Abstract
For controlling the viscous fingering in water-alternating gas injection, addition of foam with formation water is more favorable. Use of foam surfactant is one potential solution for reducing gas mobility. The main objective of this research is to generate stable foam for gas mobility control using surfactant blend formulation. Surfactant blends synergistically exhibit better foaming properties than those of individual surfactants. Surfactant blends improve the foam stability and reduces the destabilizing effect of crude oil. Using foam stabilizers may improve foam stability and apparent viscosity; both of these factors are important for improving gas mobility. Alpha olefin Sulfonate (AOSC14-16) was selected as main surfactant, Octylphenol Ethylene Oxide (TX-100) and Lauryl Amido Propyl Amine oxide (LMDO) were selected as additives. Aqueous stability test was performed at 96 °C. Foam stability test was performed in the absence and presence of crude oil. The foam stability and longevity was recorded above the liquid level. Liquid drainage and Foam half-life were noted with respect to time. The mobility reduction factor of three formulations was performed with CO2 by using Berea sandstone cores at 96 °C and 1400 psi. Experimental result showed that surfactant blend of 0.6 % AOS + 0.6 % LMDO was more stable in presence of crude oil and reduced more gas mobility as compared to an individual surfactant of 0.6 % AOS. The maximum generated foam volume and foam half time indicated better performance of the foaming agent. The surfactant blend formulation plays an important role in controlling gas mobility. Strong stability by these formulations indicates that the foam surfactant formulation is of great significance in the field of enhanced oil recovery.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In water-alternating gas injection, gas is used as a driving force to enhance oil recovery. The advantage of gas injection is its better microscopic sweep efficiency as compared to water flood (Lake 1989). The challenge associated with injected gas is poor volumetric sweep efficiency. Therefore, the injected gas does not contact a large fraction of crude oil (Wellington and Vinegar 1988; Rosen 2004). In the application of WAG or gas injection, the high mobility and low density of gas leads the gas to flow in channels through the high permeable zones and rise to the top of the reservoir by gravity segregation. Foam surfactants are a potential solution for the above mentioned challenges. It can improve the sweep efficiency by increasing the effective viscosity and decreasing the relative permeability of the gas (Sunmonu and Onyekonwu 2013). Foams are compressible fluids due to the gas presence and can undergo compression and decompression cycles because of the elasticity of liquid films. These films are stabilized by surfactant molecules concentrated at the gas/surfactant solution interface (Al-Attar 2011).
Surfactants have been used for lowering the IFT and are used for tertiary oil recovery for more than 35 years, mostly in United States of America, in depleted oil reservoirs after water flooding (Lv et al. 2011). Foam is a mixture of gas, water and foaming agent (Surfactant). There are different ways to generate foam. Each method has its own way of introducing gas into the foaming solution. For example, a small amount of foaming agent that contacts with gas can generate foam by disturbing or shaking a liquid (Sheng 2013). Foam longevity depends on the stability and is influenced by the following: surfactant concentration, salt concentration, solid/liquid adsorption, gas diffusion through foam films, gravitational drainage, surface forces, capillary forces, fluctuations (Klitzing and Müller 2002; Aronson et al. 1994).
Good stable foam should be needed for reducing CO2 mobility in the porous media. Several published literature suggested that, the strong foam can be generated at relatively high oil saturations (Aarra et al. 1997; Mannhardt and Svorstøl 1997, 2001). This may be measured by Mobility Reduction factor (MRF). MRF is the ratio of pressure drop caused by the simultaneous flow of gas and liquid through the rock (core samples) in the presence and absence of surfactant in the aqueous phase (Stevenson 2012).
The combination of different type of surfactants synergistically exhibit better foaming properties than those of its individual components. Blend of anionic and nonionic foam forming a surfactant formulation can improve the CO2 mobility control at high salinity with percentage of divalent ions. The formulation can be used at high temperature with minimum cost of foamy surfactants. This study presents the stability of foam surfactant blend in absence and presence of crude oil at atmospheric conditions. Further, these surfactant formulations are analyzed by core flooding at reservoir conditions. Mobility reduction factor is measured through differential pressure across the sandstone core samples by injecting CO2 before and after foam surfactant formulations.
Literature review
Mobility control is achieved by WAG and SWAG due to simultaneous flow of two phases (Stephenson et al. 1993; Robie et al. 1995; Sohrabi et al. 2000). In these processes, simultaneous injection of gas and surfactant slug may result in foam generation. Foam can increase the apparent viscosity of gas and thus increase the gas saturation. The increase in gas saturation results in decrease of oil saturation and relative permeability of oil. Thus, the foam generation improves mobility control in the process (Farnazeh and Sohrabi 2013). Figure 1 presents the concept of reducing gas mobility. Gas bubbles are surrounded by thin films of liquid (lamellae). These lamellae surrounded by foam bubbles may be stationary or in motion. Ability of foam to reduce gas mobility depends strongly on its texture (bubble size or number of lamellae per unit volume). The gas trapped by stationary lamellae is immobilized and reduces the gas saturation. Stationary lamellae alter gas flow paths and block the movement of gas flow resulting in the reduction of gas relative permeability. Lamellae in motion along the pore walls increase resistance to flow of the flowing gas. This cannot increase the actual gas viscosity due to only moving a portion of gas, increasing resistance to flow. The effect of moving lamellae resulted in an increase in apparent gas viscosity. Therefore, a foam surfactant reduces the gas mobility by reducing the gas relative permeability and increasing the apparent gas viscosity (Friedmann et al. 1991; Kloet et al. 2009).
The world’s largest application of foam assisted WAG was implemented at the Snorre, North Sea sandstone reservoirs. Foam assisted WAG has qualified foam as a gas mobility agent for North Sea reservoirs. Foam is generated by injecting gas and surfactant solution in a surfactant alternating gas (SAG) mode. In the North Sea AOSs (Alpha Olefin Sulfonate) have been successfully used as foaming agents for controlling gas mobility (Skauge et al. 2002; Blaker et al. 2002; Aarra et al. 2002).
Tsau et al. (1999) have used surfactant blends to improve gas mobility control in CO2 flooding. Farzaneh and Sohrabi, (2013) have observed that, the blend of anionic and nonionic surfactant showed better foaming stability, mobility reduction and less adsorption than that generated by an anionic surfactant alone. Andrianov et al. (2012) have reported a laboratory study of foam for improving immiscible WAG injection. Formulation of surfactant blend was tested in order to see the effect of generated good foam and its stability in oil. They concluded that, foam increases oil recovery by 10 % . Schramm and Green (1995)have carried out MRF test at atmospheric condition. Wassmuth et al. (1999) have performed study on scale up evaluation and simulation of mobility control foams for improved oil recovery. They used short and long Berea core samples at temperature 23 °C and pressure 1000 psi. N2 gas was used for injection. Chevron Chaser GR1080 with 0.5 wt% was used. They worked on modeling and MRF at different injection rates. In addition result was compared with different generated foams (Wassmuth et al. 1999).
Methodology
Material
Foam surfactants
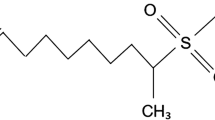
Alpha Olefin Sulfonate AOSC14-16 and Lauryl Amido Propyl Amine oxide (LMDO) were provided by Stepan Company USA. Octylphenol ethoxylate surfactant (Triton X-100) was purchased from Sigma-Aldrich.
Salts for synthetic brine (injection water)
Sodium Chloride (Nacl) was purchased from Fischer Company U.K., Magnesium chloride (MgCl2·6H2O), Potassium chloride (KCL), Sodium bicarbonate (NaHCO3), Sodium sulfate (Na2So4) and Calcium Chloride (CaCl2·2H2O) were purchased from R & M. Chemicals U.K. Table 1 presents the composition of synthetic brine.
Crude oil
Crude oil was collected from an oil field offshore of Malaysia. The density of oil was measured as 0.7886 g/cm3 (49.2307 Ib/ft3) at 96 °C and 1400 psi by Anton Par density meter and viscosity was measured as 1.591 cp by High Temperature High Pressure Electromagnetic Viscometer (HTHP EV-1000). The specific gravity of crude oil is 0.7889, and degree API is 37.7. The specific gravity and degree API is calculated by using following formula.
Specific gravity
where \( \gamma_{o} = \) Specific gravity of oil \( \rho_{o} = \) Density of oil (Ib/ft3) \( \rho_{w} = \) Density of water (62.4 Ib/ft3).
Molecular weight of crude oil is 189.850 kg/Kmol (Moradi et al. 2014). Table 2 presents the crude oil composition at atmospheric conditions.
CO2 gas
Pure CO2 gas was selected. The density of CO2 at 96 °C and 1400 psi was measured as 0.18 g/cm3. Viscosity at 96 °C was measured as 0.018 cp.
Berea sandstone properties
Berea sandstones were selected due to the hardness of its quartz grains bounded by silica. These core samples possess a chemical resistance to the erosive action of the acidic chemical. Further, silica bond does not deteriorate with temperature change and time. These types of Berea sandstone are considered as an excellent sandstone for lab experiments, particularly in enhanced oil recovery (EOR). Table 3 presents the properties of Berea core samples used in MRF experiments.
Foam stability test procedure
For screening the surfactant blend solutions for their ability, foam stability tests were performed at atmospheric conditions. These tests provide ideas of a possible interaction between the target crude oil and the particular blend of surfactant formulation. Figure 2 shows the schematic diagram of foam generation process. 20 ml of the foam surfactant solution was transferred into a 100 ml graduated cup. Compact stirrer attached with Mettler Toledo 50 was used. The cup was fixed in the Mettler Toledo. The stirrer was used with speed of 90 % (3420 rpm) with uniform agitation for 5 min. After 5 min, stirrer was stopped and foam height at time equal to zero was recorded. The cup was unfixed from the system and covered with an aluminum sheet and placed on table. Liquid drainage time was noted when liquid drained out and reached 50 ml. Foam half-time of generated foam was noted (Duan et al. 2014). Further, foam stability and longevity was noted above liquid level with respect to time and foam height in 100 ml graduated cup.
The same procedure was repeated with addition of 1 ml crude oil before mixing in the solution. Due to waxy crude oil, the test was performed at 50 °C by using hot air through a dryer. Oil was dispersed in the surfactant solution during mixing at 90 % (3420 rpm) with uniform agitation for 5 min. During the measurement process, crude oil with foam surfactant formulation was considered as (Llave and Olsen 1994).
Mobility reduction factor
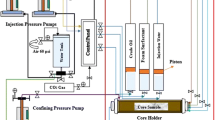
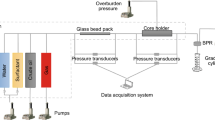
MRF test was performed by HTHP relative permeability test system (RPS) 8000–10,000, TEMCO, Inc. USA. Figure 3 presents the MRF experiments performed by using high temperature high pressure HTHP relative permeability system. MRF is the ratio of pressure drop caused by the simultaneous flow of gas and liquid through the rock (core samples) in presence and absence of surfactant in aqueous phase (Hirasaki et al. 1997; Mannhardt et al. 2000; Schramm 1994; Rosman and Kam 2009). The Mobility reduction factor may be calculated as;
To generate the foam in the Berea sandstone core samples, experiments were performed with following method at temperature 96 °C and 1400 psi.
Before starting the MRF experiments, core samples were vacuumed with brine for 48 h. Figure 4 presents the schematic diagram of MRF experiments performed at reservoir conditions. Core sample was settled in the core holder. Accumulators A, B and C were filled with CO2, brine and foam surfactant. These all were settled inside the oven at temperature 96 °C. Other required data such as weight of saturated and dry core sample were put in the software. The fluid (brine, CO2 and foam surfactant) were pumped from the accumulator to the core by a syringe type pump. The effluent was collected in the graduated cylinder. Each fluid was injected when differential pressure (Δp) reached steady state. The main four slugs (brine, CO2, surfactant, CO2) were injected to determine the foam presence and propagation through the Berea core samples.
Results and discussion
Foam stability
Before foam stability experiments, these surfactant formulations were tested at 96 °C in presence of brine water for 3–4 weeks. No precipitations were seen by using these formulations. Foam stability tests were performed at room temperature and atmosphere pressure. Foam stability and longevity of foam surfactant blend formulations are presented in the Table 4. These formulations were measured in absence and presence of crude oil.
Figure 5 presents the foam generated in absence and presence of crude oil by 0.6 % AOS (MK1). At the initial time 70 ml foam was generated from 20 ml solution of MK1. The foam height decreased slowly. After 90 min, the foam height of MK1 was recorded as 40 ml above the liquid level. In presence of crude oil, the foam height was recorded as 70 ml at the initial time. The solution showed good interaction in the presence of crude oil. The durability of this surfactant solution was recorded after 90 min as 3 ml in presence of crude oil.
Figures 6, 7 present the foam generated in absence and presence of crude oil by using foam surfactant blend of 0.2 % AOS + 0.2 % TX-100 (MK2) and 0.6 % AOS + 0.6 % LMDO (MK3). At the initial time 65 ml foam was generated from 20 ml blend solution of MK2 and 75 ml foam from blend MK3. Once the foam was generated in the 100 ml cup, the foam volume was reduced because liquid drains through the lamellae due to the force of gravity. The lamellae in the upper layer of the foam were thinner than the lower layer of the foam due to the gravity drainage. After 90 min the foam height of MK2 blend was noted as 30 ml above the liquid level in the cup whereas, 20 ml was noted from MK3. When these two formulations of surfactant blends were tested in presence of crude oil, the foam height was noted as 35 ml from MK2 and 75 ml from MK3 at the initial time. MK3 surfactant blend formulation generated strong foam as compared to surfactant formulation of MK2 in the presence of crude oil. The durability of these surfactant solutions was observed. After 30 min, the foam height was noted as 3 ml from surfactant blend of MK2 whereas surfactant blend MK3 showed 20 ml in presence of crude oil. Surfactant blend of MK3 generated foam volume greater than individual surfactant of MK1. Foam stability was increased by MK3 because its hydrophobic group of a straight chain surfactant is moved to a more central position in the molecules as proved by Rossen (2004). Another reason is use of co-surfactant as an additives with 0.6 %AOS (MK1).
The maximum foam height in presence of crude oil was recorded by surfactant blend MK3 as compared to surfactant formulations of MK1 and MK2. Foam height generally increases with increase in surfactant concentration. The lower the surface tension of the aqueous solution the greater appears to be the foam volume. The foam volume is produced when a given amount of work is done on a surfactant aqueous solution to generate foam. Foam height increases with increase in the length of the chain, because interaction cohesion increases with increase in the length of hydrophobic group. Further, the liquid drainage in absence and presence of crude oil by this surfactant blend of MK3 was slower as compared to surfactant formulation of MK1 and MK2. Slower liquid drainage by this surfactant blend was due to the presence of small bubbles in generated foam. Figure 8 presents the generated foam in presence of crude oil by surfactant blend of MK3.
Foam half time
Half time of generated foam is referred as a time at which height of the foam column reaches half of its initial value. Larger half time of generated foam corresponds to a more stable foam. In the absence of crude oil, individual surfactant 0.6 % AOS exhibited the most stable foam with foam half time of 90 min and surfactant blend formulations 0.2 % AOS + 0.2 % TX100, and 0.6 % AOS + 0.6 % LMDO were recorded as 50 and 30 min. In presence of crude oil foam half time of an individual surfactant was 10 min and two surfactant blend formulations were recorded as 6 and 20 min, respectively. In presence of crude oil, the half time by tested blended and individual formulations was found to be less as compared to the foam generated in absence of crude oil. This indicated that the oil phase weakens the generated foam stability. However, the effect of oil on foam stability depends on surfactant type.
The foam generated by surfactant formulations was most stable in the aqueous phase but largely influenced by oil. Their foam half time in presence of crude oil is smaller than that in the absence of crude oil. This showed that the generated stable foam without oil does not necessarily imply equally stable foam in presence of crude oil. The foaming volume and foam half time, in presence of crude oil, from three foam surfactant formulations are decreased. This is because of surfactant molecules move from oil/water to oil phase. Therefore, in presence of crude oil, the foam stability decreased. Surfactant blend formulation can be ranked as a function of their foam half time in presence of crude oil as 0.6 % AOS + 0.6 % LMDO > 0.6 % AOS.
Gas mobility reduction
The effect of foam on gas mobility was measured by using core flood tests. Berea sandstone core samples were used for mobility reduction factor calculations. CO2 was injected before and after surfactant injection at reservoir conditions. CO2 differential pressure before and after foam surfactant was monitored.
Figure 9 shows the differential pressure of MK1 core sample as a function of time. Differential pressure was increased when second slug of CO2 was injected. Increase in differential pressure indicates that SAG injection process has generated sufficient foam inside core to reduce the gas mobility and improve sweep efficiency. The increase in MRF was observed due to high concentration of surfactant solution. Foam generated by this surfactant is considered as strong. Generated foam may contain large number of lamellae with small bubble size by this surfactant formulation. It also reduces gas mobility because of finely textured foam.
Figure 10 shows the differential pressure of MK2 core sample as a function of time. The differential pressure was increased when second slug of CO2 was injected after surfactant blend of 0.2 % AOS + 0.2 % TX-100. Small increase in differential pressure of second CO2 slug after surfactant injection indicates that SAG injection process has generated less foam inside core to reduce the gas mobility and improve sweep efficiency. The small increase in MRF was observed due to low concentration of foam surfactant solution. Foam generated by this surfactant blend formulation was not considered as strong foam due to low concentration. It provides a moderate reduction in gas mobility due to coarsely textured foam.
Same behavior of pressure differential profile was observed by using surfactant blend MK3 as recorded in core MK1. Figure 11 shows differential pressure of MK3 as a function of time. The increase in MRF was observed due to high concentration of foam surfactant solution with additives. Strong foam was generated by this surfactant blend formulation.
MRF result showed that new blended surfactant formulations generated foam in core samples at reservoir conditions. It proved one of the statements by Sheng (2013) that, MRF value greater than one shows the presence of foam in the core at reservoir conditions. MRF range is considered as 1–10,000. If MRF is less than one (MRF <1), it means there is no foam present in the core (Sheng 2013). It is clear that, the MRF of three measured foam surfactant formulations increases with increase of injected surfactant volume. The MRF of blended foam surfactant formulation (MK3) was greater than individual measured surfactant (MK1). Higher pressure differential (Δp) values from MRF experiment indicate greater reduction of gas relative permeability and increase in the effective gas viscosity. The greatest mobility reduction typically occurs when the surfactant is dissolved in CO2 phases. These results are confirmed with obtained results by Xing et al. (2010) using Berea sandstone cores. The maximum MRF was recorded by surfactant blend of 0.6 % AOS + 0.6 % LMDO (MK3).
Conclusions
The more stable foam was observed in presence of crude oil by the surfactant blend of 0.6 % AOS + 0.6 % LMDO as compared to individual surfactant of 0.6 % AOS.
-
Foam stability increases with increasing surfactant concentration. The blended formulation improves the foam stability.
-
In presence of crude oil, the half time by tested surfactant blend and individual formulations were found to be less as compared to the foam generated in absence of crude oil.
-
Higher pressure differential (Δp) values by MRF experiments indicated greater reduction of gas relative permeability (gas mobility). Strong stability by these formulations indicated that the foam surfactant formulations are of great significance in the field of enhanced oil recovery.
References
Aarra M, Ormehaug P, Skauge A (1997) Foams for GOR control-improved stability by polymer additives. In: IOR 1997-9th European Symposium on Improved Oil Recovery
Aarra M, Skauge A, Martinsen H (2002) FAWAG: a breakthrough for EOR in the North Sea. In: SPE Annual Technical Conference and Exhibition
Al-Attar HH (2011) Evaluation of oil foam as a displacing phase to improve oil recovery: a laboratory study. J Petrol Sci Eng 79:101–112
Andrianov A, Farajzadeh R, MahmoodiNick M, Talanana M, Zitha PL (2012) Immiscible foam for enhancing oil recovery: bulk and porous media experiments,”. Ind Eng Chem Res 51:2214–2226
Aronson A, Bergeron V, Fagan ME, Radke C (1994) The influence of disjoining pressure on foam stability and flow in porous media. Colloids Surf A 83:109–120
Blaker T, Aarra MG, Skauge A, Rasmussen L, Celius HK, Martinsen HA et al (2002) Foam for gas mobility control in the Snorre field: the FAWAG project. SPE Reser Eval Eng 5:317–323
Duan X, Hou J, Cheng T, Li S, Ma Y (2014) Evaluation of oil-tolerant foam for enhanced oil recovery: laboratory study of a system of oil-tolerant foaming agents. J Petrol Sci Eng 122:428–438
Farnazeh S, Sohrabi M (2013) A review of the status of Foam applications in enhanced oil recovery. In: Society of Petroleum Engineers. SPE-164917. Presentation at the EAGE Annual Conference & Exhibition incorporating SPE Europe held in London
Friedmann F, Chen W, Gauglitz P (1991) Experimental and simulation study of high-temperature foam displacement in porous media. SPE Reserv Eng 6:37–45
Hirasaki G, Miller C, Szafranski R, Lawson J, Akiya N (1997) Surfactant/foam process for aquifer remediation. In: International Symposium on Oilfield Chemistry
Klitzing RV, Müller H-J (2002) Film stability control. Curr Opin Colloid Interface Sci 7:42–49
Kloet M, Renkema WJ, Rossen WR (2009) Optimal Design Criteria for SAG Foam Processes in Heterogeneous Reservoirs. In: EUROPEC/EAGE Conference and Exhibition
Kovscek A, Radke C (1994) Fundamentals of foam transport in porous media. In: Schramm L (ed) Foams: fundamentals and applications in the petroleum industry, ACS Symposium Series, Washington DC, pp 115–163
Lake LW (1989) Enhanced oil recovery. Prentice Hall, United States
Llave FM, Olsen DK (1994) Use of mixed surfactants to generate foams for mobility control in chemical flooding. SPE Reserv Eng 9:125–132
Lv W, Bazin B, Ma D, Liu Q, Han D, Wu K (2011) Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J Petrol Sci Eng 77:209–218
Mannhardt K, Svorstøl I (1997) Foam propagation in Snorre reservoir core-effects of oil saturation and ageing. In: 9th European Symposium on Improved Oil Recovery
Mannhardt K, Svorstøl I (2001) Surfactant concentration for foam formation and propagation in Snorre reservoir core. J Petrol Sci Eng 30:105–119
Mannhardt K, Novosad J, Schramm L (2000) Comparative evaluation of foam stability to oil. SPE Reserv Eval Eng 3:23–34
Moradi B, Awang M, Bashir A, Sabil KM (2014) Effects of alcohols on interfacial tension between carbon dioxide and crude oil at elevated pressures and temperature. J Petrol Sci Eng 121:103–109
Robie D Jr, Roedell J, Wackowski R (1995) Field Trial of Simultaneous Injection of CO2 and Water, Rangely Weber Sand Unit, Colorado. In: SPE Production Operations Symposium
Rosen MJ (2004) Surfactants and interfacial phenomena. Wiley
Rosman A, Kam S (2009) Modeling foam-diversion process using three-phase fractional flow analysis in a layered system. Energy Sour Part A 31:936–955
Schramm LL (1994) Foam sensitivity to crude oil in porous media. ACS Adv Chem Ser 242:165–200
Schramm LL, Green W (1995) The influence of Marangoni surface elasticity on gas mobility reductions by foams in porous media. Colloids Surf A 94:13–28
Sheng J (2013) Enhanced oil recovery field case studies. Gulf Professional Publishing, Texas, USA
Skauge A, Aarra M, Surguchev L, Martinsen H, Rasmussen L (2002) Foam-assisted WAG: experience from the Snorre Field. In: SPE/DOE Improved Oil Recovery Symposium
Sohrabi M, Henderson G, Tehrani D, Danesh A (2000) Visualisation of oil recovery by water alternating gas (WAG) injection using high pressure micromodels-water-wet system. In: SPE Annual Technical Conference and Exhibition
Stephenson DJ, Graham AG, Luhning RW (1993) Mobility control experience in the Joffre Viking miscible CO2 flood. SPE Reserv Eng 8:183–188
Stevenson P (2012) Foam engineering: fundamentals and applications. Wiley
Sunmonu RM, Onyekonwu M (2013) Enhanced oil recovery using foam injection; a mechanistic approach. In: SPE Nigeria Annual International Conference and Exhibition
Tsau J-S, Syahputra AE, Yaghoobi H, Grigg RB (1999) Use of sacrificial agents in CO2 foam flooding application. In: SPE Annual Technical Conference
Wassmuth F, Cameron A, Schramm L, Taylor K, Krasey R (1999) A waterflood and chemically improved oil recovery evaluation for the pembina bear lake unit. J Can Pet Technol 38:1–9
Wellington SL, Vinegar HJ (1988) Surfactant-induced mobility control for carbon dioxide studied with computerized tomography. In: Surfactant-based mobility control. ACS symposium series, Chapter 17, vol 373. American Chemical Society, pp 344–358
Xing D, Wei B, Trickett K, Mohamed A, Eastoe J, Soong Y et al (2010) CO2-soluble surfactants for improved mobility control. In: SPE Improved Oil Recovery Symposium
Acknowledgments
We would like to acknowledge the Universiti Teknologi PETRONAS for supporting this research work. The authors would like to thank Stepan Company USA who provided the surfactant samples for research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Memon, M.K., Shuker, M.T. & Elraies, K.A. Study of blended surfactants to generate stable foam in presence of crude oil for gas mobility control. J Petrol Explor Prod Technol 7, 77–85 (2017). https://doi.org/10.1007/s13202-016-0243-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-016-0243-9