore, the filter cake can be described as a filtration membrane whose feed is the mixture of clay and the seawater and whose permeate is the SmartWater. The overall phenomenon of cake filtration that is happening during the process could be pictorially represented using Fig. 1.
The transport properties were measure using the filtration experiments with a filter press setup to represent the cake filtration technique. Properties were measured at laboratory conditions to determine the flux and ions rejection. The filter press was used with nitrogen gas providing the necessary pressure. The prepared sample was stirred and poured into the cell fitted with filter paper underneath. The assembly was mounted on the filter press, and a graduated cylinder was placed underdrain tube to receive filtrate. 100 psi pressure was applied, and the test period was started at the same time. The filtrate volume was recorded at 5-min intervals for 60 min.
The salt rejection was calculated using the expression in Eq. 1
where \(R\) is the rejection, \(C_{\text{i}}\) and \(C_{\text{f}}\) are the initial and final ion concentrations, respectively.
Results and discussion
Characterization of raw clay
Compositional analysis was carried out to determine the elemental composition analysis of the clay that was used. Eight samples which were taken from various locations were evaluated. The results of XRD are shown in Fig. 2.
From this figure, it can be observed that the peaks for all the eight samples are quite similar to one another and the Wyoming clay. The results on this figure indicate that all the samples are similar in composition and that clay taken from any of these eight locations can be used for the intended purpose. Also, the results of the XRD indicate that the clay used in this work and the Wyoming clay have some components in common. The prominent peaks that were observed around 2θ = 27 and 60 indicate the presence of quartz. The two peaks observed at 21 and 40 may be interpreted as the presence of Felspar. Some other visible peaks at 20, 35, 37, 55, 62, 74, and 76 correspond to montmorillonite as reported for the Wyoming clay (Falode et al. 2008). The peak at 2θ = 46 is an indication of the presence of calcite.
The SEM/EDS images of selected samples from various locations are shown in Fig. 3. The images clearly revealed the morphological similarity between the clay samples. The results of the EDS analysis for the eight samples are shown in Fig. 4. The elemental analysis shown in this figure shows a similarity among all the samples. The figure reveals oxygen to be the most dominant element followed by silicon. The results also showed that the clay contains metals such as Na, Mg, Al, K, Ca, Ti, Fe, Cu, and Zn. Generally, clays are often classified based on the Na and Ca content as Ca-based and Na-based clay. To classify the clay sample used in this study, the ratio of these two metals was evaluated. The ratio Na/Ca of this clay is shown in Table 1. The results from this table showed that this ratio is greater than unity for the samples. This shows that the clay is more of Na-based clay than Ca.
Analysis of activated clay
The capability of the activated clay to perform the required tuning of the chemistry of seawater is a direct measure of its characteristic property. Sample conductivity can be regarded as a quick indicator of water quality since it is related to the sum of all ionized solutes or total dissolved solids (TDS) content of the water. Therefore, the gradual removal of the metallic ions from the residual sample was monitored by measuring the electrical conductivity of the supernatant that was obtained after washing and centrifuging the activated clay samples. The change in the values of the conductivity of the sample supernatant is shown in Figs. 5 and 6. Figure 5 contains the conductivity of the first supernatant (the activated clay solution as it was removed from the microwave oven) immediately after treatment. Six vessels were used to perform microwave treatment. Two out of the six samples were then chosen, and their conductivity values were monitored with respect to the number of washing. Results of the conductivity of the supernatant corresponding to the number of washing are shown in Fig. 6. Activated clay samples were washed ten times to remove binding metallic ions. From this figure, it can be observed that the conductivity became practically constant after the fourth washing. This is an indication that all the free cations have been washed away during the first four washing (Fig. 7).
Particle size measurement
Particle size analysis was done for the commercial bentonite clay, raw clay, and the activated clay samples. The results of the particle size analysis revealed activated clay samples with heterogeneous particle size distribution. The average size of the commercial bentonite clay was observed to be 1 µm with a specific surface area of 102,184 cm2/cm3. The raw clay powder has an average size of 7 µm with a specific surface area of 42,071 cm2/cm3, while the activated clay has an average size of 12 µm and a specific surface area of 18,987 cm2/cm3. Clearly, the commercial bentonite particles are the smallest but have the highest surface area. On the other hand, the activated clay has the highest particle size with the lowest surface area. The initial particle of the raw clay was observed to have influenced the particle of the activated clay. The microwave treatment, therefore, was observed to have resulted in an increase in particle size which consequently reduced the surface area. The activated particle size increased by about 71%, while the specific surface area dropped by 55% as compared with the raw clay. Despite the lowest surface area, the activated clay particles demonstrated the best ability to control the transport properties of the clay as will be explained in the subsequent sections.
FTIR analysis
FTIR was performed for structural determination of samples by interpreting characteristic spectra ranges which resulted from molecular vibrations of a specific structural group. The FTIR results are shown in Fig. 8. For all the samples, small peaks that were observed around 500 and 920 are characteristics bands for the stretching and bending vibrations of the hydroxyl group (OH) contained within the clay. The OH bending vibrations have been observed at ≈ 920 cm−1 for AlAlOH, ≈ 880 cm−1 for AlFeOH, and ≈ 850 cm−1 for AlMgOH (Gates et al. 2017). The spectra at around 3620 and 647, 668 and 667 cm−1, can be attributed to the OH stretching and bending for minerals containing Mg(II) and Li(I) in the octahedral sheet (Madejová et al. 2009). The spectra at 3620 cm−1 were found only for the raw clay and the bentonite. This spectrum disappeared after the activation and hence was not found in the activated clay due to the removal of certain metallic ions such as Mg. The method of activation used in this work was carefully performed to preserve the metals that are contained within the clay thereby preserving the clay structure. The presence of the spectra at 647 and 668 cm−1 for raw clay and activated clay, respectively, is an indication of the presence of a small amount of metal which may mean that the structure of the clay is not destroyed. Bands were observed to also occur at 993, 1060, and 991 for raw clay, activated clay, and bentonite, respectively. In many FTIR results, the bands around these peaks have been attributed to Si–O stretching vibration of the tetrahedral sheet of clay (Madejová et al. 2009).
On a general note, the peaks of bentonite and raw clay are much similar. This is in line with the results of XRD in Fig. 2. Moreover, there is a clear difference between the peaks of the three curves between 1000 and 2000 cm−1. The absence of peaks within this range for the activated clay can infer the absence of certain metallic ions and functional groups from the samples. Also, the gradual decrease or the disappearance of certain spectra can be interpreted as diminishing in the content of the atoms which causes that particular spectra (Madejová et al. 2009).
Furthermore, the shift in Si–O stretching was reported to be due to the formation of an amorphous silica phase (Komadel 2016). This fact was later corroborated by the results of the transport properties which will be explained in a later section of this manuscript.
Based on the FTIR results, it can be inferred that the microwave treatment has no significant effect on the structure of the silicate within the clay bentonite.
Energy-dispersive X-ray spectroscopy (EDS)
The EDS was done to evaluate the elemental compositions of the clay before and after the activation process. The results of this analysis are shown in Figs. 9 and 10. The results in Fig. 9 show that the raw clay contains a large amount of Si and O. The major metallic ions are Al and Fe, while other components such as Mg, Na, Cl, Ca, Ti, Cu and Ka are also present but in relatively smaller percentages. It was evident in Fig. 10 that all the metals have been removed except Al which is about 4 wt% of the activated clay (Figs. 11, 12).
XRF analysis
The XRF measurement was also performed to complement our understanding of the structure and composition of the clay obtained after treatment. The mean values of these measurements are the averages of atomic weight percent obtained from 5 readings. The results obtained from the XRF measurement for the elemental composition are shown in Table 2. As shown in this table, the activation process has removed acid-soluble species in the raw clay leaving mainly the insoluble ones (Si). Element such as magnesium was observed to have been removed totally. Other elements (apart from Si) constitute less than 3% of the amount of the elements present in the activated clay.
The results of the XRF measurement for the composition of oxide are shown in Table 3. The activated clay is observed to be mainly SiO2. The SiO2 compositions of the three samples are 63.941, 55.558, and 96.258 for the bentonite, raw clay, and the activated clay, respectively. The activated clay has the highest percentage of SiO2 due to the removal of most of the constituent metals.
Transport properties
The transport properties were evaluated with respect to the solute’s concentration and individual concentration of ions. Mud was prepared from bentonite and blends of bentonite, clay, and activated clay. Freshwater and synthetic seawater were used in preparing the mud. The mud samples were used to form filter cake which was used as the filter media. Table 4 displays the bulk solute concentration of the filtrate obtained from pure bentonite mud and a mixture of activated clay and bentonite mud. The concentration was measured in terms of conductivity. The results from this table showed that the filter cake has some capability for rejecting the solutes and tune the chemistry of the water used in preparing the mud. The filter cake property is expected to change with time. Therefore, the filtrate sample was collected and tested after every 5 min. For pure bentonite, there was a decrease in the concentration of the brine solution by about 5% within the first 5 min of filtration. It was observed that this change in concentration increased to 6.5, 7.5, and 8.3 at 10, 30, and 40 min, respectively. From the 40th minute onward, the increase was noticed to be very insignificant. This is an indication of some equilibrium filter cake properties and the average conductivity between 40 and 60th min was found to be 8.5 mS/cm.
The use of charged particles for tuning the chemistry of the seawater to produce SmartWater was investigated using in-house prepared activated clay. The effect of the activated clay on solutes transport is shown in Table 4. It was observed that the filter cake containing activated clay had better control over the solutes transport than the ones containing only pure bentonite. The concentration of the solute in the permeate (filtrate) was found to drop by 12.7, 13.3, 13.4, and 13.1% for the first 5, 20, 30, and 40 min, respectively. Unlike the pure bentonite filter cake, the concentration of the permeating seawater was found to be almost constant above the 10th min. The average change in concentration between 20 and 60 was found to be 13.6%.
The results of this table clearly revealed that there is a change between the chemistry of the initial seawater and the filtrate after passing through the formed filter cake. However, there is no clear indication as to which particular ion has been removed from the seawater as it passes through the filter cake. To get the clear knowledge of the specific ions that were removed from the seawater, another analysis was conducted to identify individual ions contained in the filtrate and the seawater using the ion chromatography. The test conducted on individual ions was done for Na+, K+, Mg2+, and Ca2+. The results are shown in Fig. 5 and Fig. 6. The figures contain the percentage cation rejection by the pure bentonite filter cake and activated clay filter cake, respectively. The results obtained confirmed that all the filter cakes exhibit some abilities to control the transport of the ions that are contained in the seawater passing through them. However, the extent of the control differs from one filter cake to another. In Fig. 5, which contains the results for pure bentonite filter cake, an equilibrium concentration was observed as early as around 5–10 min into the filtration experiment (for some ions such as K+ and Mg2+). For example, the rejection of Mg2+ was 49.5% at 5 min. It later increased to 54.8 at 10 min. There were no significant changes in its concentration afterward. The concentration at 60 min is 55.2, while the average concentration between 10 and 60 min is 54. The rejection values obtained for Na+ and Ca2+ were 0.54 and 100% of the initial brine concentration, respectively, within the first 5 min. This indicates that the pure bentonite cake has a very minimal restriction on the transport of Na+, while it exhibits very high restriction on Ca2+ within this time.
Generally, there appeared to be some fluctuations in the concentration of Na+ with time. The rejection recorded for this ion does not follow any regular pattern except toward the end of the experiment. For example, the rejection values recorded at 50 and 60 min are 6.0 and 10.9, respectively. The rejection of Ca2+ was also observed to drop by about 13% at the 10th minute as compared to the value at the 5th minute. From 10 min, the rejection was observed to increase gradually from 87.8% at 10 min to 99.5% at 30 min. For the pure bentonite filter cake, rejection for Mg2+ is about half that for Ca2+. Sodium ions (Na+) are the least rejected of all the ions despite been the ions with the highest concentration in the original brine solution.
The filter cake obtained by the addition of 5wt % activated clay to pure bentonite was observed to have a significant effect on Na+, Mg2+, and Ca2+. The transport property of Na+ through the mixed-matrix was found to follow a more regular pattern than for the pure bentonite cake. A regular trend of behavior was seen from 10 min up to 60th minute. The rejection was observed to increase gradually with time within this period. There seems to be no significant effect on the transport property of K+ by the addition of the activated clay. The rejection increased by a range of 57–72.5% compared to the rejection obtained for the pure bentonite. Conversely, the rejection of Ca2+ was observed to drop due to the addition of the clay.
The addition of 5 wt% of activated clay increased the retention of Na+ and Mg2+ to 6.5 and 85.5%, respectively. Measurements were made for up to 60 min. The change in the rejection observed after 5 min was insignificant except for Na+ in which the recorded value is 9.3% at 60 min. It can, therefore, be concluded that the capability of the cake to produce SmartWater could be sustained for a longer period.
The cation exchange is one of the basic properties of bentonite which can arise from negative charge creation caused by the isomorphous substitution in the octahedral and tetrahedral sheets of montmorillonite (the predominant clay mineral in bentonite). The negative charges are therefore balanced by exchangeable cations. The preference to replace cations present in clay minerals by external ions is dominated by some factors among which is the preference of larger inorganic cation to smaller inorganic cation. For smectite, the preference is as follows:
In this work, the obtained rejection results (for the pure bentonite filter cake) closely followed the above trend for certain ions (Ca2+ > Mg2+ and K+ > Na+). Moreover, the trend was reversed by the addition of the activated clay (Mg2+ > Ca2+ and K+> Na+). The reaction site that was created from the activation process seems to possess more affinity for the Mg2+ than Ca2+ ions. Details explanation of the mechanism of what is happening needs to be further investigated. The effect of charged (sulfonated) polymers on the diffusion of sodium chloride was reported by Geise et al. (2012). The property of the charged polymers is comparable to that of bentonite. These polymers have fixed charges that contain an excess of counter-ions than needed to maintain electro-neutrality in the polymer. Therefore, such a cation exchange material would sorb in more cations than anions. Closely related principle was employed to investigate the effect of activated clay on the transport of solutes used in this work.
Another factor is the preference of bentonite for heterovalent cations over monovalent cations (Muurinen 2011). Also, the binding coefficients on montmorillonite-based bentonite for Na+ and K+ are 1 and 2 M−1 (Nir et al. 1986), respectively, while that of Mg2+ and Ca2+ are 2 and 4–40 M−1 (Rytwo et al. 1996), respectively. Taking these factors into consideration, the most preferred cation for cation exchange would be Ca2+, while the least preferred for cation exchange would be Na+. This is also evident in the percentage rejection with Ca2+ being the most rejected (100%), while Na+ was the least rejected (0.54%) in the first 5 min. This level of preferential rejection was consistently observed throughout the experiment. The binding coefficient of K+ and Mg2+ on montmorillonite is the same, indicating an almost equal preference for an exchange on bentonite and this is also observed in their close percentage rejection in this work.
The residue obtained following the activation of the clay was mainly silica. The incorporation of silica into the bentonite induces surface and structural modification which enhances the cation rejection property of the modified material for sodium and magnesium ions with a 1103.5% and 72.5% increase in rejection for Na+ and Mg2+ within the first 5 min, respectively.
It is proposed that the main mechanism behind the cation rejection is cation exchange in bentonite, while the little contribution from other processes such as surface precipitation, adsorption, and dissolution may be involved as proposed by Bergaya and his group (Bergaya et al. 2006).
Conclusion and recommendations
The work therein presents a new approach for producing SmartWater via cake filtration for application in enhanced oil recovery. The filter cake exhibits preferential rejection capabilities in the sequence Ca2+ > Mg2+ > K+ > Na2+ for the cake formed from pure bentonite, while the sequence is Mg2+ > Ca2+ > K+ > Na2+ for the cake in which activated clay has been added. Thus, the presence of the activated clay serves to reverse the selectivity of the pure bentonite filter cake with respect to Ca2+ and Mg2+. The outcome of this work is expected to open a new frontier in tuning the chemistry of seawater for SmartWater production. It will also enhance our ability to engineer other potential cheap and locally available materials which can be tailored toward enhancing the chemistry of seawater for SmartWater application in water flooding.
References
Abu-Eishah SI (2008) Removal of Zn, Cd, and Pb Ions from water by Sarooj clay. Appl Clay Sci 42(1–2):201–205
Adewole JK, Sultan A (2014) A study on processing and chemical composition of date pit powder for application in enhanced oil recovery. In: Defect and diffusion forum. Trans Tech Publ, vol 353, pp 79–83
Al-Mutairi SM, Kokal SL (2011) EOR potential in the middle east: current and future trends. In: SPE EUROPEC/EAGE annual conference and exhibition, 2011: society of petroleum engineers
Al-Shahrani S (2012) Treatment of wastewater contaminated with nickel using Khulays activated bentonite. Int J Eng Techn 12(4):14–18
Al-sofi AM, Yousef AA (2013) Insight into smart-water recovery mechanism through detailed history matching of coreflood experiments. In: JJ Sheng (ed.) SPE reservoir characterization and simulation conference and exhibition, 2013: Society of Petroleum Engineers
Austad T (2013) Water-based EOR in carbonates and sandstones: new chemical understanding of the EOR potential using “smart water”. In: Enhanced oil recovery Field case studies. Elsevier, Amsterdam, pp 301–335
Bergaya F, Lagaly G, Vayer M (2006) Chapter 12.10 cation and anion exchange. In: Bergaya F, Theng BKG, Lagaly G (eds) Developments in clay science, vol 1. Elsevier, Amsterdam, pp 979–1001
Chao T, Sanzolone R (1992) Decomposition techniques. J Geochem Explor 44(1–3):65–106
EPA D (1996). Method 3050B. Acid digestion of sediments, sludges, and soils. Revision 2. Test Methods for Evaluating Solid Wastes: Physical/Chemical Methods, EPA SW-846Section a, 3050B-3051e3050B
Falode O, Ehinola O, Nebeife P (2008) Evaluation of local bentonitic clay as oil well drilling fluids in Nigeria. Appl Clay Sci 39(1–2):19–27
Fathi SJ, Austad T, Strand S (2012) Water-based enhanced oil recovery (EOR) by” Smart Water” in Carbonate Reservoirs. In: SPE EOR conference at oil and gas West Asia, 2012: society of petroleum engineers
Gachuz-Muro H, Sohrabi M (2014) Smart water injection for heavy oil recovery from naturally fractured reservoirs. In: SPE heavy and extra heavy oil conference: Latin America, Society of Petroleum Engineers
Gates W, Kloprogge JT, Madejova J, Bergaya F (2017) Infrared and Raman spectroscopies of clay minerals, vol 1. Elsevier, Amsterdam
Geise GM, Falcon LP, Freeman BD, Paul DR (2012) Sodium chloride sorption in sulfonated polymers for membrane applications. J Membr Sci 423:195–208
Gupta R, Smith GG, Hu L, Willingham T, Lo Cascio M, Shyeh JJ et al (2011) Enhanced waterflood for carbonate reservoirs-impact of injection water composition. In: SPE Middle East oil and gas show and conference, 2011: Society of Petroleum Engineers
Komadel P (2016) Acid activated clays: materials in continuous demand. Appl Clay Sci 131:84–99
Liu Z (2015) Chapter 4—supply and demand of global energy and electricity. In: Liu Z (ed) Glob Energy Interconnect. Academic Press, Boston, pp 101–182
Madejová J, Pálková H, Pentrák M, Komadel P (2009) Near-infrared spectroscopic analysis of acid-treated organo-clays. Clays Clay Miner 57(3):392–403
Mahmoud M, Elkatatny S, Abdelgawad KZ (2017) Using high-and low-salinity seawater injection to maintain the oil reservoir pressure without damage. J Petrol Explor Prod Technol 7(2):589–596
Mamonov A, Puntervold T, Strand S (2017) EOR by smart water flooding in sandstone reservoirs-effect of sandstone mineralogy on initial wetting and oil recovery. In: SPE Russian petroleum technology conference, 2017: Society Of Petroleum Engineers
Manrique EJ, Muci VE, Gurfinkel ME (2007) EOR field experiences in carbonate reservoirs in the United States. SPE Reservoir Eval Eng 10(06):667–686
Muurinen A (2011) Measurements on cation exchange capacity of bentonite in the long-term test of buffer material (LOT). (POSIVA-WR--11-10). Finland It is a Working Report which was pubished online at https://inis.iaea.org/search/search.aspx?orig_q=RN:43107417
Nair RR, Protasova E, Bilstad T (2016) Smart water for enhanced oil recovery by nano-filtration. J Pet Environ Biotechnol 7:1–8
Nir S, Hirsch D, Navrot J, Banin A (1986) Specific adsorption of lithium, sodium, potassium, and strontium to montmorillonite: observations and predictions 1. Soil Sci Soc Am J 50(1):40–45
Puntervold T, Strand S, Austad T (2009) Coinjection of seawater and produced water to improve oil recovery from fractured North Sea chalk oil reservoirs. Energy Fuels 23(5):2527–2536
Rytwo G, Banin A, Nir S (1996) Exchange reactions in the Ca–Mg–Na-montmorillonite system. Clays Clay Miner 44(2):276–285
Sarvestani AD, Ayatollahi S, Moghaddam MB (2019) Smart water flooding performance in carbonate reservoirs: an experimental approach for tertiary oil recovery. J Petrol Explor Prod Technol 9:2643–2657
Strand S, Høgnesen EJ, Austad T (2006) Wettability alteration of carbonates—effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf A 275(1–3):1–10
Strand S, Austad T, Puntervold T, Høgnesen EJ, Olsen M, Barstad SMF (2008) “Smart water” for oil recovery from fractured limestone: a preliminary study. Energy Fuels 22(5):3126–3133
Acknowledgements
The authors will like to thank the Center for Integrative Petroleum Research, College of Petroleum Engineering and Geosciences, King Fahd University Petroleum Minerals, Saudi Arabia, for her supports. Our appreciation also goes to Dr. Abdullah S. Sultan (College of Petroleum Engineering and Geosciences), Messrs Hatim Dafalla, and Abdulrashid I. Mohammed, Center for Engineering Research, KFUPM, Mr. Musa O. Najimu, University of South Carolina, USA, and Mr. Kazeem Muhammad, University of Birmingham, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adewole, J.K., Kazeem, T.S. & Oyehan, T.A. Tuning the chemistry of seawater with activated clay: an application in SmartWater enrichment for enhanced oil recovery. J Petrol Explor Prod Technol 10, 3905–3916 (2020). https://doi.org/10.1007/s13202-020-00943-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-00943-z