Abstract
Microplastic pollution endangers both aquatic and terrestrial ecosystems. Their spread across the food chain also endangers human health. Wastewater treatment plants (WWTPs) can be viewed as the final barrier between microplastics and the environment. In addition, it is well-known that water mites are abundant parasites in aquatic ecosystems, and nearly all insect orders with aquatic stages are considered potential hosts for at least one water mite species. However, no studies have been conducted to test the direct and indirect effects of parasites on population dynamics in freshwater ecosystems or the role of predators in shaping the behavior and life histories of aquatic organisms. Thus, this work aimed to study the seasonal abundance, distribution, composition, and risk assessment of MPs in surface water, aquatic insects (Coroxide and Notonectidae), and for the first time, water mites (Hydrachnidiae), as well as the effect of water mites’ parasitism on the number of MPs ingested by aquatic insects in two of the most polluted wastewater sites (S1 and S2) in Sohag Governorate, Egypt. The two wastewater sites receive different wastewater inputs (domestic and industrial). The results showed that the MPs abundance in surface water was higher in S2 than in S1 during the four seasons of the year, where the microplastic abundance in surface water was 2.05 ± 0.79 and 3.01 ± 0.9 particles/L in S1 and S2, respectively. Also, MPs were significantly higher in S2 in two insect taxa (Corixidae and Notonectidae) that are known to be infected by water mites. In contrast, the number of MPs was lower in S1, where water mites were absent. In addition, our results showed that adult water mites accumulated MPs. Overall, the 500–2000 µm size range was the most prevalent for both wastewater sites. Fibers were the most common MP morphotype discovered, followed by fragments. The dominant colors of MP were blue, red, and black. Furthermore, FTIR spectroscopy revealed the existence of three distinct polymers, namely polyester (PES), polypropylene (PP), and polyethylene (PE). To the best of our knowledge, this is the first study to examine the effect of water mites’ parasitism on the number of MPs ingested by aquatic organisms. However, Further research is needed to confirm our suggestion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
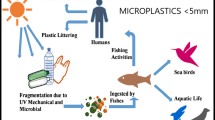
Microplastic contamination has been recorded all over the world, from the poles to the equator, and from the ocean’s surface to the deepest abyss (Blettler et al. 2018; Eerkes-Medrano and Thompson 2018; Li et al. 2018; Mendoza and Balcer 2019; Peng et al. 2018a, b; Rochman et al. 2015; Tursi et al. 2022). Microplastics are plastic particles ranging in size from 0.05 to 5 mm (Arthur et al. 2009). They are transferred from the terrestrial ecosystems via biotic and abiotic mechanisms, or they reach the aquatic environment directly through things that contain microplastics (Li et al. 2018; Ory et al. 2017). After that, microplastics are further classified as primary microplastics (plastic beads used in air blasting and cosmetic items) or secondary microplastics (plastic fragments made from bigger plastic particles) (Sharma et al. 2023). According to Burns et al. (2018), MPs may be categorized into several form categories with names like fragments, fibers, films, foam, and beads. MPs’ physical features, including density, shape, and size, may affect how they move across various environments and how they disperse (Hartmann et al. 2019). Stormwater runoff, industrial waste, and household rubbish are just a few of the pathways via which microplastics may enter an aquatic ecosystem (Horton and Dixon 2018; Nel and Froneman 2018). Based on Li et al. (2018), the mean abundance of MPs in freshwater systems varied from almost none to several million pieces per cubic meter. Since Gregory, (1977) discovered the first discovery of microplastics off the coast of New Zealand, the microplastic study has grown exponentially around the world.
Most of the research has classified each sampling site according to the predominant human activities that surround it to analyze the source of microplastics qualitatively (Lin et al. 2022). Furthermore, wastewater treatment plants (WWTPs) are well recognized as point sources of microplastics because huge volumes of microplastic-containing effluents are continually discharged (Grbić et al. 2020), even though the majority of the microplastics are removed from the influents (Talvitie et al. 2017). As a result, WWTPs are frequently associated with significant levels of microplastic contamination. Recent research, for example, discovered greater microplastic concentrations in WWTP effluents compared to those in a reference location (Magnusson and Norén 2014).
Organisms consume MPs unwillingly by eating prey that already includes MPs or incidentally by mistaking them for natural food (such as plankton) (Boerger et al. 2010; Davison and Asch 2011). MPs can change the biology of tissues when they are swallowed and accumulate in them. Microplastic exposure can have a variety of physiological effects on reproductive success (Ahrendt et al. 2020), metabolic changes (Hamed et al. 2019), behavior (Rochman et al. 2013), growth (Ahrendt et al. 2020), and histopathology (Hamed et al. 2020). Microplastics have also been demonstrated to collect trace metals and infections (Khdre et al. 2023; McCormick et al. 2014; Nakashima et al. 2012). As a result, the presence of MPs in aquatic ecosystems is thought to act as a conduit for the transfer of diseases, metals, and persistent organic pollutants to higher trophic chains (Gholizadeh and Patimar 2018; McCormick et al. 2014). Aquatic insects have important ecological functions in both the land and water environments as main consumers, detritivores, pollinators, and predators (Khedre et al. 2023a, b). Consequently, aquatic insects play a crucial role in preserving the ecological cycles. Due to their functions as disease vectors or bioindicators, several groups had their ecology well researched. Despite their inconsistent contribution to global biodiversity, freshwater aquatic insects have often not been a focus of diversification (Idris et al. 2020). Aquatic insects such as caddisflies, mayflies, stoneflies, beetles, dragonflies, true flies, and certain moths are among the groups of insects represented in streams by their larvae. These aquatic insects serve an important function in connecting the freshwater food chain between producers and higher consumers (Mohd Rasdi et al. 2012).
It is well-known that water mites are abundant parasites in aquatic ecosystems, nearly all insect orders with aquatic stages are considered as potential hosts for at least one water mite species (Smith and Oliver 1986). The life cycle of water mites consists of six stages (Smith 1988): the egg, larvae, protonymph, deutonymph, tritonymph, and adult (mostly predators) (Zhavoronkova 2006). Water mite parasitism is necessary for larval feeding and completing its development (Wohltmann et al. 2001). Because the water mites drain nutrients from their hosts, they affect the host condition and consequently host fitness as well (Paul 2022). So far, reduced mating success (Rehfeldt 1995) and reduced reproductive output (Rolff 1999) of aquatic insect hosts have been reported as direct consequences of mite inspiration. Few studies have been conducted to test the direct and indirect effects of parasites on population dynamics in freshwater ecosystems (Ebert et al. 2000) and the role of predators in shaping the behavior and life history of aquatic organisms (Eklöv, 2000).
As an alternative, several research studies have collected environmental microplastics using a one-time sampling technique, which showed just a snapshot of microplastic contamination at that time point (Naji et al. 2017; Wagner and Lambert 2018; Khedre et al. 2023c, d) and may give incorrect data on microplastic abundance. Studies on the temporal and geographical distributions of microplastics in the environment are available (Fan et al. 2022; Xia et al. 2021), but there are not many that specifically address microplastics detected in living organisms.
Two of the most contaminated wastewater sites in the Sohag Governorate were chosen for this investigation. Every day, a considerable volume of wastewater is dumped into these areas. Furthermore, wastewater can be used to irrigate land utilized for agriculture, leading MPs to be transported to agricultural land’s surface, which is a big concern. The current study aims to (i) assess the microplastics levels of the wastewater using a multicompartment approach for surface water, aquatic insects, and water mites throughout the four seasonal of the year to determine possible microplastics risks, where this study presents new information about MPs in three different edible aquatic fauna family: Coroxide, Notonectidae, Hydrachnidiae (water mites) collected from two wastewater sites; (ii) answer the following questions: a) what is the relationship between the microplastic loads in aquatic fauna and the different aquatic systems that differ in microplastic pollution?, b) what is the effect of the water mites’ parasitism on the number of MPs ingested by aquatic insects? and c) Finally, what is the ability of the aquatic insect and mite species to be used as qualitative and quantitative biomonitoring for microplastics in freshwater? (iii) identify shapes, colors, size, and polymeric characteristics of MPs extracted from water and aquatic fauna; and (IV) assess the potential risks of MPs through multiple indices. Furthermore, our findings were intended to provide information regarding the seasonal variation of MPs in the wastewater, hence increasing understanding of the seasonal effect on MP pollution and risk levels in freshwater ecosystems. Consequently, our findings will help policymakers and the government, in partnership with international organizations, implement suitable management strategies to minimize the waste of plastic.
Materials and methods
Sampling sites
The sampling area is in Sohag City, which is located in Upper Egypt, in the middle of the Nile Valley (about 125 km long) (Fig. 1A, B). Two wastewater disposal plants have been established in Sohag Governorate. One on the western plateau, designated the West wastewater treatment plant (S1), and the other in the east plateau, named the East wastewater treatment plant (S2), were chosen for sampling in the four seasons of 2022 (Fig. 1C). The two sites’ characteristics are summarized in Table 1.
Sample collection
Surface water and Aquatic fauna (aquatic insects and mites) samples were collected from the two wastewater sites (S1 and S2) across four seasons in 2022. 75 L of surface water samples were taken from five randomly chosen points at the same times and points as the aquatic fauna samples, using the grab sampling approach as outlined by McNeish et al. (2018), which was then kept in 5-L clean glass containers for laboratory examination. Using a pond net (200 μm mesh size; 0.30 m × 0.30 m opening), aquatic species were collected. Following collecting, fieldworkers performed sorting and preliminary taxonomical identification.
Separation and identification of aquatic fauna
Using a dissecting binocular and a light microscope in the laboratory, the gathered samples were divided into groups based on their taxonomical groupings before being preserved once more in 70% ethanol. When practicable, taxonomical identification was carried out down to the genus level, in accordance with Cook (1974) and Hirashima et al. (1989).
Extraction of microplastics from surface water and aquatic fauna
Each 5-L water sample received 150 ml of 10% KOH to help break down the organic debris overnight, in accordance with the Dahms et al. (2020) procedure. Then, water samples were filtered using cellulose nitrate filter paper (0.45 µm pore) in a vacuum filtration. To be sure there were no MPs remaining after each wash, the containers were rinsed five times with filtered distilled water.
Using Naji et al. (2018) methodology, the MPs in the aquatic insects and mites were separated, where five samples, each with 10 individuals, were prepared from each taxon. Before digestion, the wet weight of each individual and sample (in milligrams) was determined. Individuals from each sample were placed in a glass beaker and digested by adding 20 ml of 30% H2O2 (strong oxidizing acids to break down biological tissues). Each digested sample was filtered via 0.45 µm filter paper.
Microplastic identification and characterization.
The number, form, and color of the MPs were identified using a dissecting microscope equipped with a digital camera (Carl Zeiss Suzhou Co.). Additionally, the MPs’ dimensions (length and width) were measured using the ImageJ software. The chemical composition of the MPs was determined using (ATR-FTIR, Alpha Bruker Platinum, 1–211-6353). MPs were classified based on physical properties like color, shape, and size, and quantified using FTIR. The data were processed using the OPUS programme (Bruker Optics GmbH). The polymer type was determined by comparing the acquired spectra to known reference spectra (Primpke et al. 2018).
Experiment quality assurance
All samples were stored in sealed vials or Petri dishes to avoid contamination. Wearing cotton laboratory coats and gloves, the experimenters did not utilize any plastic products. Every container was cleaned using Milli-Q water before use. Every solution used in the investigation was run through three filters before use. Three procedural blanks were run to ensure there was no contamination from the laboratory setting. Throughout the course of the inquiry, the blanks did not contain any microplastic particles. For a day, three Petri dishes were placed close to the workstation to gather airborne particles and determine the level of airborne contamination. Because we only took one sample of cotton fiber, procedural contamination had little effect on the investigation’s findings.
Risk assessment of microplastics
The pollution load index (PLI), which was used to calculate the risk of microplastic contamination (Kasamesiri et al. 2023), was created using the following formulae (Wang et al. 2020).
where Co is the background MP concentration and Ci is the MP concentration at sample site j. The global records for water (0.28 particles/m3, 0.00028 particles/L) were used as the basis for the MP reference values (Guo et al. 2021).
The polymer risk index (H), as determined by Li et al. (2020), is as follows:
where Sn is the polymer hazard score determined by Lithner et al. (2011), Pn is the percentage of each polymer type at each sample location. H was classified into four levels by Lithner et al. (2011) and Guo et al. (2021):
The ecological and toxicological effects of MPs have been evaluated using RI (Peng et al. 2018a; Ranjani et al. 2021).
Tj stands for MPs’ toxicity coefficient. Five RI contamination limits were determined by Guo et al. (2021).
Statistical analysis
Descriptive statistics (means and standard deviations) were used to characterize the main characteristics of MP concentrations in water and aquatic fauna collected at various seasons and sites. Statistical analysis was used to assess the basic assumptions about seasonal fluctuation in the number, shape, color, and polymer type of MPs in water and aquatic fauna. Season, location, taxon, and size are all independent factors. The data were analyzed using Shapiro–Wilk and Levene’s tests to assess normality, homogeneity, and equal variance. To analyses normally and evenly distributed data, one-way ANOVA was employed. For skewed and unequally distributed data, the nonparametric Mann–Whitney U test was utilized. P values < 0.05 were deemed significant differences. The data were analyzed using IBM SPSS (version 22, IBM Corp., Armonk, NY, USA).
Results
Seasonal abundance and characterization of microplastics in surface water
Data presented in Fig. 2 show the water MP abundance at the two wastewater sites throughout the different seasons. The mean number of MPs throughout the system was 2.55 ± 1.1 particles/L. S2 had a higher abundance of MPs (3.1 ± 0.9 particles/L) throughout the investigated period. The mean highest values of MPs were recorded in the winter season (3.27 ± 0.06 and 4.09 ± 1.1 particles/L in S1 and S2, respectively). High significant differences were obtained among the different seasons in each site (F = 7.4, P = 0.004). Moreover, MP abundance was significantly higher in S2 than in S1 over all seasons (F = 9.3, P = 0.019).
The microplastic shapes detected in the surface water of the two wastewater sites were fibers and fragments only (Fig. 3). Annually, fibers were the shape of the most prevalent particles observed in S1 and S2 accounting for 88% and 84%, respectively (Fig. 4A). Statistically, no significant differences in the abundance of fibers and fragments were recorded between S1 and S2 throughout the investigated year (F = 4.2, P = 0.086).
The sizes of MP particles (fibers and fragments) obtained from these sites did not exceed 3000 µm. The percentage of seasonal variations in the different MP size classes showed fluctuations during the study period (Fig. 4B). The most abundant MP size classes were in the range of 1501- 2000 µm (27%) and 2001-2500 µm (26%) in S1. However, in S2 the frequent sizes were in the range of 1001-1500 µm (29%) followed by 1501–2000 µm (21.5%). Statistical analysis showed a significantly higher abundance of the smaller MP size classes (180-2000 µm) than the greater (2001–3000 µm) throughout the year (F = 4.7, P = 0.02). Also, significant differences in the abundance of MP size classes between the two sites were observed (F = 6.1, P = 0.009).
Fibers and fragments were introduced in a wide spectrum of colors, including red, blue, black, green, violet, orange, yellow, and white. The frequency distribution of MP colors in the two sampling sites in the different seasons was illustrated in Fig. 4C. Most of the fibers and fragments appeared blue (38%). Red and black were the second most abundant colors of the total MP particles and represented 24% and 22%, respectively. The annual proportion of blue color was significantly higher in S2 than that in S1 (F = 5 P = 0.016). However, the red color was significantly more abundant in S1 than in S2 (F = 5.3, P = 0.015).
Three types of polymers were identified by FTIR spectroscopy: polyester (PES), polyethylene (PE), and polypropylene (PP) (Fig. 3). Polymer concentrations observed in the water samples varied considerably among the different seasons and between the two sites (Fig. 4D), where the microplastic polymers in the winter, spring, summer, and autumn seasons were predominantly PES (75%,94%, 92%, and 90%, respectively), followed by PP and PE in winter and spring, and PE and PP in summer and autumn. A significantly higher abundance of PES was found in surface water during the year when compared with other polymers (F = 11.2, P = 0.001). PP and PE were significantly more abundant in winter (F = 6.82, P = 0.041) when compared with the other seasons. According to the two sites of wastewater, the most abundant polymers of MPs throughout the year were PES (88% and 84%, respectively), followed by PE and PP in S1 (7% and 5%, respectively) and PP and PE in S2 (10% and 6%, respectively). No significant differences in the abundance of PES were found among the two sites (F = 3.21, P = 0.09) throughout the year. However, significantly the PP was more abundant in S2 than in S1 (F = 6.4, P = 0.009).
Microplastics load indices
The PLI values of MP pollution during various seasons are displayed in Table 1. As can be observed in Table 2, the two wastewater sites’ calculated PLI values over various seasons were extremely high, indicating a very high pollution discharge level. The greatest PLI values were also recorded in the winter. Additionally, in every season, PLI values were greater in S2 than in S1. Table 2 lists the H values for MP contamination at the two wastewater sites over various seasons. In Fig. 4D, the percentages of the identified polymer used to calculate the H are shown. Approximately equal percentages of each polymer were found in each of the four seasons. As a result, obtained H values were nearly comparable between seasons. A medium danger to the environment is indicated by the H values of the polymers in various seasons, which would fall under category III. This is due to the polymers’ toxic properties. In the two wastewater sites, the highest H of MPs was identified during the autumn at S1 and in winter at S2. Additionally, the RI index of microplastic at the two sites indicated the very highest degree of danger (degree V).
Size of the collected fauna taxa
Based on the mean body wet weight (w.w.) of the different taxa collected from the two wastewater sites, the smallest mean size was obtained for water mites (Hydrachnidiae) (0.27 ± 0.033 mg), followed by Coroxidae (5.7 ± 0.8 mg), and Notonectidae (6.8 ± 0.6 mg). Statistically, significant differences in the size of the individuals among the three taxa were recorded (F = 8.4, P = 0.007).
Seasonal abundance and characterization of MPs in the different fauna taxa at the two wastewater sites
Corixidae
A total of 139 MP particles were recorded from 400 adults across the two sites. The number of MP particles in S1 and S2 was 60 and 79, respectively. Summer had the highest number of MPs per individual in both S1 and S2 (0.41 ± 0.18 and 0.63 ± 0.07 particles/ind, respectively) followed by winter (0.34 ± 0.012 and 0.47 ± 0.07 particles/ind, respectively). Spring had the lowest number in S1 (0.16 ± 0.01 particles/ind), however, in S2 the lowest number was recorded in autumn (0.17 ± 0.03 particles/ind) (Fig. 5A, B). Significant seasonal differences in MP loads per individual were observed in both sites (F = 13.2, P = 0.001). Moreover, the MP load per individual was significantly higher in the S2 than in the S1 in the four seasons (F = 16.7, P = 0.0013).
All MPs ingested by Corixidae adults were fibers (100%) (Fig. 6A). Fiber’s lengths ranged from 382 to 961 µm with an average of 655 ± 234 µm. The microplastics in the size range of 501-1000 µm accounted for the highest percentage (78%) (Fig. 6B) and were mostly blue (65%) followed by red (16.5%) and black (12%) (Fig. 6C). The only polymer in Corixidae adults was polyester (Fig. 6D). The proportion of contaminated adults with MPs was 75% and 85% in S1 and S2 of all samples collected throughout the study period, respectively.
Notonectidae
From 400 adults a total of 263 MP particles were extracted across two sites. In S1 and S2, the number of MP particles was 123 and 140, respectively. Where, the highest number of MPs per individual in S1 and S2 was observed in summer (0.61 ± 0.08 and 0.82 ± 0.1 particles/ind, respectively) followed by winter (0.511 ± 0.08 and 0.77 ± 0.1 particles/ind, respectively) and the lowest number was recorded in autumn (0.39 ± 0.16 and 0.54 ± 0.09 particles/ind, respectively) (Fig. 5A, B). Significant seasonal differences in MP loads per individual were observed at both sites (F = 11.45, P = 0.0051). Moreover, the MP load per individual was significantly higher in the S2 than those in the S1 among the four seasons (F = 13, P = 0.01).
Most MPs ingested by Notonectidae adults were fibers and ranged from 85 to 88% of all ingested MP particles in the two wastewater sites, and the fragments represented the remaining proportion (Fig. 6A). According to the length, fibers ranged from 614 to 1105 µm with an average of 797 ± 184 µm; while, fragments were between 107 and 167 µm with an average of 137 ± 46 µm. Overall, the microplastics in the size range of 501-1000 µm accounted for the highest percentage (65.5%) (Fig. 6B) and were mostly blue (60.5%), red (20%), and black (17%) (Fig. 6C). The dominant polymer in the adults was polyester (86.5%), followed by polypropylene (7.5%) and polyethylene (6%) (Fig. 6D). The percentage of adults contaminated with MPs was 75% and 85% in S1 and S2 of all samples collected throughout the study period, respectively.
Hydrachnidiae
Adults of the family Hydrachnidiae (water mites) were recorded only in S2 during the investigated year. A total of 6.9 MP particles were extracted from 200 adults. In summer, the highest number of MPs was recorded (0.042 ± 0.015 particles/ind) followed by winter (0.034 ± 0.013 particles/ind), and the lowest number was in spring (0.02 ± 0.004 particles/ind) (Fig. 5B). Statistical analysis showed a significant seasonal difference in MP loads per individual (F = 9.4, P = 0.02).
The fibers were the only MPs ingested by Hydrachnidiae adults (100%) (Fig. 6A). The lengths of fibers ranged from 165 to 315 µm with an average of 258 ± 73 µm. Also, the microplastics in the size range of 165-500 µm accounted for the highest proportion (96%) (Fig. 6B), and the most colors were red (73%) (Fig. 6C). The polymer in Hydrachnidiae adults was polyester (100%) (Fig. 6D).
Discussion
This study is the first report on the seasonal and annual abundance of microplastic concentrations and characterization extracted from surface water, Coroxidae, Notonectidae, and Hydrachnidiae (water mites), and the first observation of the effect of the water mites’ parasitism on the number of MPs ingested by aquatic insects in two wastewater sites in Sohag Governorate, Egypt. The results revealed that MPs were observed in all samples obtained from the two sites of wastewater, which indicates that MPs are widespread in different aquatic systems. These results support the earlier studies showing that MPs are ubiquitous in aquatic systems all over the world (Horton and Dixon 2018; Mani and Burkhardt-Holm 2020; Woodall et al. 2014).
Wastewater treatment plants remain poorly managed in developing countries (Mema 2010), and wastewater discharge from these systems is considered an important source of MP pollution in aquatic ecosystems (Browne 2015; Chang 2015). Large amounts of MP particles are discharged from these systems into the waterway daily (Eriksen et al. 2013; Mason et al. 2016; Murphy et al. 2016). S1 and S2 are situated near several treatment plants whose effluents carry MPs into these sites. MP abundance was higher in S1 than in S2, and the density of MPs in these sites varied in different seasons. The variation might be associated with the amount of sewage effluent discharged to the basins from the WWTPs. A significant increase in surface water MP abundance was recorded in S2 compared to S1 across all seasons. This may be attributed to the fact that S2 receives the waste of many factories including textile mills. S1 had lower MP abundance compared to S2 despite its contamination with agricultural plastic products. This confirms that the relative abundance of MPs is closely correlated with the presence of spatial sources of pollution (Irfan et al. 2020).
Fibers are among the dominant shapes of MPs observed in aquatic systems ranging from sea beds to small inland freshwater lakes (Cesa et al. 2017). The present results confirmed the previous study, where the microfibers represented a higher proportion in S1 and S2 throughout the year. The dominance of fibers in surface water suggests that the source of MPs is more related to direct effluents from garment washing processes. Napper and Thompson, (2016) reported that a single wash of about 6 kg of clothes can release nearly 500,000 fibers in its waste effluent, resulting in higher fiber particles in the environment. Moreover, Mintenig et al. (2017) reported that data on treated wastewater in Germany revealed that fibers dominated in more than 80% of the samples. Furthermore, Luo et al. (2019) reported that urban effluent, such as domestic pollution, was considered a key factor contributing to microfiber abundance. They also demonstrated that fibers are more likely to be observed close to the shoreline, where effluents are discharged. This may be an additional explanation for the dominance of fibers in the present results. In addition to domestic effluents, they can directly receive airborne fibers. This process can increase the concentrations of fibers in the environment (Brahney et al. 2020; Luo et al. 2019). The second frequent shape in the present study was fragments. S2 water samples contained a higher proportion of fragment particles compared to S1. This may be due to site-specific factors that may affect MP densities in each site (Eerkes-Medrano et al. 2015; Klein et al. 2015).
The present result indicated that small particles were dominant and constituted a larger proportion of the MPs in our samples. Surface water samples from the two wastewater sites contain small particles (500–2000 µm). The small-size classes represented 77.6% of the total surface water samples. A significantly higher proportion of small MPs in the wastewater sites suggests that the MPs have been subjected to degradation mechanisms due to physical and chemical stresses from the environment (Wicaksono et al. 2021a; Zhang et al. 2016), as well as higher rates of biodegradation coinciding with increased microorganisms in these sites (Hoellein et al. 2017). Additionally, the closed environment of wastewater basins may increase the degradation of MPs through long-term accumulation (Eerkes-Medrano et al. 2015). The dominance of small-sized particles in the sampling areas may also be due to the high abundance of fiber particles in the samples. These findings have been recorded in many freshwater systems such as Laurentian Great Lakes USA (Driedger et al. 2015), Hövsgöl Lake, Mangolia (Free et al. 2014) Taihu Lake, China (Su et al. 2016), and Rhine River, Europe (Mani et al. 2019). Generally, an inverse relationship between MP size and concentration was a common finding in freshwater MP studies (Battula et al. 2021; Fan et al. 2021; Schmidt et al. 2018). However, larger plastic particles (1501–2500 µm) only accounted for the low ratio (24%) in the present samples, respectively. This trend of size distribution was more or less similar to that of other water systems, where the highest frequency of size was 250–1000 µm followed by (2000–3000 µm) (Nguyen et al. 2020). Furthermore, Barrows et al. (2018) found a global prevalence of MPs less than 1.5 mm (77%) throughout the ocean waters.
Eight different MP colors were identified in the present samples. Abundant colored MPs have been recorded in many studies (Gallitelli et al. 2021; Luo et al. 2019; Yuan et al. 2019). A great variety of colored plastic products are frequently used in modern life; and therefore, large quantities of plastic waste are produced and consumed. After degradation, these wastes can finally result in colored MPs (Yuan et al. 2019). Microplastic color can give information to speculate on the source and weathering process (Wicaksono et al. 2021a, b). For instance, the yellowish color of MPs can indicate the photooxidation and weathering processes of MPs (Liu et al. 2020). Moreover, the lower abundance of white color from the obtained samples indicates the low period of particle placements in the present sites because, by increasing MP placement time in aquatic systems, the effects of sunlight lead to the change of their colors to white (Rasta et al. 2021). Also, data obtained from research conducted by Singh et al. (2021) indicated that white-colored particles are mainly related to PS foam. This finding supports our data showing that foam particles are absent from wastewater sites. The most common MP color found was blue, accounting for 38% of the total samples in surface water. The second most frequent colors in our study sites were red and black, represented by 24% and 22.5%, respectively. Previous studies showed that the dominant species typically included blue (Barrows et al. 2018; Lahens et al. 2018; Strady et al. 2021), black (Qin et al. 2020; Sang et al. 2021), and red (Nguyen et al. 2021; Wicaksono et al. 2021a). However, the presence of a low percentage (1%) of yellow MPs indicates that the degradation of plastic waste does not appear where yellow MPs are highly degrading products (Liu et al. 2020).
The chemical composition of the polymer reflected the different sources of plastic pollution (Ballent et al. 2016). Chemical analysis of MP revealed that the proportion of PES was the highest in the two sampling sites. Domestic sewage derived from point sources plays an important role in MP pollution. For example, effluent from WWTPs contributes to high levels of MP in surface water (Conley et al. 2019), which could explain the higher abundance of polyester in both sites. PE and PP were found at the two sampling sites in different proportions according to the different seasons. They are used worldwide as material for various disposable items such as plastic bags, drinking straws, and bottle caps, which are used in large amounts every day and subsequently accumulate in aquatic environments after being thrown (Andrady 2011; Antunes et al. 2018). Moreover, PP is the most applied plastic in urban, industry food packaging, and agriculture (Shabaka et al. 2022). Therefore, they could be extremely observed in aquatic ecosystems (Zhao et al. 2015). From the previous results, it can be concluded that MP characteristics (physical and chemical) could be considered important factors in pollution fingerprinting (Luo et al. 2019).
PLI is used to determine the extent of MP contamination (Pan et al. 2021). PLI had the greatest and lowest values in winter and spring, respectively, and the highest values of PLI were recorded in S2, these findings attributed to PLI values increasing with increasing MP abundance, and PLI values were connected to the number of MPs up to now. Similar findings were observed in a runoff study at Bushehr Port (Hajiouni et al. 2022). Because the MPs problem is becoming more serious, evaluating MPs risks will help to a more thorough understanding of the state of MPs pollution, migratory routes, and potential harm to human health, and aid in improving ecosystem safety (Xu et al. 2018). MP polymers’ hazardous effects are determined by their chemical constituents (Lithner et al. 2011). Polymers found in MP particles have been linked to environmental risks (Xu et al. 2018). MPs have been consumed by a variety of taxa, including fish (Lusher et al. 2013), prawns (Daniel et al. 2020), crabs (Watts et al. 2014), mussels (Von Moos et al. 2012), and aquatic insects (Khedre et al. 2023d). The current findings indicate that MPs leaching from wastewater have the potential to cause environmental contamination and should be regarded as a possible issue. The findings of this study agreed with those of studies conducted in India on coral reef ecosystems and beach sediments in the Gulf of Mannar (Patterson et al. 2022). The highest H of MPs was found in autumn at S1 and in winter at S2, which is most likely because of the season’s greater percentage of polyethylene and higher hazard score for polyethylene than for other detected polymers. Seasonal variations in MP types can be attributed to several variables, including industrial activity, the amount of sewage effluent that WWTPs dump into the basins, and agricultural cultivation. As a result, different MP polymer types can have an impact on pollution levels (Wang et al. 2021).
Our results showed that backswimmer notonectid adults accumulated MPs. Notonectidae are important predators dominating the food webs (Dettner and Ecology 2019; Papáček and Soldán, 2008) and can play a major role in shaping the structure and the abundance of the zooplankton population in several freshwater environments (Hampton et al. 2000). They have a broad diet that includes several aquatic organisms such as crustaceans, tadpoles, and aquatic insects (Fischer et al. 2012, 2013; Jara et al. 2012; Khedre et al. 2024a, b). In our study, 75% of all notonectid samples contained MPs in S1. However, in S2 this proportion increased to 85% during the investigated period. This finding suggests that the absence of MPs in samples indicates decreased contamination of the site in a specific area (Iannilli et al. 2023; Mistri et al. 2022). This may be explained by the fact that a high concentration of MPs in an aquatic environment leads to a high MP encounter rate resulting in an increased feeding rate (Scherer et al. 2017). Moreover, MP load/individual was significantly higher in S2 compared to S1, which was consistent with higher MP levels in S2. In this regard, Simakova et al. (2022) reported that a higher number of MP particles was consumed by aquatic insects where the concentration of MPs was higher. Furthermore, no correlation between water MP loads and notonectid MP loads (P > 0.05) was observed in the different seasons. This might be attributed to the different prey that the notonectid adults had fed on, which not only included aquatic but also terrestrial organisms trapped on the water surface, such as bees, ants, and mosquitoes adults (Quiroz-Martínez and Rodríguez-Castro 2007).
Corixidae (Cenocorixa sp.), which belong to the Heteroptera order presented one of the most dominant taxa in the wastewater sites throughout the investigated period. They are commonly described as predators; their feeding strategy differs from that of notonectid adults as a result of the absence of the standard piercing beak present in Notonectids. They ingest living materials (diatoms, algae, protozoans, small insects) when they stir up debris, and microscopic organisms in the bottom of the water body (Kriska 2023). The differences in their mouthparts and consequently their feeding mechanism, in addition to the differences in the size of adults of both species may explain the variation in both the qualities and quantities of MPs and the mean MP load/individual between corixid and notonectid adults. Huang et al. (2022) and Watts) mentioned that the anatomy of mouth parts and the physiology of organisms likely affect the ingestion of MPs. It is important to note that Corixidae are eaten by fish, birds, and humans (Srayko et al. 2022). Therefore, they may act as vectors that introduce MPs to food webs and another aquatic system since the adults can fly and disperse widely, and rapidly invade new habitats (Souty-Grosset et al. 2016).
Our results showed that water mites also accumulated MPs. Water mites are found globally in a great variety of freshwater habitats. They have a complex life cycle that has co-evolved with important freshwater insect groups (Smith et al. 2010). Adult water mites are predators of different prey including copepods, mosquito larvae, chironomid larvae, and ostracods (Pozojević et al. 2019). Larval water mites are common ectoparasites of aquatic and semiaquatic insects including water bugs, dragonflies, mayflies, and water beetles (Smith 1988). The mean MP load per individual detected in our samples was 0.03 ± 0.01 particles/ind. This suggests that water mites may accumulate MPs either through feeding on chironomid larvae (López-Vázquez et al. 2022) that have previously ingested MPs or by consuming MPs by mistake if the particles resemble their prey.
Moreover, our results suggest that the presence of water mites affects the number of MPs ingested by aquatic insects that live in the same site. MPs were significantly higher in S2 in two insect taxa (Corixidae and Notonectidae) that are known to be infected by larval water mites (Smith et al. 2010). In contrast, the number of MPs was lower in S1, where water mites were absent. These differences may have two possible explanations. First, the presence of higher MP concentrations in S2 than in S1 may affect the MP loads/individual (Scherer et al. 2017). Second, the presence of parasite infection by water mites in S2 has been linked to MP contamination (Dixon 2016; Hernandez-Milian et al. 2019; Parker et al. 2021).
In our study, larval water mites were detected in adult corixids. This suggests that higher parasite loads may increase the susceptibility of individual hosts to have higher MP loads, or influence the ingestion of MPs (Parker et al. 2020, 2022). In this regard, Ponton et al. (2011) reported that compensatory changes in feeding were observed in infected insects and are likely to involve physiological mechanisms modifying the quantity of ingested food. For instance, increased food uptake by infected insects can compensate for the extra energy required or nutrient costs imposed by growing parasites. Further research is needed to confirm our suggestion, the effect of parasitism by mites on MP ingestion in the host.
Regarding the shape of MPs detected in the different taxa, fibers were the main MP type and accounted for 85–100% of the total MP. This was caused by the higher abundance of fibers observed in surface water samples in the two sampling sites compared with the fragments. This could be related to the fact that fibers have the smallest width as reported in the present study and the previous data (Pirc et al. 2016). This was similar to the previous findings which indicated that fibers were the most dominant MPs in most freshwater invertebrates, for instance, Naji et al. (2018) reported that MP fibers represented more than 50% of the total MPs in molluscs from the northern region of the Persian Gulf. Hurley et al. (2017) reported that 87% of the MPs ingested by freshwater annelid (Tubifex tubifex) were microfibers. Akindele et al. (2020) and Akindele) demonstrated that fibers were the most abundant MPs in both abiotic and biotic samples. (Bertoli et al. 2022) recorded fibers in 48.5% of their investigated taxa including different insect orders, e.g., Odonata, Heteoptera, Coleoptera, Diptera, Trichoptera, and Plecoptera. The abundance of fibers in the different taxa has been attributed to their diverse sources, e.g., washing domestic wastewater, fisheries, and other anthropogenic activities (Mason et al. 2016; Murphy et al. 2016).
Besides MP shape, the size of MPs is another characteristic that influences the interaction between MPs and aquatic taxa (Scherer et al. 2017). The present results showed different size ranges of MP (< 500-2500 µm) in the different taxa. Small microfibers (< 500-1000 µm) were the predominant size in most examined insects. Statistical analysis showed that MPs in both size classes < 500 µm, and 501-1000 µm were significantly higher than in their surrounding MPs water (P < 0.05). Small microfibers < 500 µm represented a high proportion of the total MPs in Hydrachnidiae (96%). However, Notonectidae ingested MP particles in the upper size range (1000-1500 µm). The reason for the MP size variability in these taxa may be the significant variability in the body size of Notonectidae compared with Hydrachnidiae. A similar finding was reported by Scherer et al. (2017) who demonstrated that MP size preference depends on the size of specimens where larger organisms ingested larger MPs since the morphology of mouthparts may allow the ingestion of large MP particles that pass through the alimentary canal. As previously reported for freshwater mussels, more MPs of smaller sizes were ingested in both field and laboratory samples, which indicates that the ingestion of MPs is size-dependent (Qu et al. 2018). It is interesting to note that some MPs extracted from the examined taxa were smaller in size than those detected in water samples. This suggests that MP size reduction may occur inside their gut because of biodegradation. Likewise, Yang et al. (2014) reported that some bacterial strains, such as Bacillus. and Entrobacter alsuriae break down polyethylene MPs in the gut of wax worms (Plodia interpunctella). Moreover, Immerschitt and Martens, (2021) stated that mechanical fragmentation of MPs may take place in the gizzard of Odonata nymphs by their chitinous teeth.
To our best knowledge, smaller particles of MPs may cause more harmful effects than larger ones particularly to lower trophic organisms as these organisms capture any food source of appropriate size without selection (Nguyen et al. 2020).
Regarding the MP colors detected in the examined taxa, the most abundant colors were blue followed by red and black. One possible explanation is that the dominance of blue-colored MPs in the surrounding environment increases the possibility of their ingestion by different insects. An alternative explanation is that the organisms prefer, or are attracted to this color, which may look like their food. This is supported by the fact that blue-green algae are considered food resources for collector-gatherer groups (Parker et al. 2022), which may lead to the accumulation of high loads of blue MPs within the present taxa. The high proportion of red microfibers in water mites (70%) in S2 suggests that these organisms could actively select red MPs like their prey. Previous studies reported that chironomid larvae (characterized by red color and filament shape) are one of the best prey for water mites (Vasquez et al. 2022). Ory et al. (2017) and Wicaksono et al. (2021b) confirmed that aquatic biota may feed on MPs that are like their prey in color and shape. The prevalence of blue, red, and black MPs in the present taxa was also observed in other freshwater invertebrates (Bour et al. 2018; Lenz et al. 2023; Parker et al. 2022).
The chemical composition of MPs must be well characterized to offer a better understanding of the negative impact of MPs on aquatic biota (Bagheri et al. 2020). Dominant polymer types observed in the aquatic fauna of specific regions reflected the abundance of these polymer types in the water of the same region (Munari et al. 2017). In the present study, PES was the most common polymer in the examined taxa.
Notonectid seemed to accumulate three polymer types (PES, PP, and PE) corresponding to the polymers in the wastewater. This suggests that notonectid could be best employed as MP qualitative bioindicators in freshwater.
Conclusion
As far as we know, this is the first study that provides data about the seasonal abundance, distribution, composition, and risk assessment of MPs in surface water, aquatic insects (Coroxide and Notonectidae), and water mites (Hydrachnidiae) in two of the most polluted wastewater sites in Sohag Governorate, Egypt. As well as the effect of water mites’ parasitism on the number of MPs ingested by aquatic insects. Our results suggest that the presence of water mites affects the number of MPs ingested by aquatic insects that live in the same site. Also, our results showed that water mites accumulated MPs. However, further research is needed to confirm our suggestion. Furthermore, the current study can serve as a warning sign for MP buildup in all compartments of the aquatic environment. We give a unique possibility for reaching conclusions on the relationship between pollution levels and interior microplastics using aquatic insects. This is critical and beneficial for policymakers in developing a more comprehensive strategy for microplastic mitigation, especially when certain species or ecosystems are vulnerable to microplastics. Additional research on the bioaccumulation of MPs and associated contaminants (such as heavy metals and persistent organic matter) within various higher trophic levels in various aquatic systems should be carried out to comprehend the implications and risks of MPs as well as the toxicity caused by the adsorption presence of these contaminants in freshwater biota.
Data Availability
All data analyzed in this manuscript is included in the published article.
References
Ahrendt C, Perez-Venegas DJ, Urbina M, Gonzalez C, Echeveste P, Aldana M, Pulgar J, Galbán-Malagón C (2020) Microplastic ingestion causes intestinal lesions in the intertidal fish Girella laevifrons. Mar Pollut Bull 151:110795
Akindele EO, Ehlers SM, Koop JH (2019) First empirical study of freshwater microplastics in West Africa using gastropods from Nigeria as bioindicators. Limnologica 78:125708
Akindele EO, Ehlers SM, Koop JH (2020) Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria. Environ Sci Pollut Res 27:33373–33379
Alimba CG, Faggio C (2019) Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ Toxicol Pharmacol 68:61–74
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62(8):1596–1605
Antunes J, Frias J, Sobral P (2018) Microplastics on the portuguese coast. Mar Pollut Bull 131:294–302
Arthur C, Baker J, Bamford H (2009) Proceedings of the international research workshop on the occurrence. Effects Fate Micropl Marine Debris 8:9–11
Bagheri T, Gholizadeh M, Abarghouei S, Zakeri M, Hedayati A, Rabaniha M, Aghaeimoghadam A, Hafezieh M (2020) Microplastics distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay. Caspian Sea Chemosphere 257:127201
Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe FJ (2016) Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull 110(1):383–395
Barrows APW, Cathey SE, Petersen CW (2018) Marine environment microfiber contamination: global patterns and the diversity of microparticle origins. Environ Pollut 237:275–284
Battula, S., Kumar, M., Panda, S.K., Pavan, K., Rao, U., 2021. In-situ microplastic detection sensor based on cascaded microring resonators, OCEANS 2021: San Diego–Porto. IEEE, pp. 1–5.
Bertoli M, Pastorino P, Lesa D, Renzi M, Anselmi S, Prearo M, Pizzul E (2022) Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: novel insights in a riverine ecosystem. Sci Total Environ 804:150207
Blettler MCM, Abrial E, Khan FR, Sivri N, Espinola LA (2018) Freshwater plastic pollution: recognizing research biases and identifying knowledge gaps. Water Res 143:416–424
Boerger CM, Lattin GL, Moore SL, Moore CJ (2010) Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar Pollut Bull 60(12):2275–2278
Bour A, Avio CG, Gorbi S, Regoli F, Hylland K (2018) Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ Pollut 243:1217–1225
Brahney J, Hallerud M, Heim E, Hahnenberger M, Sukumaran S (2020) Plastic rain in protected areas of the United States. Science 368(6496):1257–1260
Browne MA (2015) Sources and pathways of microplastics to habitats. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer International Publishing, Cham, pp 229–244. https://doi.org/10.1007/978-3-319-16510-3_9
Burns EE, Boxall AB (2018) Microplastics in the aquatic environment: evidence for or against adverse impacts and major knowledge gaps. Environ Toxicol Chem 37(11):2776–2796
Carbery M, O’Connor W, Palanisami T (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int 115:400–409
Cesa FS, Turra A, Baruque-Ramos J (2017) Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci Total Environ 598:1116–1129
Chang M (2015) Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar Pollut Bull 101(1):330–333
Cheung PK, Hung PL, Fok L (2019) River microplastic contamination and dynamics upon a rainfall event in Hong Kong. China Environ Processes 6:253–264
Conley K, Clum A, Deepe J, Lane H, Beckingham B (2019) Wastewater treatment plants as a source of microplastics to an urban estuary: removal efficiencies and loading per capita over one year. Water Res 3:100030
Cook DRJMAEI (1974) Water mite genera and subgenera. Water Res 21:1–860
Daniel DB, Ashraf PM, Thomas SN (2020) Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala. India Sci Total Environ 737:139839
Davison P, Asch RG (2011) Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar Ecol Prog Ser 432:173–180
Dettner K (2019) Defenses of water insects. In: Del-Claro K, Guillermo R (eds) aquatic insects: behavior and ecology. Springer International Publishing, Cham, pp 191–262. https://doi.org/10.1007/978-3-030-16327-3_9
Dixon BR (2016) Parasitic illnesses associated with the consumption of fresh produce—an emerging issue in developed countries. Curr Opin Food Sci 8:104–109
Driedger AG, Dürr HH, Mitchell K, Van Cappellen P (2015) Plastic debris in the Laurentian great lakes: a review. J Great Lakes Res 41(1):9–19
Ebert D, Lipsitch M, Mangin KL (2000) The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. Am Nat 156(5):459–477
Eerkes-Medrano D, Thompson R (2018) Occurrence, fate, and effect of microplastics in freshwater systems. Microplastic contamination in aquatic environments. Elsevier, Amsterdam, pp 95–132. https://doi.org/10.1016/B978-0-12-813747-5.00004-7
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82
Eklöv P (2000) Chemical cues from multiple predator-prey interactions induce changes in behavior and growth of anuran larvae. Oecologia 123:192–199
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77(1–2):177–182
Fan J, Zou L, Zhao G (2021) Microplastic abundance, distribution, and composition in the surface water and sediments of the Yangtze River along Chongqing City. China J Soils Sediments 21:1840–1851
Fan Y, Zheng J, Deng L, Rao W, Zhang Q, Liu T, Qian X (2022) Spatiotemporal dynamics of microplastics in an urban river network area. Water Res 212:118116
Fischer S, Pereyra D, Fernández L (2012) Predation ability and non-consumptive effects of Notonecta sellata (Heteroptera: Notonectidae) on immature stages of Culex pipiens (Diptera: Culicidae). J Vector Ecol 37(1):245–251
Fischer S, Zanotti G, Castro A, Quiroga L, Vargas DV (2013) Effect of habitat complexity on the predation of Buenoa fuscipennis (Heteroptera: Notonectidae) on mosquito immature stages and alternative prey. J Vector Ecol 38(2):215–223
Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B (2014) High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull 85(1):156–163
Gallitelli L, Cera A, Cesarini G, Pietrelli L, Scalici M (2021) Preliminary indoor evidences of microplastic effects on freshwater benthic macroinvertebrates. Sci Rep 11(1):720
Gholizadeh M, Patimar R (2018) Ecological risk assessment of heavy metals in surface sediments from the Gorgan Bay, Caspian Sea. Mar Pollut Bull 137:662–667
Grbić J, Helm P, Athey S, Rochman CM (2020) Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res 174:115623
Gregory MR (1977) Plastic pellets on New Zealand beaches. Mar Pollut Bull 8(4):82–84
Guo Z, Boeing WJ, Xu Y, Borgomeo E, Mason SA, Zhu Y-G (2021) Global meta-analysis of microplastic contamination in reservoirs with a novel framework. Water Res 207:117828
Hajiouni S, Mohammadi A, Ramavandi B, Arfaeinia H, De-la-Torre GE, Tekle-Röttering A, Dobaradaran S (2022) Occurrence of microplastics and phthalate esters in urban runoff: a focus on the Persian Gulf coastline. Scie Total Environ 806:150559
Hakanson L (1980) An ecological risk index for aquatic pollution control. A Sedimentol Approach Water Res 14(8):975–1001
Hamed M, Soliman HAM, Osman AGM, Sayed AE-DH (2019) Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 228:345–350
Hamed M, Soliman HAM, Osman AGM, Sayed AE-DH (2020) Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ Sci Pollut Res 27(13):14581–14588
Hampton SE, Gilbert JJ, Burns CW (2000) Direct and indirect effects of juvenile Buenoa macrotibialis (Hemiptera: Notonectidae) on the zooplankton of a shallow pond. Limnol Oceanogr 45(4):1006–1012
Hartmann NB, Huffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M (2019) Are we speaking the same language? ACS Publications, Recommendations for a definition and categorization framework for plastic debris
Hernandez-Milian G, Lusher A, MacGabban S, Rogan E (2019) Microplastics in grey seal (Halichoerus grypus) intestines: are they associated with parasite aggregations? Mar Pollut Bull 146:349–354
Hoellein TJ, McCormick AR, Hittie J, London MG, Scott JW, Kelly JJ (2017) Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw Sci 36(3):491–507
Horton AA, Dixon SJ (2018) Microplastics: An introduction to environmental transport processes. Wiley Interdiscip Rev Water 5(2):e1268
Huang D, Chen H, Shen M, Tao J, Chen S, Yin L, Zhou W, Wang X, Xiao R, Li R (2022) Recent advances on the transport of microplastics/nanoplastics in abiotic and biotic compartments. J Hazard Mater 438:129515
Hurley RR, Woodward JC, Rothwell JJ (2017) Ingestion of microplastics by freshwater tubifex worms. Environ Sci Technol 51(21):12844–12851
Iannilli V, Passatore L, Carloni S, Lecce F, Sciacca G, Zacchini M, Pietrini F (2023) Microplastic toxicity and trophic transfer in freshwater organisms: ecotoxicological and genotoxic assessment in Spirodela polyrhiza (L) Schleid and Echinogammarus veneris treated with polyethylene microparticles. Water 15(5):921
Idris NH, Shafie NS, Mohd Snawi MM, Azlan Rozali MF, Bahri AS, Shukor AM (2020) Macroinvertebrates Checklist in River Intakes of Pergau Lake, Jeli, Kelantan. IOP Conference Series Earth Environ Sci 549(1):012048
Immerschitt I, Martens A (2021) Ejection, ingestion and fragmentation of mesoplastic fibres to microplastics by Anax imperator larvae (Odonata: Aeshnidae). Odonatologica 49(1–2):57–66
Irfan T, Khalid S, Taneez M, Hashmi MZ (2020) Plastic driven pollution in Pakistan: the first evidence of environmental exposure to microplastic in sediments and water of Rawal Lake. Environ Sci Pollut Res 27:15083–15092
Jara FG, Perotti MG, Dieguez MDC (2012) Distribution of backswimmers in shallow ponds of Patagonia and their predatory role on a common tadpole–copepod assemblage. NZJ Mar Freshwater Res 46(4):459–473
Kasamesiri P, Panchan R, Thaimuangphol W (2023) Spatial-temporal distribution and ecological risk assessment of microplastic pollution of inland fishing ground in the ubolratana reservoir. Thailand Water 15(2):330
Khdre AM, Ramadan SA, Ashry A, Alaraby M (2023) Chironomus sp. as a bioindicator for assessing microplastic contamination and the heavy metals associated with it in the sediment of wastewater in sohag governorate. Egypt Water Air Soil Pollut 234(3):161
Khedre AM, Ramadan SA, Ashry A, Alaraby M (2023a) Pollution of freshwater ecosystems by MPs: a short review on degradation, distribution, and interaction with aquatic biota. Sohag J Sci 8:289–295
Khedre AM, Ramadan SA, Ashry A, Alaraby M (2023b) Assessment of microplastic accumulation in aquatic insects of different feeding guilds collected from wastewater in Sohag Governorate. Egypt Mar Freshw Res 74(8):733–745
Khedre AM, Ramadan SA, Ashry A, Alaraby M (2023c) Seasonal variations of microplastic in sediment, Chironomus sp. larvae, and chironomid tubes in two wastewater sites in Sohag Governorate. Egypt Environ Sci Pollut Res 30:125846–125865
Khedre AM, Ramadan SA, Ashry A, Alaraby M (2023d) Ingestion and egestion of microplastic by aquatic insects in Egypt wastewater. Environ Qual Manag 33(1):135–145
Khedre AM, Ramadan SA, Ashry A, Alaraby M (2024a) Abundance and risk assessment of microplastics in water, sediment, and aquatic insects of the Nile River. Chemosphere 26:141557
Khedre AM, Ramadan SA, Ashry A, Alaraby M (2024b) Interactions between microplastics and Culex sp. larvae in wastewater. Water Environ Res 96(2):e11003
Klein S, Worch E, Knepper TP (2015) Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany. Environ Sci Technol 49(10):6070–6076
Kriska G (2023) Freshwater invertebrates in Central Europe: a field guide. Springer Nature
Lahens L, Strady E, Kieu-Le T-C, Dris R, Boukerma K, Rinnert E, Gasperi J, Tassin B (2018) Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ Pollut 236:661–671
Lenz M, Brennecke D, Haeckel M, Knickmeier K, Kossel E (2023) Spatio-temporal variability in the abundance and composition of beach litter and microplastics along the Baltic Sea coast of Schleswig-Holstein. Germany Mar Pollut Bull 190:114830
Li J, Liu H, Chen JP (2018) Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374
Li R, Yu L, Chai M, Wu H, Zhu X (2020) The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Sci Total Environ 708:135025
Lin C-T, Chiu M-C, Kuo M-H (2022) A mini-review of strategies for quantifying anthropogenic activities in microplastic studies in aquatic environments. Polymers 14(1):198
Lithner D, Larsson Å, Dave G (2011) Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ 409(18):3309–3324
Liu P, Zhan X, Wu X, Li J, Wang H, Gao S (2020) Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 242:125193
López-Vázquez J, Rodil R, Trujillo-Rodríguez MJ, Quintana JB, Cela R, Miró M (2022) Mimicking human ingestion of microplastics: Oral bioaccessibility tests of bisphenol A and phthalate esters under fed and fasted states. Sci Total Environ 826:154027
Luo H, Xiang Y, He D, Li Y, Zhao Y, Wang S, Pan X (2019) Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci Total Environ 678:1–9
Lusher AL, McHugh M, Thompson RC (2013) Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67(1–2):94–99
Magnusson, K., Norén, F., 2014. Screening of microplastic particles in and down-stream a wastewater treatment plant
Mani T, Burkhardt-Holm P (2020) Seasonal microplastics variation in nival and pluvial stretches of the rhine river-from the swiss catchment towards the North Sea. Sci Total Environ 707:135579
Mani T, Primpke S, Lorenz C, Gerdts G, Burkhardt-Holm P (2019) Microplastic pollution in benthic midstream sediments of the Rhine River. Environ Sci Technol 53(10):6053–6062
Mason SA, Garneau D, Sutton R, Chu Y, Ehmann K, Barnes J, Fink P, Papazissimos D, Rogers DL (2016) Microplastic pollut. is widely detected in US municipal wastewater treatment plant effluent. Environ Pollution 218:1045–1054
McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48(20):11863–11871
Mema V (2010) Impact of poorly maintained wastewater sewage treatment plants-lessons from South Africa: Wastewater management. Resource 12(3):60–65
Mendoza LMR, Balcer M (2019) Association of hazardous compounds with microplastics in freshwater ecosystems. Microplastics in Water and Wastewater; IWA Publishing: London, UK pp 15–25
Meng W, Sun H, Su G (2023) Plastic packaging-associated chemicals and their hazards–an overview of reviews. Chemosphere 331:138795
Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G (2017) Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res 108:365–372
Mistri M, Sfriso AA, Casoni E, Nicoli M, Vaccaro C, Munari C (2022) Microplastic accumulation in commercial fish from the Adriatic Sea. Mar Pollut Bull 174:113279
Mohd Rasdi Z, Fauziah I, Ismail R, Mohd Hafezan S, Fairuz K, Hazmi AD, Che Salmah MR (2012) Diversity of aquatic insects in keniam River National Park, Pahang, Malaysia, Asian. J Agri Rural Develop 2(3):312
Munari C, Infantini V, Scoponi M, Rastelli E, Corinaldesi C, Mistri M (2017) Microplastics in the sediments of terra nova bay (Ross sea, Antarctica). Mar Pollut Bull 122(1–2):161–165
Murphy F, Ewins C, Carbonnier F, Quinn B (2016) Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol 50(11):5800–5808
Naji A, Esmaili Z, Khan FR (2017) Plastic debris and microplastics along the beaches of the Strait of Hormuz. Persian Gulf Mar Pollut Bull 114(2):1057–1062
Naji A, Nuri M, Vethaak AD (2018) Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ Pollut 235:113–120
Nakashima E, Isobe A, Kako SI, Itai T, Takahashi S (2012) Quantification of toxic metals derived from macroplastic litter on ookushi beach, Japan. Environ Sci Technol 46(18):10099–10105
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar Pollut Bull 112(1–2):39–45
Nel H, Froneman P (2018) Presence of microplastics in the tube structure of the reef-building polychaete Gunnarea gaimardi (Quatrefages 1848). Afr J Mar Sci 40(1):87–89
Nguyen QAT, Nguyen HNY, Strady E, Nguyen QT, Trinh-Dang M (2020) Characteristics of microplastics in shoreline sediments from a tropical and urbanized beach (Da Nang, Vietnam). Mar Pollut Bull 161:111768
Nguyen NB, Kim M-K, Le QT, Ngo DN, Zoh K-D, Joo S-W (2021) Spectroscopic analysis of microplastic contaminants in an urban wastewater treatment plant from Seoul. South Korea Chemosphere 263:127812
Ory NC, Sobral P, Ferreira JL, Thiel M (2017) Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci Total Environ 586:430–437
Pan Z, Liu Q, Jiang R, Li W, Sun X, Lin H, Jiang S, Huang H (2021) Microplastic pollution and ecological risk assessment in an estuarine environment: the dongshan bay of China. Chemosphere 262:127876
Papáček M, Soldán T (2008) Structure and development of the reproductive system in Aphelocheirus aestivalis (Hemiptera: Heteroptera: Nepomorpha: Aphelocheiridae). Acta Entomol Mus Natl Pragae 48(2):299–318
Parker BW, Beckingham BA, Ingram BC, Ballenger JC, Weinstein JE, Sancho G (2020) Microplastic and tire wear particle occurrence in fishes from an urban estuary: influence of feeding characteristics on exposure risk. Mar Pollut Bull 160:111539
Parker B, Andreou D, Green ID, Britton JR (2021) Microplastics in freshwater fishes: occurrence, impacts and future perspectives. Fish and Fisher 22(3):467–488
Parker B, Andreou D, Pabortsava K, Barrow M, Green ID, Britton JR (2022) Microplastic loads within riverine fishes and macroinvertebrates are not predictable from ecological or morphological characteristics. Sci Total Environ 839:156321
Patterson J, Jeyasanta KI, Laju RL, Booth AM, Sathish N, Edward JKP (2022) Microplastic in the coral reef environments of the Gulf of Mannar, India-characteristics, distributions, sources and ecological risks. Environ Pollut 298:118848
Paul S (2022) Consequences of ectoparasite infection in damselflies. Macquarie University, Sydney, NSW
Peng G, Xu P, Zhu B, Bai M, Li D (2018a) Microplastics in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ Pollut 234:448–456
Peng X, Chen M, Chen S, Dasgupta S, Xu H, Ta K, Du M, Li J, Guo Z, Bai S (2018b) Microplastics contaminate the deepest part of the world’s ocean. Geochem Perspect Lett 9(1):1–5
Pirc U, Vidmar M, Mozer A, Kržan A (2016) Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ Sci Pollut Res 23:22206–22211
Ponton F, Chapuis M-P, Pernice M, Sword GA, Simpson SJ (2011) Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in drosophila melanogaster. J Insect Physiol 57(6):840–850
Pozojević I, Juršić L, Vučković N, Dorić V, Gottstein S, Ternjej I, Mihaljević Z (2019) Is the spatial distribution of lentic water mite assemblages (Acari: Hydrachnidia) governed by prey availability? Exp Appl Acarol 77:487–510
Primpke S, Wirth M, Lorenz C, Gerdts G (2018) Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal Bioanal Chem 410:5131–5141
Qin Y, Wang Z, Li W, Chang X, Yang J, Yang F (2020) Microplastics in the sediment of lake Ulansuhai of Yellow river basin. China Water Environ Res 92(6):829–839
Qu X, Su L, Li H, Liang M, Shi H (2018) Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci Total Environ 621:679–686
Quiroz-Martínez H, Rodríguez-Castro A (2007) Aquatic insects as predators of mosquito larvae. J Am Mosq Control Assoc 23(sp2):110–117
Ranjani M, Veerasingam S, Venkatachalapathy R, Mugilarasan M, Bagaev A, Mukhanov V, Vethamony P (2021) Assessment of potential ecological risk of microplastics in the coastal sediments of India: a meta-analysis. Mar Pollut Bull 163:111969
Rasta M, Rahimibashar MR, Ershad A, Jafroudi HT, Kouhbane ST (2021) Characteristics and seasonal distribution of microplastics in the surface waters of southwest coast of the Caspian Sea (Guilan Province, Iran). Bull Environ Contam Toxicol 107(4):671–676
Rehfeldt GE (1995) Natürliche feinde, parasiten und fortpflanzung von libellen: predators, parasites and reproductive behaviour of dragonflies. Aqua et Terra-Medien für Landschaftsökologie und Naturschutz.
Rezania S, Park J, Din MFM, Taib SM, Talaiekhozani A, Yadav KK, Kamyab H (2018) Microplastics pollution in different aquatic environments and biota: a review of recent studies. Mar Pollut Bull 133:191–208
Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagioti HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC (2013) Classify plastic waste as hazardous. Nature 494(7436):169–171
Rochman CM, Tahir A, Williams SL, Baxa DV, Lam R, Miller JT, Teh F-C, Werorilangi S, Teh SJ (2015) Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep 5(1):1–10
Rolff J (1999) Parasitism increases offspring size in a damselfly: experimental evidence for parasite-mediated maternal effects. Anim Behav 58(5):1105–1108
Sang W, Chen Z, Mei L, Hao S, Zhan C, Bin Zhang W, Li M, Liu J (2021) The abundance and characteristics of microplastics in rainwater pipelines in Wuhan. China Sci Total Environ 755:142606
Scherer C, Brennholt N, Reifferscheid G, Wagner M (2017) Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci Rep 7(1):1–9
Schmidt LK, Bochow M, Imhof HK, Oswald SE (2018) Multi-temporal surveys for microplastic particles enabled by a novel and fast application of SWIR imaging spectroscopy–Study of an urban watercourse traversing the city of Berlin. Germany Enveron Pollut 239:579–589
Shabaka S, Moawad MN, Ibrahim MI, El-Sayed AA, Ghobashy MM, Hamouda AZ, El-Alfy MA, Darwish DH, Youssef NAE (2022) Prevalence and risk assessment of microplastics in the Nile Delta estuaries:“The Plastic Nile” revisited. Sci Total Environ 852:158446
Sharma S, Bhardwaj A, Thakur M, Saini A (2023) Understanding microplastic pollution of marine ecosystem: a review. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-28314-1
Simakova A, Varenitsina A, Babkina I, Andreeva Y, Bagirov R, Yartsev V, Frank Y (2022) Ontogenetic transfer of microplastics in bloodsucking mosquitoes aedes aegypti L.(Diptera: Culicidae) Is a potential pathway for particle distribution in the environment. Water 14(12):1852
Singh N, Mondal A, Bagri A, Tiwari E, Khandelwal N, Monikh FA, Darbha GK (2021) Characteristics and spatial distribution of microplastics in the lower Ganga River water and sediment. Mar Pollut Bull 163:111960
Smith BP (1988) Host-parasite interaction and impact of larval water mites on insects. Annu Rev Entomol 33(1):487–507
Smith IM, Oliver D (1986) Review of parasitic associations of larval water mites (Acari: Parasitengona: Hydrachnida) with insect hosts. Can Entomol 118(5):407–472
Smith IM, Cook DR, Smith BP (2010) Water mites (Hydrachnidiae) and other arachnids, Ecology and classification of North American freshwater invertebrates. Elsevier, Amsterdam, pp 485–586
Souty-Grosset C, Anastacio PM, Aquiloni L, Banha F, Choquer J, Chucholl C, Tricarico E (2016) The red swamp crayfish Procambarus clarkii in Europe: impacts on aquatic ecosystems and human well-being. Limnologica 58:78–93
Srayko SH, Jardine TD, Phillips ID, Chivers DP (2022) Seasonal mass migration of water boatmen (Hemiptera: Corixidae) as a wetland–river linkage and dietary subsidy to riverine fish. Ecosystems 25(7):1571–1588
Strady E, Dang TH, Dao TD, Dinh HN, Do TTD, Duong TN, Duong TT, Hoang DA, Kieu-Le TC, Le TPQ (2021) Baseline assessment of microplastic concentrations in marine and freshwater environments of a developing Southeast Asian country. Viet Nam Mar Pollut Bull 162:111870
Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H (2016) Microplastics in taihu lake. China Enveron Pollut 216:711–719
Talvitie J, Mikola A, Setälä O, Heinonen M, Koistinen A (2017) How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res 109:164–172
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen 33:566–575
Tursi A, Baratta M, Easton T, Chatzisymeon E, Chidichimo F, De Biase M, De Filpo G (2022) Microplastics in aquatic systems, a comprehensive review: origination, accumulation, impact, and removal technologies. RSC Adv 12(44):28318–28340
Vasquez AA, Bonnici BL, Yusuf SH, Cruz JI, Hudson PL, Ram JL (2022) Improved chironomid barcode database enhances identification of water mite dietary content. Diversity 14(2):65
Von Moos N, Burkhardt-Holm P, Köhler A (2012) Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol 46(20):11327–11335
Wagner M, Lambert S (2018) Freshwater microplastics: emerging environmental contaminants? Springer Nature
Wang S, Zhang C, Pan Z, Sun D, Zhou A, Xie S, Wang J, Zou J (2020) Microplastics in wild freshwater fish of different feeding habits from beijiang and pearl river delta regions, south China. Chemosphere 258:127345
Wang G, Lu J, Li W, Ning J, Zhou L, Tong Y, Liu Z, Zhou H, Xiayihazi N (2021) Seasonal variation and risk assessment of microplastics in surface water of the Manas River Basin. China Ecotoxicol Environ Saf 208:111477
Watts AJ, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS (2014) Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ Sci Technol 48(15):8823–8830
Wicaksono EA, Werorilangi S, Galloway TS, Tahir A (2021a) Distribution and seasonal variation of microplastics in tallo river, makassar, eastern indonesia. Toxics 9(6):129
Wicaksono EA, Werorilangi S, Tahir A (2021b) The influence of weirs on microplastic fate in the riverine environment (case study: Jeneberang River, Makassar City, Indonesia), IOP conference series: earth and environmental science. IOP Publishing, p. 012054.
Wohltmann A, Witte H, Olomski R (2001) Organismal patterns causing high potential for adaptive radiation in Parasitengonae (Acari: Prostigmata), Halliday, RB, Walter DE, Proctor, HC, Norton, RA & Colloff, MJ, Acarology: proceedings of the 10th International Congress. CSIRO publishing, Melbourne, Australia, pp. 83–99.
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GLJ, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC (2014) The deep sea is a major sink for microplastic debris. Royal Soc Open Sci 1(4):140317
Xia F, Yao Q, Zhang J, Wang D (2021) Effects of seasonal variation and resuspension on microplastics in river sediments. Environ Pollut 286:117403
Xu P, Peng G, Su L, Gao Y, Gao L, Li D (2018) Microplastic risk assessment in surface waters: a case study in the Changjiang Estuary. China Mar Pollut Bull 133:647–654
Yang J, Yang Y, Wu W-M, Zhao J, Jiang L (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48(23):13776–13784
Yuan Y, Xiang M, Liu C, Theng BK (2019) Chronic impact of an accidental wastewater spill from a smelter, China: a study of health risk of heavy metal (loid) s via vegetable intake. Ecotoxicol Environ Saf 182:109401
Zhang K, Su J, Xiong X, Wu X, Wu C, Liu J (2016) Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau. China Environ Pollut 219:450–455
Zhao S, Zhu L, Li D (2015) Microplastic in three urban estuaries. China Environ Pollut 206:597–604
Zhavoronkova O (2006) Oviposition and development of larvae in the water mite Hydrachna cruenta (Acariformes, Hydrachnidae). Entomol Rev 86:S107–S117
Acknowledgements
We would like to thank members of the Chemistry analysis Labs of the Science Faculty at Sohag University for their support of FTIR.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The research received no external funding and depended completely upon the facilities offered by Sohag University. Article processing charge (APC) is completely covered according to Springer Nature’s fully open access agreements (Transformative Agreement between Springer Nature and Science, Technology & Innovation Funding Authority (STDF) in cooperation with Egyptian Knowledge Bank (EKB), started from 01 January 2022, and my institute (Sohag University) is one of the participating institutes.
Author information
Authors and Affiliations
Contributions
AK and SR planned the study and wrote the manuscript. AA and MA carried out field sampling. MA and AA performed data analysis and contributed to the manuscript reviewed by AK and SR All authors have read and agree to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to publish
All authors agree to publish their research data pertinent to the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khedre, A.M., Ramadan, S.A., Ashry, A. et al. Can water mites’ parasitism influence the number of microplastics ingested by aquatic insects?. Appl Water Sci 14, 134 (2024). https://doi.org/10.1007/s13201-024-02192-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02192-5