Abstract
Groundwater is the only freshwater source for agriculture and domestic use in the Khulais region of Saudi Arabia (SA). Anthropogenic activities, particularly agricultural runoff and lithogenic sources cause groundwater contamination, posing health risks to all generations (infant, child, and adult) via ingestion, dermal, and inhalation exposure routes. The configuration of non-carcinogenic and carcinogenic health concerns posed by contaminated water is, thus, a time-sensitive requirement. This study uses multivariate statistical techniques to assess health risks and to identify health impacts and pollution sources. Sampling of groundwater at nineteen sampling sites was carried out in two seasons (winter and summer) of 2021. The samples were analyzed for major ions and toxic metals. Results show that the average hazard quotient (HQ) and health hazard index (HHI) in infants was higher than 1 for most of toxic metal in both the seasons. The health risks associated with ingestion were significantly higher than those associated with the dermal and inhalation pathways. The aquifer’s carcinogenic risk ranged from high to extremely high in terms of chromium (Cr), nickel (Ni), and lead (Pb) concentrations. The estimated health risk values may have a negative impact on people’s health, which suggests that active aquifer management should be implemented. Multivariate statistical analyses were accomplished to determine the primary contamination sources in the aquifer system. The total dissolved solid (TDS) correlated strongly among chloride (Cl−), sulphate (SO42−), sodium (Na+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), iron (Fe), strontium (Sr), fluoride (F−) and bromide (Br−), which caused by seawater intrusion within winter and summer seasons. The hierarchical cluster analysis (HCA) identifies two clusters and one independent case within the summer and winter seasons. One-way analysis of variance (ANOVA) showed significant variations in the concentrations of Mg2+, Aluminum (Al), Cobalt (Co), Cr, Ni, and Pb in winter, while TDS, electrical conductivity (EC), Cl−, SO42−, Na+, Mg2+, Ca2+, Sr, F− and Br− in summer. This research demonstrates that aquifer cleanup and management protocols should be implemented, encouraged, and maintained. Applying the best hygienic practices and pre-clean-up of polluted groundwater is recommended before application for different purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is a valuable natural resource essential to the long-term development of many countries worldwide. To ensure access to high-quality water, the quality and quantity of groundwater must be evaluated and monitored over time (Yan et al. 2022, Kumar et al. 2023a). Many recent studies have focused on groundwater quality monitoring and evaluation (Hofmann et al. 2015; Khan et al., 2020a, 2020b; Kumar et al. 2021; Kumar et al. 2023b). The water quality of the aquifer mainly depends on the lithology of the particular region (Yetiş et al. 2019), land use land cover (Reddy et al. 2018), and groundwater age (Sakakibara et al. 2019). The groundwater system is degraded by human activities such as domestic practices (sanitary practices and waste disposal), mining, agriculture, industrialization expansion, and urbanization (Ukah et al., 2020; Khan et al., 2022). Numerous recent findings on groundwater quality (Chen et al. 2017) have concluded that unplanned municipal development, agriculture activities, and a lack of hydrochemical knowledge are among the causes of poor groundwater quality. In recent decades, irresponsible groundwater management and overexploitation have led to numerous environmental issues, including groundwater contamination, subsidence, and groundwater table decline (Xia 2002).
Agriculture and human consumption were primarily dependent on aquifer resources. Unhygienic conditions contribute to approximately 80% of diseases caused by contaminated groundwater (Singh et al. 2009; Vasanthavigar et al. 2012). The aquifer’s high concentration of trace metals threatens the quality of groundwater, soils, plants, and human health. Ingestion of toxic elements through drinking water is considered a route to negative health exposure. High levels of arsenic (As), Cr, Cd, Ni, and Pb in an aquifer can cause cancer and severe damage to human health, including kidney disease, high blood pressure, liver ailment, skin irritation, diarrhoea, stomach diseases, and infections in children (Wagh et al. 2018; USEPA 2001a, b; WHO guidelines 2011, Nguendo-Tongsi 2011). Toxic metals migrated to soils, bioaccumulate in plants, animals, and water, and then via the food chain enter the human body (Khan et al. 2017; Lazhar 2018; Khademi et al. 2019; Zhao et al. 2019; Kumar et al. 2023c). They degrade human health and result in numerous diseases and infections. Humans were exposed to toxic metals through ingestion (drinking), dermal, and inhalation routes. Dosage, exposure duration, and personality traits determined the health probability rate. Health risk determination was performed to assess the impact of heavy metals on human beings using the polluted aquifers (Congke et al. 2020; Çelebi et al. 2014). Redox potential conditions and pH influenced the concentration of Cr in the aquifer (Coyte et al. 2019). Ratnalu and Dhakate 2021 discovered that anthropogenic contamination of the aquifer was the primary cause of cancer in India.
In SA, renewable water resources, treated water, desalinated water and nonrenewable fossil water are used to fulfil the water requirements of various sources. The shallow aquifer plays a significant role in the overall water usage and is recharged by precipitation and flash floods. Similarly, precipitation is the most critical contributor (2045 MCM) to SA’s water storage (Zaharani et al. 2011; Masoud et al. 2018). Approximately 84% of the country’s groundwater is consumed by agriculture (MoWE 2014; Chowdhury and Al-Zahrani 2015). The shallow aquifer’s groundwater storage is depleted due to over-abstraction, which has reduced its quantity and degraded the quality (FAO 2009; Zaidi et al. 2015). In addition, groundwater quality degradation is commonly attributed to landfills, irrigation return flow, sewage systems, dumping sites, and seawater intrusion in the coastal region of SA (Subyani 2005; Al-Arifi et al. 2013; Bamousa and El Maghraby 2016). Alquwaizany et al. (2019) studied the organic compound contamination in groundwater by analysing 993 water samples collected from 13 provinces of SA. They reported that 9.67% of wells in residential, industrial, and agricultural sites were contaminated with organic compounds. Al-Ahmadi and El-Fiky (2009) studied the shallow aquifer at Wadi Marwani using major ions. They reported a high concentration of nitrate in the groundwater in residential areas due to domestic wastewater. El-Hames et al. (2011) assessed the groundwater quality using geographic information system (GIS) and reported that groundwater is only suitable for agriculture due to its high salinity. Al-Ahmadi (2012) investigated groundwater quality and found that mineral weathering, rock-water interaction, and agricultural activity affected water quality. Al-Ahmadi (2013) evaluated the hydrochemistry of Wadi Sayyah groundwater and reported that mineral weathering and evaporation are the predominant geochemical processes regulating water chemistry in this region and that the water is suitable for drinking and irrigation.
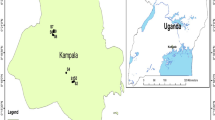
Khulais is a governorate in Makkah, the southernmost province of Saudi Arabia. Since, it served as a rest stop for pilgrims travelling between Makkah Al Mukaramah and Madinah Al Munawarah, it was historically considered an important region. It is approximately 30 kms to the coast of the Red Sea (Fig. 1). Surface waterways are scarce, and the only source of water is groundwater. Both natural and human influences are detrimental to the shallow aquifer. The hydrological input is inferior to the output and as a result, its ecosystem is unbalanced, resulting in significant groundwater contamination due to the intrusion of seawater and the effects of agriculture. Rapid depletion of groundwater resources, caused by declining groundwater levels, pollution, or a combination of the two, is the most severe and obvious problem and utilizing them is becoming too costly, resulting in poor agricultural outcomes.
In the past, few researchers have examined the groundwater in the Khulais region. Hussain et al. (1993a, b) discuss the groundwater availability and aquifer capacity in the Khulais Plain, which includes the downstream portions of Wadis Murawani, Abu Hulaifa, and Ghiran. Alyamani et al. (1994) demonstrate the application of factor analysis to determine and classify groundwater chemistry in the Khulais region. Qaid and Saleem (2016) assessed the Physical, Chemical, and Biological Properties of Ground Water on a spatial scale. As a critical region for the nation regarding agricultural activities and groundwater supply, there has been a lack of research on water quality and the accompanying health risk. This study aims (i) to evaluate the potential health risk from selected toxic metals on infants and adults during drinking, dermal, and inhalation exposure; (ii) to protect human health, environment, and conservation of water resources in highly populated cities; and (iii) to identify the lithogenic and anthropogenic sources using multivariate statistical methods. The outputs will aid in elucidating the consequences of aquifer pollution and health risks and can be used to mitigate the environment. Applying toxic metals and environmental risk parameters provides comprehensive health risks in trace elements ingestion through drinking and irrigation water. Suitable preparation for mitigation is necessary, and large-scale analysis along with the distribution of wells among neighbours is suggested for decreasing human health exposure.
Study area
The Khulais is located approximately 110 km north-east of Jeddah, SA, between the latitudes of 22°00′–22°15′ N and the longitudes of 39°05′–39°30′ E (Fig. 1). The groundwater is mainly used for domestic and agricultural purposes in this region. The average winter and summer temperatures ranging from 20 to 24 °C in winter and 30 to 34 °C in summer (Khan et al. 2022). West (plain region) and east (mountainous region) received 60 mm/year and 170 mm/year of precipitation, respectively (Gabr et al., 2017).
A complex basement supports the Cretaceous-Tertiary sedimentary succession. The eastern and western structural margins were composed of basement rocks at a high elevation (Bazuhair et al., 1992). The lithology of the aquifer is composed of Tertiary and Quaternary deposits (Fig. 2). The transmissivity, permeability, and porosity ranges of an unconfined aquifer ranged between 90 to 5800 m2/day, 7 to 1035 m/day, and 25 to 35%, respectively (Hussein et al., 1993). The aquifer is primarily recharged by precipitation, which during the sampling period of 2021 ranges from 10 to 38 mm/year (average) in winter and a few mm in summers.
Materials and methods
Groundwater samples collection
Figure 1 depicts nineteen selected and monitored sampling locations in the region under study. During the summer and winter of 2021, groundwater samples were collected from each sampling site’s borehole, protected, and transferred to the laboratory using standard precautions (APHA, 1998). The containers were first treated with nitric acid (HNO3) to prevent contamination and then rinsed twice with groundwater at the sampling sites. To reduce the margin of error, samples were filtered within two days of collection and stored in a refrigerator at 4 °C to prevent the growth of organic matter. A 0.45 m cellulose nitrate membrane was utilized to filter each sample. Digital meters (HACH Instruments) were used to measure each water sample’s pH and EC. Using an automatic TDS meter (HACH Instruments) and a mercury thermometer (HACH Instruments), TDS and temperature were measured, respectively. A Metrohm 850 Professional IC ion chromatographer was used to determine the concentration of cations and anions. The IC was calibrated using a standard solution of cation and anion eluents. Various cations and anions had an IC detection limit of 0.1 mg/L. The concentrations of heavy metals were determined using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) (Agilent ICP 720ES) following the Environmental Protection Agency-recommended method (EPA, Method 3005A). Pure nitric acid and reagents were used from well-known Merck (Darmstadt, Germany). The correlation coefficients of calibration lines for toxic metals were > 0.96. The analysis reproducibility control needs a triplicate chemical analysis for each toxic metal. The standard known concentration solution was analyzed after every five samples for accuracy and precision. These analyses (heavy metals and ions) were conducted at King Abdulaziz University’s Center of Excellence in Desalination Technology in Jeddah, SA.
Human health risk assessment
Exposure pathways assessment
A health risk assessment determines the danger posed to humans by contaminated groundwater. Oral ingestion (drinking aquifer), dermal absorption (skin), and inhalation (mouth and nose) contribute to toxic metal exposure (Deng et al. 2019; USEPA 1989; Xu et al. 2018). The non-carcinogenic (average daily dose [ADD], mg/kg/day) metals received via three exposure routes are as follows:
where ADD Ingestion = Average Daily Dose by Ingestion (mg/kg/day), ADD Dermal = Average Daily Dose by Skin (mg/kg/day), ADD Inhalation = Average Daily Dose by Inhalation (mg/kg/day), C = concentration of toxic metals, IR = water intake rate, ED = exposure duration, EF = exposure frequency, BW = average body weight, AT = time of exposure to the contaminants, ESA = exposed skin area, SAF = skin adhesion factor (Kp), ABSd = dermal absorption factor, IRinh = ingestion rate, PEF = particle emission factor.
Note
ADD values estimate the duration, frequency, and duration of exposure to specific toxic metals.
In this research, some uncertainties did not keep in mind, and they represent a limitation for the validity of the health risk determination. The weight of the human (male, female) and daily drinking water consumption was not considered. They differ from place to place, even in the same country, for instance, who live in rural, urban, and desert areas.
Non-carcinogenic risk assessment
Non-carcinogenic risk represented by HQ and Hazard index (HI), describes the possible risk to people by summing toxic metals. They estimated for single and total toxic metal exposure via ingestion, dermal, and inhalation. The health hazard index HHI) is the sum of the health hazards associated with ingestion, dermal, and inhalation exposure. It clarifies the cumulative risk associated with particular trace elements (n). The determination is based on the equations listed below:
where RFD = Reference Dose (mg/kg/day) for each toxic metal as tabulated in Table 1.
HQ or HI or HHI > 1: Non-cancer probability influence on people health; HQ or HI or HHI < 1: No consequences impact by groundwater ingestion, dermal, and inhalation.
Carcinogenic risk assessment
Carcinogenic risk is the cancer probability posed by carcinogenic hazards throughout a lifetime (Zhaoyong et al. 2019). It follows the following equations:
where CR = carcinogenic risk ingestion of toxic elements in aquifer, CSF = slope factors (mg/kg/day) and tabulated in Table 1, TCR = total carcinogenic risks.
Note
If CR or TCR < 1 * 10−6: no risk; > 1 * 10−4: harmful to people (USEPA 1989).
The Pb, Ni, and Cr slope factor was only applied to evaluate carcinogenic risk due to no slope factor data for the other toxic metals. The slope factor, actually, changes from one metal to another.
The heavy metal evaluation index (HEI) can evaluate the carcinogenic risk of an aquifer if the water quality is clarified correctly (Edet and Offiong 2002). It follows from the following equation:
where Hc = metal concentration in aquifer (mg/l), Hmac = maximum contaminant level for the toxic metal.
The aquifer quality carcinogenic influence was assessed by degree of pollution (Cdeg). It is estimated by the following equations (Wagh et al. 2018):
where, Cdeg = degree of pollution, Cfi = pollution factor for toxic metal, Cai = metal concentration in aquifer (mg/l), Cni = maximum contaminant level for the toxic metal.
The values of these parameters in materials and methods section are as tabulated in Table 1 and Table S1 and S2.
The health risk assessment was only evaluated using the measured toxic metals (Table S1 and S2) and ignored the organics and other toxic metals (not measured), which dissolved in the groundwater. Therefore, the level of health risk may be higher than those determined in the current work.
Statistical techniques used
To identify hydrogeochemical processes and solute sources, as well as to interpret datasets, multivariate statistical techniques were employed (Kazi et al. 2009; Khan et al. 2016a, b, c; Khan et al. 2017; Khan et al. 2020a, b). HCA, correlation matrix, and factor analysis are the most common techniques. The correlation coefficient (r) between the parameters was calculated using the Pearson correlation technique. Using the Pearson linear correlation coefficient, bivariate statistics were also performed to determine the correlations and strength of correlation between pairs of variables (Sivakumar et al. 2014). The r values 0.5, 0.5 to 0.7, and > 0.7, respectively, indicate a weak, moderate, and strong correlation (Oinam et al. 2012).
The HCA includes Q and R modes. The Q mode determines the spatial relationship between sampling sites, while the R mode classifies the parameters into groups based on their similarity (Banoeng-Yakubo et al. 2009). Using the dendrogram, the number of clusters with combined similarity levels of observations is determined (Lokhande et al. 2008). The dendrogram provides a graphical representation of the clustering process by presenting an image of groups and their proximity with a dramatic reduction in the dimensionality of the original data. (Bodrud-Doza et al. 2016). In this study, HCA was conducted using the squared Euclidean distance and the Ward method in the Q mode.
Using factor analysis (FA), variations in groundwater quality caused by natural and human processes are identified. The contribution of less significant parameters is diminished by simplifying the data structure derived from principal component analysis (PCA) (Nosrati and Van Den 2012). PCA transforms the original variable into a new variable, called the principal components, using orthogonal transformation (PCs). These are regarded as linear combinations of the initial variables and uncorrelated variables (axes). The extracted PCs are consistent with the fact that the highest variance is assigned to the first component, followed by the second highest variance, and so on (Purushothaman et al. 2014). To produce knowledge dependent on the most significant parameters with minimal loss of original data, PCA-defined axes are rotated, and new variables called varifactor (VF) can be created (Rogerson 2001). Consequently, a varimax rotation process facilitates and improves FA comprehension (Sakizadeh and Ahmadpour 2016). In this examination, the measured data for correlation analysis (CA) and FA were normalized to prevent misclassification due to the use of distinct measurement units.
ANOVA is a statistical technique used to evaluate potential contrasts between a scale level–dependent variable and a nominal level variable with at least two categories. This study utilized a one-way ANOVA to determine and quantify the correlations and differences between clustering variables. Molugaram and Rao (2017) provides a comprehensive explanation of the ANOVA.
Software used for statistical analysis
ArcGIS 10.3 version, SPSS 23 (IBM Corporation, Windows version) and Excel 2013 (a part of the Microsoft Office Suite) were used for statistical and other analysis. The AquaChem program determines the saturation indices and mixing proportions among groundwater samples with different salinity.
Results and discussion
Human health risk assessment
Non-carcinogenic health risks
The non-carcinogenic results of health risk for trace elements in groundwater for ADD Ingestion, ADD Dermal, and ADD Inhalation, for infants, children and adults were evaluated using trace elements viz., B, Ba, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn, F, Br, NO2, and NO3 for winter; Fe, strontium (Sr), Br, Selenium (Se), F, NO2, and NO3 for summer; are presented in Tables S3, and S4. They are characterized by increasing risk order as of infants > child > adults. The HQ for infants, children, and adults clarifies that ingestion is more dangerous than dermal and inhalation exposures (Table 2). These trends are consistent with the findings of Adimalla and Wang 2018; Diami et al. 2016; Hu et al. 2017; Zhaoyong et al. 2019; Adimalla 2019; Rajeshkumar et al. 2018. The non-carcinogenic HQ of aquifer to exposed to trace elements for infants through ingestion was 0.13 to 0.51 (Ba), 0.015 to 0.7 (Co), 0.9 to 12.6 (Cr), 2.73 to 30.9 (Cu), 0.012 to 0.069 (Fe), 0.13 to 23.68 (Mn), 0.07 to 1.68 (Ni), 22.5 to 267.78 (Pb), 0.0015 to 0.053 (Zn), 3.44 to 7.4 (F), and 0.76 to 202.38 (NO3) (Table 2). It reflects that HQ of Cu, Pb, and NO3 are the highest risk among the toxic metals and might be attributed to a high concentration in groundwater through agricultural and industrial wastewaters. The average HQ and HHI in infants through ingestion was greater than 1 concerning Cr (5.14), Cu (15.6), Mn (2.8), Pb (110.9), F (3.9) and NO3 (41.2) in winter, and F (3.6) and NO3 (56.6) in summer. Similarly, the average HQ in children through ingestion was greater than 1 concerning Cr (3.4), Cu (10.4), Mn (1.9), Pb (11.1), F (2.6), and NO3 (4.1) in the winter season, and F (2.4) and NO3 (8.5) in summer season (Table 2). They confirm strong detrimental influences on both infants and children with the presence of non-carcinogenic risks.
The high HQ mean concentration of Pb in drinking water causes high blood pressure, impaired kidney function, and reproductive issues (USEPA 2016). Winter’s elevated mean HQ of Pb (110.9) can amplify the severity of its effects (WHO 2019). The high HQ mean concentration of Mn in drinking water was very sustainable for brain and neurological disorders (Congke. et al. 2020). The mean HQ concentrations by ingestion (in winter) for infants, children, and adults were 110,9, 11,1, and 3.7, respectively (Table 2). Due to their low weight, infants consume a disproportionate amount of Pb-contaminated water, which increases their risk of exposure. The high mean HQ value for Cu (15.6) is indicative of acute exposure, including gastrointestinal disturbances (nausea and vomiting) (National Research Council 2000). Dermal and inhalational influence pathways were significantly lower than ingestion influence pathways. The HQ values were extremely low (< 1) in both the dermal and inhalation pathways (Tables 2 and S1), indicating a low risk of skin disease infection and a low health risk for infants and children.
The average HQ and HHI increase order is Pb > NO3 > Cu > Cr > Fe > Ni > Ba > Co > Fe > Zn for infants, whereas Pb > Cu > NO3 > Cr > F > Mn > Ni > Ba > Co > Fe > Zn in child and adults during winter season. In the summer season, the increasing order is NO3 > F > Se > Fe for infant, child and adult cases. It reflects a high risk for infants, children, and adults regarding Pb, NO3, and Cr levels in groundwater. The bioaccumulation of these toxic metals in the human body has detrimental effects. The release of these toxic metals by agricultural and sanitary wastewaters would increase the risk of bioaccumulation in the study area. NO3, Pb, and Cu were the most significant toxic metal contributors to HQ and HHI (Table 2). Pb and NO3 had the highest average HQ for infants among toxic metals from agricultural and lithogenic sources. Therefore, this research clarifies the lifetime management of Pb and NO3 during the infant and childhood stages. The HHI is the cumulative risk of trace metals in the aquifer; if it is < 1, there is no significant risk of non-carcinogenic influences (Congke et al. 2020). The HHI values for infants were higher than those in children. The latter was higher than those in adults. It indicates that infants and children were more vulnerable to the non-carcinogenic risks posed by toxic metals.
Hazard index (HI)
In winter, the northern, southwestern, and southeastern parts posed a high risk to human health from Cr ingestion, whereas no to moderate exposure was observed in the rest of the study area (Fig. 3a). Mn posed moderate to high health risks through ingestion whereas low to negligible risks through inhalation and dermal (Fig. 3b). Cu exposure by ingestion shows low to moderate health risk in the northeastern region. In contrast, the rest of the region was at high risk (Fig. 3c). Ingestion of Pb posed greater health risks all over the study area (Fig. 3d).
Most of the study area shows a high risk concerning NO3 concentration in groundwater in the winter and summer seasons, except few samples, which show no risk level (Fig. 4a, b). Exposure to drinking high concentration of NO3 water led to methemoglobinemia, colorectal cancer, thyroid disease, stomach cancer, spontaneous abortion, gastric problems, diabetes, oesophagal disorders and neural tube defects (Espejo-Herrera 2015; Taneja et al. 2017). Ward et al. (2018) stated that high NO3 concentration in drinking water has a negative impact on pregnancy periods such as spontaneous abortion, fetal deaths, prematurity, intrauterine growth retardation, low birth weight, congenital malformations, and neonatal deaths. Most winter and summer groundwater samples reveal a low F risk in aquifers (Fig. 4c, d). In the central part of the study area, aquifer’s F concentrations posed a moderate threat (Fig. 4c, d). The high concentration of F ion in drinking water replaces Ca elements in bones and teeth, thereby increasing dental risk, skeletal fluorosis, a decline in brain and kidney function, and osteoporosis in individuals, particularly the elderly (Choi et al. 2012).
Figures 5, 6 and 7 displays the summed HI for toxic elements such as Ba, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn, F, NO2, and NO3 (ƩHI) in winter and summer for infants, children, and adults exposed via ingestion, dermal, and inhalation pathways. During the winter and summer seasons, a high risk (> 10) is observed for infants and children in the southeastern and central-southern regions of the study area (Fig. 5a–d). The Cr, Ni, Pb, Br, and NO3 concentrations are also significantly higher than the maximum contaminant limits of 0.05 mg/l, 0.02 mg/l, 0.01 mg/l, 0.5 mg/l, and 45 mg/l, which has the potential to cause long-term kidney, liver, children’s disease, and bone damage in long-term usages in drinking water. It could be due to agricultural, seawater intrusion, or sanitary wastewater and demonstrates unacceptable levels of non-carcinogenic health risks from Cr, Ni, Pb, Br, and NO3.
Low to moderate risk was concentrated in the northern and central regions during the summer for children (Fig. 5d), whereas the high risk was observed in the southeastern region (Fig. 5d). In the central and southern regions, the health risk in adults shows high in winter, whereas in the northeast, it was moderate to low (Fig. 5e). In summer, the health risk in adults shows low to negligible in the majority of investigated areas (Fig. 5f).
Figures 6 and 7 depict the HI distribution for toxic metals in drinking water through dermal and inhalation contacts in infants, children, and adults. The HI distribution values for the dermal and inhalation pathways never exceeded the level of concern for infants, children, and adults.
Carcinogenic health risks
CR and TCR values were greater than 1 × 10−4 (USEPA 1989, 2002) and 1 × 10−3 (Paweczyk 2013; Haque et al. 2018) for Cr, Ni, and Pb elements during the winter season (Table 3).
These elevated values might pose cancer risks for exposed infants, children, and adults through ingestion (Fig. S1). The aquifer was classified as grade VII for Cr and Ni, which pose an extremely high risk and must be remedied (Table 4). It increases the carcinogenic effect based on dose of exposure (USEPA 2016).
However, Ni poses the greatest potential carcinogenic health risk (Fig. S1), while the average TCR for infants, children, and adults was 0.027, 0.018, and 0.006, respectively (Table 3). Cr is lustrous and contributes to respiratory difficulties, lung cancer, irritation, and lung, nose, and throat damage (Ratnalu and Dhakate 2021). Ingestion of carcinogenic elements increased the potential cancer risk for infants and children. When water resources are scarce, the use of aquifers for drinking may pose serious health risks to humans. Infants and children had a higher TCR than adults, indicating that they are significantly more susceptible to CR from Cr, Ni, and Pb concentrations in the aquifer. Children are susceptible to CR because they consume excessive amounts of water, food, and air relative to their body weights (WHO, 2017). During the early stages of growth, exposure to toxic metals in infants and children can cause irreversible harm (Peek et al. 2018). To decrease the carcinogenic and non-carcinogenic effects of these toxic metals, aquifer remediation and control measures should be implemented.
Heavy metal evaluation index (HEI) and degree of contamination (Cdeg)
During the winter and summer seasons, the HEI rises in the central region and falls in the northeastern region, respectively (Fig. 8a, b). Winter had higher HEI values than summer (Fig. 8a, b). It may be because viruses and bacteria, which are more prevalent in summer than in winter, consume most of the toxic metals in summer.
The average HEI values for the winter and summer seasons are 91.70 and 23.8, respectively (Tables 5 and 6). Ingestion of groundwater poses a low to high carcinogenic risk. In the winter and summer, the Cdeg of toxic metals increases in the central region and decreases in the northeastern and southeastern areas, respectively (Fig. 8c, d). According to Egbueri and Mgbenu’s (2020) classification, the majority of groundwater samples have Cdeg values greater than 80, indicating poor drinking water quality, particularly for Al, Cr, Ni, Pb, and Br (winter) and Sr and Br (summer). The average Cdeg values for the winter and summer seasons are 79.7 and 18.8, respectively (Tables 5 and 6). It was determined that the aquifer posed a high carcinogenic risk. In general, the majority of toxic metal concentrations exceeded the standards.
Multivariate statistical analyses
Correlation analysis (CA)
Table 7 shows descriptive information regarding the hydrogeochemical parameters. During the winter season, Pearson's method is used to conduct correlation analysis, which is summarized in Table S5. Hydrogeochemical parameters of the groundwater are not influenced by the pH, because there is no significant relationship has been observed (Table S5). There is a strong correlation of TDS (R2 = 0.83–1, Table S5) with EC, Cl, SO4, Na, K, Mg, Ca, F, and Br elements. During the summer, the TDS correlated strongly with Cl, SO4, Na, K, Mg, Ca, Fe, Sr, F, and Br (Table S6). It is mainly due to seawater intrusion during the winter and summer. In winter, the TDS correlated with HCO3, Cu, and Fe metals (R2 = 0.45–0.65, Table S5), which might result from rock-water interaction caused by precipitation and partially through anthropogenic activity.
The lack of a relationship between the bicarbonate (HCO3) ion and the major and trace elements in the summer (Table S6) results from the low rainfall and high evaporation caused by the rise in temperature. In winter, the Fe concentration is strongly correlated with Al (R2 = 0.73), Cr (R2 = 0.7), and Cu (R2 = 0.89) (Table S5) due to the desorption of these metals from the soil. Most Fe in the aquifer is derived from water–soil interaction under redox conditions. In the winter, the NO3 element is correlated with the HCO3 ion (R2 = 0.6, Table S5), which can be attributed to fertilizer use in agricultural wastewater. The strong correlation between F and Br (R2 = 0.8, Table S5) suggests that both ions originated from seawater intrusion. During the summer, the Se concentration in the aquifer was only correlated with SO4 (R2 = 0.6), confirming the lithogenic origin of Se ions.
Principal component analysis (PCA)
The rotated factor investigation assists in identifying the winter and summer aquifer pollution sources (Figs. 9 and 10). Four principal components (PCs) were extracted and explained about 86% and 91.5% of the total variances in winter and summer, respectively. The PC1 has positive loadings for TDS, EC, Cl, SO4, HCO3, Na, K, Mg, Ca, F, and Br during the winter (Fig. 9). These elements originate from the intrusion of seawater into the coastal aquifer as a result of excessive pumping. During the summer, the PC1 has positive loadings for TDS, EC, Cl, SO4, Na, K, Mg, Ca, Fe, Sr, F, and Br loadings (Fig. 10). In both the seasons, the PC1 is due to seawater intrusion. The PC2 in winter has positive loadings for SO4, Al, B, Ba, Co, Cr, Cu, Fe, Ni, Pb, and Zn (Fig. 9). It might be due to the interaction between soil and rock water through sorption desorption processes.
PC2 has negative loading with elevation in winter (Fig. 9), indicating that the low topography (wadi) is influenced by soil (sorption/desorption processes), whereas the high elevation reflects hard rocks. It can be confirmed that PC2, negative loading is due to soil–water interaction in winter. However, PC2 has positive loadings for HCO3, SO4, and Se (Fig. 10) in summer, indicating that precipitation plays a role in the release of these ions from the sulphate hard rocks. The HCO3 ion in aquifers reflects the effect of precipitation on the dissolution of CO2 and its subsequent transformation into HCO3 (Egbueri 2019; McDonald 2006). The HCO3 ion was attributed to atmospheric CO2 (rainfall), decomposition of organic matter in soil, and anthropogenic sources. It can confirm that PC2 is due to the precipitation in summer.
In winter, PC3 has positive NO3 and HCO3 loadings, reflecting agricultural activity (Fig. 9). Therefore, agriculture is the main factor for PC3 in winter. PC3 has positive loading with F whereas negative loadings with elevation and HCO3 (Fig. 10), confirming the influence of soil–water interaction (wadi) precipitation, respectively.
PC4 has positive Fe and negative pH loadings in winter (Fig. 9), indicating the effects of pH on the sorption and desorption of aquifer Fe concentration. Therefore, pH is the main factor for PC4 in winter. In summer, PC4 has only positive NO3 loading, indicating agricultural activity (Fig. 10).
The study area is distinguished by sanitary wastewater, agricultural activity, witness over pumping, seawater intrusion, and rock–water interaction through joints and fractures. These toxic metals were released into aquifers by the application of composts and agricultural chemicals. Fig. S2 depicts the relationships between the PC1, PC2, and PC3.
Hierarchical cluster analysis (HCA)
The hydrogeochemical similarity of groundwater samples is classified into numerous classes. The Ward linkage technique was implemented using squared Euclidean distance and z-score standardization (Egbueri 2020, b). During the winter and summer, the HCA is divided into two clusters and one independent case (Fig. 11a and b).
Cluster I is divided into groups 1 and 2 during the winter. In contrast, cluster II is divided into groups 3 and 4. (Fig. 11a). In the summer, cluster I is subdivided into groups 1, 2, and 3. In contrast, cluster II consists of group 4 and one independent case (Fig. 11b). Group 4 had the highest mean TDS, EC, Cl, SO4, Mg, Ca, Na, and Br concentrations compared to the other groups during winter and summer (Fig. S2), indicating the impact of seawater intrusion and rock–water interaction. In winter, group 4 had the highest mean TDS content, followed by group 3 and then groups 1 and 2. In summer, group 4 had the highest mean TDS content, followed by group 2 and then groups 1 and 3 (Fig. 12 and S3). Group 2 in winter and group 3 in summer had the lowest TDS (mean) (Fig. 12 and S3) and were located in the northeastern part (high topography). It may be due to the high rainfall that replenishes the aquifer system. In the winter, Group 3 has the highest mean NO3 concentration and is located in the south, whereas, in summer, it is distributed in the south and central region (Fig. 12 and S3).
It might be due to the agricultural activity and is significantly more widespread in summer than in winter. In winter, group 4 has the highest mean concentrations of B, Ba, Co, Cr, Al, Ni, Pb, Zn, and Cu compared to the other groups (Fig. 13), which may be partially derived from seawater intrusion, lithogenic, and human sources. In winter, group 2 has the lowest mean concentrations of B, Co, Cr, Al, Fe, Ni, Pb, Zn, and Cu (Fig. 13).
Group 2 is located in an area with a high topography and high precipitation, which dilutes groundwater quality. Groups 3 and 4 have the lowest and highest concentrations of F, Sr, Se, and Fe during the summer (Fig. 14), respectively. Groups 3 and 4 are situated in the northeast (heavy precipitation) and the central part (seawater intrusion), respectively. The independent case (sample 8) has winter and summer mean TDS concentrations of 15,235 and 18,620, respectively (Fig. 11a and b). It is the well with the highest TDS concentration, which is caused by seawater intrusions and the opening of Quaternary alluvial wadis into the Red Sea with no damming of hard rocks. As a result, the hydrogeological connection between the aquifer and sea was encouraged.
One-way ANOVA investigation
Using one-way ANOVA, the four groups were divided into two clusters for the winter and summer seasons. In the winter, the elevation, TDS, EC, Cl, SO4, Mg, Ca, Na, Al, B, Ba, Co, Cr, Cu, Fe, Ni, Pb, Zn, and Br of the four groups have significant differences (sig < 0.05) (Table 8).
On the other hand, the pH, NO3, HCO3, K, Mn, and F shows insignificant differences (sig > 0.05) between the four groups (Table 8). The highest significance differences (sig = 0) were observed for elevation, Mg, Al, Co, Cr, Ni, and Pb (Table 8), reflecting the influence of topography and soils on hydrogeochemistry. In the summer, there are significant differences in elevation, pH, TDS, EC, Cl, NO3, SO4, Mg, Ca, Na, K, Al, Sr, F, and Br between four groups, whereas differences in HCO3, Fe, and Se are minimal (Table 8). Altitude, TDS, EC, Cl, SO4, Na, Mg, Ca, Sr, F, and Br show the highest significant differences (Table 8).
Conclusion and recommendation
Human exposure to toxic metals through lithogenic and anthropogenic pollution sources in the coastal aquifer of the Khulais region of SA is evaluated. The maximum contaminant concentrations for Cr, Ni, Pb, Br, and NO3 in drinking water exceeded the prescribed limit. The HHI increasing order Pb > NO3 > Cu > Cr > Fe > Ni > Ba > Co > Fe > Zn (infants); Pb > Cu > NO3 > Cr > F > Mn > Ni > Ba > Co > Fe > Zn (children and adults) is observed during the winter season. The TCR was high concerning Cr, Ni, and Pb concentrations in the aquifer. High NO3 health risk covers most of the study area within the winter and summer seasons. The most area of investigation represented by Cdeg values greater than 80, indicating poor drinking water quality, particularly for Al, Cr, Ni, Pb, and Br (winter) and Sr and Br (summer). The TDS correlated strongly among Cl, SO4, Na, K, Mg, Ca, Fe, Sr, F, and Br, which attributed to seawater intrusion in winter and summer. Four PCA were extracted in summer and winter, namely seawater intrusion, rock water interaction, agricultural activity, and pH impact. The HCA identifies two clusters and one independent case. The degree of aquifer contamination posed a high carcinogenic risk regarding toxic metals. The health risk assessment shows the aquifer water is not good for drinking, touching (dermal), and inhaling. The statistical analyses outline the aquifer contamination caused by seawater intrusion, rock–water interaction, and human activities (mainly agriculture). The aquifer should be monitored periodically to check the contaminants and TDS concentration trend. Specific remediation procedures should be applied to aquifers containing high levels of NO3, Cr, and Pb to prevent future contamination. The excessive pumping should be reduced to decline the rate of seawater intrusion. The harvesting point of rainfall collection should inject the aquifer to shift the aquifer toward the sea, thereby decreasing the seawater intrusion rate and increasing agricultural yields through a reduction in TDS groundwater concentration. In future, more effort is required to reduce the health risk through the purification process and control toxic metal discharge, mainly from agricultural fertilizers. In addition, wastewater treatment plants must be applied appropriately to protect the aquifer system and reduce human health risks.
References
Adimalla N (2019) Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum Ecol Risk Assess: Int J. https://doi.org/10.1080/10807039.2018.1508329
Adimalla N, Wang H (2018) Distribution, contamination, and health risk assessment of heavy metals in surface soils from northern Telangana India. Arab J Geosci 11(21):684. https://doi.org/10.1007/s12517-018-4028-y
Al-Ahmadi ME (2012) Interpretation of groundwater quality using factor analysis, Wadi Rabigh area, Western Saudi Arabia. J Environ Hydrol 20:1–12
Al-Ahmadi ME (2013) Hydrochemical characterization of groundwater in wadi Sayyah, Western Saudi Arabia. Appl Water Sci 3:721–732. https://doi.org/10.1007/s13201-013-0118-x
Al-Ahmadi ME, El-Fiky AA (2009) Hydrogeochemical evaluation of shallow alluvial aquifer of Wadi Marwani, western Saudi Arabia. J King Saud Univ Sci 21:179–190. https://doi.org/10.1016/j.jksus.2009.10.005
Al-Arifi NS, Al-Agha MR, El-Nahhal YZ (2013) Environmental impact of landfill on groundwater, south east of Riyadh, Saudi Arabia. J Nat Sci Res 3:118–128
Alquwaizany AS, Alfadul SM, Khan MA, Alabdulaaly AI (2019) Occurrence of organic compounds in groundwater of Saudi Arabia. Environ Monit Assess 191:601. https://doi.org/10.1007/s10661-019-7723-6
Alyamani MS, Bazuhair AS, Hussein MT (1994) Interpretation of groundwater chemistry by factor analysis technique. Earth Sci 7(1):89–100
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Bamousa AO, El Maghraby M (2016) Groundwater characterization and quality assessment, and sources of pollution in Madinah Saudi Arabia. Arab J Geosci 9:536. https://doi.org/10.1007/s12517-016-2554-z
Banoeng-Yakubo B, Yidana SM, Nti E (2009) Hydrochemical analysis of groundwater using multivariate statistical methods—the Volta region Ghana. KSCE J Civ Eng 13(1):55–63
Bazuhair AS, Hussein MT, Alyamani MS, Ibrahim K (1992) Hydrogeophysical Studies of Khulais basin, Western Region, Saudi Arabia. Saudi Arabia: Unpub. Report, King Abdulaziz Uni, Jeddah
Bodrud-Doza M, Islam AR, Ahmed F, Das S, Saha N, Rahman NS (2016) Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci 30(1):19–40
CDQE Health Canada Guidelines For Canadian Drinking Water Quality—Summary Table (2020) Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, 2020
Çelebi A, Şengörür B, Kløve B (2014) Human health risk assessment of dissolved metals in groundwater and surface waters in the Melen watershed, Turkey. J Environ Sci Heal Part A 49:153–161
Chen X, Liu M, Ma J, Liu X, Liu D, Chen Y et al (2017) Health risk assessment of soil heavy metals in housing units built on brownfields in a city in China. J Soils Sedim 17(6):1741–1750
Choi AL, Sun G, Zhang Y, Grandjean P (2012) Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Persp 120(10):1362–1368. https://doi.org/10.1289/ehp.1104912
Chowdhury S, Al-Zahrani M (2015) Characterizing water resources and trends of sector wise water consumptions in Saudi Arabia. J King Saud Univ Eng Sci 27:68–82. https://doi.org/10.1016/j.jksues.2013.02.002
Congke G, Zhang Y, Peng Y, Leng P, Zhu N, Qia Y, Zhao L, Fadong L (2020) Spatial distribution and health risk assessment of dissolved trace elements in ground-water in Southern China. Sci Rep. https://doi.org/10.1038/s41598-020-64267-y
Coyte RM, McKinley KL, Jiang S, Karr L, Dwyer GS, Keyworth AJ, Davis CC, Kondash AJ, Vengosh A (2019) Occurrence and distribution of hexavalent chromium in groundwater from NorthCarolina USA. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.135135
Deng Y, Jiang L, Xu L, Hao X, Zhang S, Xu M et al (2019) Spatial distribution and risk assessment of heavy metals in contaminated paddy fields—a case study in Xiangtan City, southern China. Ecotoxicol Environ Saf 171:281–289
Egbueri JC (2019) Water quality appraisal of selected farm provinces using integrated hydrogeochemical, multivariate statistical, and microbiological technique. Model Earth Syst Environ 5(3):997–1013
Egbueri JC (2020) Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha. Nigeria. Anal Lett 53(10):1620–1638
Egbueri JC, Mgbenu CN (2020) Chemometric analysis for pollution source identification and humanhealth risk assessment of water resources in Ojoto Province, southeast Nigeria. Appl Water Sci 10:98
Diami SM, Kusin FM, Madzin Z (2016) Potential ecological and human health risks of heavy metals in surface soils associated with iron ore mining in Pahang Malaysia. Environ Sci Pollut Res 23(20):21086–21097
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a study case from Akpabuyo-Odukpani area, lower cross river basin (southeastern Nigeria). Geoj 57:295–304. https://doi.org/10.1023/B:GEJO.0000007250.92458.de
El-Hames AS, Al-Ahmadi M, Al-Amri N (2011) A GIS approach for the assessment of groundwater quality in Wadi Rabigh aquifer, Saudi Arabia. Environ Earth Sci 63:1319–1331. https://doi.org/10.1007/s12665-010-0803-0
Espejo-Herrera N, Cantor KP, Malats N, Silverman DT, Tardon A, Garcia-Closas R, Serra C, Kogevinas M, Villanueva CM (2015) Nitrate in drinking water and bladder cancer risk in Spain. Environ Res 137:299–307
FAO (2009) Groundwater management in Saudi Arabia. Food and Agriculture Organization of the United Nation
Gabr SS, Morsy EA, El Bastawesy MA, Habeebullah TM, Shaaban FF (2017) Exploration of potential groundwater resources at Thuwal Area, north of Jeddah, Saudi Arabia, using remote sensing data analysis and geophysical survey. Arab J Geosci 10(23):1–19. https://doi.org/10.1007/s12517-017-3295-3
Haque A, Jewel MA, Ferdoushi Z, Begum M, Husain MI, Mondal S (2018) Carcinogenic and non−carcinogenic human health risk from exposure to heavy metals in surface water of Padma river. Res J Environ Toxicol 12(1):18–23
Hofmann J, Watson V, Scharaw B (2015) Groundwater quality under stress: contaminants in the Kharaa River basin (Mongolia). Environ Earth Sci 73(2):629–648
Hu B, Wang J, Jin B, Li Y, Shi Z (2017) Assessment of the potential health risks of heavy metals in soils in a coastal industrial region of the Yangtze River Delta. Environ Sci Pollut Res 24(24):19816–19826
Hussein MT, Bazuhair A, Al-Yamani MT (1993a) Groundwater availability in the Khulais plain, western Saudi Arabia. Hydrol Sci J 38(3):203–213
Hussein M, Bazuhair A, Al-yamani M (1993b) Groundwater availability in the KhulaisPlain, Western Saudi Arabia. Hydrol Sci J 3S(3):6–1993. https://doi.org/10.1080/02626669309492663
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah Abdul Q (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf 72(2009):301–309
Khademi H, Gabarro´n M, Abbaspour A, Martı ´nez- S, Faz A, Acosta JA (2019) Environmental impact assessment of industrial activities on heavy metals distribution in street dust and soil. Chemosphere 217:695–705
Khan MYA, Gani KM, Chakrapani GJ (2016a) Assessment of surface water quality and its spatial variation. A case study of Ramganga River, Ganga Basin India. Arab J Geosci 9(1):1–9
Khan MYA, Khan B, Chakrapani GJ (2016b) Assessment of spatial variations in water quality of Garra River at Shahjahanpur, Ganga Basin India. Arab J Geosci 9(8):1–10
Khan MYA, Khan B, Chakrapani GJ (2016c) Assessment of spatial variations in water quality of Garra River at Shahjahanpur, Ganga Basin India. Arab J Geosci 9(8):1–10
Khan MYA, Gani KM, Chakrapani GJ (2017) Spatial and temporal variations of physicochemical and heavy metal pollution in Ramganga River—a tributary of River Ganges, India. Environ Earth Sci 76:1–13
Khan MYA, ElKashouty M, Bob M (2020a) Impact of rapid urbanization and tourism on the groundwater quality in Al Madinah city, Saudi Arabia: a monitoring and modeling approach. Arab J Geosci 13:1–22
Khan MYA, Hu H, Tian F, Wen J (2020b) Monitoring the spatio-temporal impact of small tributaries on the hydrochemical characteristics of Ramganga River, Ganges Basin, India. Int J River Basin Manag 18(2):231–241
Khan MYA, ElKashouty M, Tian F (2022) Mapping groundwater potential zones using analytical hierarchical process and multicriteria evaluation in the central eastern desert. Egypt Water 14(7):1041
Kumar A, Krishna AP (2021) Groundwater quality assessment using geospatial technique based water quality index (WQI) approach in a coal mining region of India. Arab J Geosci 14:1126
Kumar A, Mishra S, Bakshi S, Upadhyay P, Thakur TK (2023) Response of eutrophication and water quality drivers on greenhouse gas emissions in lakes of China: a critical analysis. Ecohydrology 16(1):e2483. https://doi.org/10.1002/eco.2483
Kumar A, Song H, Mishra S (2023) Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: a critical review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2023.137894
Kumar A, Mishra S, Pandey R, Yu Z et al (2023) Microplastics in terrestrial ecosystems: un-ignorable impacts on soil characterises, nutrient storage and its cycling. TrAC, Trends Anal Chem 158:116869. https://doi.org/10.1016/j.trac.2022.116869
Lazhar B, Ammar T, Lotfi M (2018) Assessment of heavy metals contamination in groundwater: a case study of the south of Setif area, east Algeria. In: Komatina D (ed) Achievements and challenges of integrated river basin management. IntechOpen, London. https://doi.org/10.5772/intechopen.75734
Li J, Dong Li FA, Zhang QY, Zhao GS, Liu Q, Song S (2013) Groundwater trace metal pollution and health risk assessment in agricultural areas. Understanding Freshwater Quality Problems in a Changing World. In: Proceedings of H04, IAHS-IAPSO-IASPEI Assembly, Gothenburg, Sweden, 361
World Health Organization Guidelines for drinking-water quality: first Addendum to the Fourth Edition, License: CC BY-NC-SA 3.0 IGO, Geneva, 2017
Lokhande PB, Patit VV, Mujawar HA (2008) Multivariate statistical analysis of groundwater in the vicinity of Mahad industrial area of Konkan region India. Int J Appl Environ Sci 3(2):149–163
Masoud MHZ, Basahi JM, Rajmohan N (2018) Impact of flash flood recharge on groundwater quality and its suitability in the Wadi Baysh Basin, Western Saudi Arabia: an integrated approach. Environ Earth Sci 77:395. https://doi.org/10.1007/s12665-018-7578-0
McDonald J (2006) Alkalinity & pH relationships. CSTN, pp 392–394
Miguel DE, Iribarren I, Chacon E, Ordonez A, Charlesworth S (2007) Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere. https://doi.org/10.1016/j.chemosphere.2006.05.065
Molugaram K, Rao GS (2017) Statistical techniques for transportation engineering. Butterworth Heinemann, Elsevier Inc, Oxford
MoWE (2014) National water strategy: to supply and protect the Kingdom’s most precious natural resource. Ministry of Water and Electricity, Kingdom of Saudi Arabia
National reasesrch Council (NRC) (2000) Copper in Drinking Water. Copyright © National Academy of Sciences. All rights reserved. ISBN: 0-309-59406-5, 162 pages, 6 × 9, (2000)
Nguendo-Tongsi HB (2011) Microbiological evaluation of drinking water in a sub-saharan urban community (Yaounde). Am J Biochem Mol Biol 1:68–81
Nosrati K, Van Den EM (2012) Assessment of groundwater quality using multivariate statistical techniques in Hashtgerd plain. Iran Environ Earth Sci 65(1):331–344
Oinam JD, Ramanathan AL, Jayalakshmi SG (2012) Geochemical and statistical evaluation of groundwater in Imphal and Thoubal district of Manipur, India. J Asia Earth Sci 48:136–149
Pawelczyk A (2013) Assessment of health risk associated with persistent organic pollutants in water. Environ Monitor Assess 185:497–508
Peek L et al (2018) Children and disasters, in handbook of disaster research, vol 8. Springer, Berlin, pp 243–262
Purushothaman P, Rao MS, Rawat YS, Kumar CP, Krishan G, Parveen T (2014) Evaluation of hydrogeochemistry and water quality in Bist-Doab region, Punjab India. Environ Earth Sci 72(3):693–706
Qaid A, Saleem M (2016) Assessment of physico-chemical and biological properties of ground water of Khulais, Province, Kingdom of Saudi Arabia. Assessment. 5(1)
Rajeshkumar S et al (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638
Ratnalu GV, Dhakate R (2021). Human health hazard evaluation with reference to chromium
Ratnalu GV, Dhakate R (2021) Human health hazard evaluation with reference to chromium (Cr+ 3 and Cr+ 6) in groundwater of Bengaluru Metropolitan City South India. Arab J Geosci 14:2472. https://doi.org/10.1007/s12517-021-08671-2
Reddy AVK, Reddy KV, Murthy PK (2018) Geomatics for land use/land cover and water quality changes. Int J Sci Res Sci Technol 4(2):1614–1618
Rogerson P (2001) A statistical method for the detection of geographic clustering. Geogr Anal 33:215–227
Sakakibara K, Iwagami S, Tsujimura M, Abe Y, Hada M, Pun I, Onda Y (2019) Groundwater age and mixing process for evaluation of radionuclide impact on water resources following the Fukushima Daiichi nuclear power plant accident. J Contam Hydrol 223:103474
Sakizadeh M, Ahmadpour E (2016) Geological impacts on groundwater pollution: a case study in Khuzestan Province. Environ Earth Sci 75(1):1–12
Singh RK, Sengupta B, Bal R, Shukla BP, Gurunadharao VS, Srivatstava R (2009) Identification and mapping of chromium (VI) plume in groundwater for remediation: a case study at Kanpur Uttar Pradesh. J Geol Soc India 74(1):49–57. https://doi.org/10.1007/s12594-009-0103-z
Sivakumar S, Chandrasekaran A, Ravisankar R, Ravikumar SM, Prince PrakashJebakumar J, Vijayagopal P (2014) Measurements of natural radioactivity and evaluation of radiation hazards in coastal sediments of east coast of Tamilnadu, India using statistical approach. J Taibah Univ Sci 8:375–384
Subyani AM (2005) Hydrochemical identification and salinity problem of ground-water in Wadi Yalamlam basin, Western Saudi Arabia. J Arid Environ 60:53–66. https://doi.org/10.1016/j.jaridenv.2004.03.009
Taiwo AW, Awomeso JA (2017) Assessment of trace metal concentration and health risk of artisanal gold mining activities in Ijeshaland, Osun State, Nigeria –part 1. J Geochem Explor 177(2017):1–10. https://doi.org/10.1016/j.gexplo.2017.01.009
Taneja P, Labhasetwar P, Nagarnaik P, Ensink JHJ (2017) The risk of cancer as a result of elevated levels of nitrate in drinking water and vegetables in Central India. J Water Health 15:602–614
Ukah BU, Ameh PD, Egbueri JC, Unigwe CO, Ubido OE (2020) Impact of effluent-derived heavy metals on the groundwater quality in Ajao industrial area, Nigeria: an assessment using entropy water quality index (EWQI). Int J Energy Water Resour 4:231–244
USEPA (United States Environmental Protection Agency) (1989) Risk assessment guidance for Superfund, Volume I: human health evaluation manual (Part A), interim final, office of emergency and remedial response. EPA/540/1-89/002. https://www.osti.gov/biblio/7037757
USEPA (United States Environmental Protection Agency) (2002) United States Environmental Protection Agency, Supplemental guidance for developing soil screening levels for Superfund sites, Appendix D-Dispersion Factors Calculations, Washington, DC, USA, OSWER93552002 4–24
USEPA (2001a) (US Environmental Protection Agency)baseline human health risk assessment, Vasquez Boulevard and I-70 Superfund site, U.S. Public Health Service, Denver, CO
USEPA (2001b) (US Environmental Protection Agency)baseline human health risk assessment, Vasquez Boulevard and I-70 Superfund site, U.S. Public Health Service, Denver, CO. (2015) 351 https://doi.org/10.1007/s10661-015-4319-7
USEPA (2016) Basic information about lead in drinking water. In: USEPA. https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinkingwater. Accessed 22 Jan 2021
USEPA (United States Environmental Protection Agency) (2014) Human health evaluation manual, supplemental guidance: update of standard default exposure factors. OSWER Directive 9200.1–120
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2012) Evaluation of groundwater suitability for domestic, irrigational, and industrial purposes: a case study from Thirumanimuttar river basin, Tamilnadu, India. Environ Monit Assess 184(1):405–420. https://doi.org/10.1007/s10661-011-1977-y
Wagh VM, Panaskar DB, Mukate SV, Gaikwad SK, Muley AA, Varade AM (2018) Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik India. Model Earth Syst Environ. https://doi.org/10.1007/s40808-018-0496-z
Ward MH, Jones RR, Brender JD, de Kok DM, Weyer PJ, Nolan BT, Villanueva CM, van Breda C (2018) Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15:1557. https://doi.org/10.3390/ijerph15071557
WHO (2017) Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva
WHO (2019) Lead poisoning and health. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health. Accessed 15 Feb 2021
WHO Guidelines for Drinking-Water Quality (2011) Environmental health criteria, 4th edn. World Health Organization, Geneva, Switzerland
Xia J (2002) A perspective on hydrological base of water security problem and its application in North China. Prog Geogr 21(6):51–526
Xu Y, Dai S, Meng K, Wang Y, Ren W, Zhao L et al (2018) Occurrence and risk assessment of potentially toxic elements and typical organic pollutants in contaminated rural soils. Sci Total Environ 630:618–629
Yan H, Hu H, Liu Y, Tudaji M, Yang T, Wei Z, Chen L, Khan MYA, Chen Z (2022) Characterizing the groundwater storage–discharge relationship of small catchments in China. Hydrol Res 53(5):782–794
Yetiş R, Atasoy AD, Yetiş AD, Yeşilnacar M (2019) Hydrogeochemical characteristics and quality assessment of groundwater in Balikligol Basin, Sanliurfa, Turkey. Environ Earth Sci 78(11):331
Zaharani KH, Al-Shayaa MS, Baig MB (2011) Water conservation inthe kingdom of Saudi Arabia for better environment: implicationsfor extension and education. Bulg J Agri Sci 17: 389–395 (2) (PDF) Impact of flash flood recharge on groundwater quality and its suitability in the Wadi Baysh Basin, Western Saudi Arabia: an integrated approach
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess 187:607. https://doi.org/10.1007/s10661-015-4828-4
Zhao K, Fu W, Qiu Q, Ye Z, Li Y, Tunney H et al (2019) Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma 337:453–462
Zhaoyong Z, Mamat A, Simayi Z (2019) Pollution assessment and health risks evaluation of (metalloid) heavy metals in urban street dust of 58 cities in China. Environ Sci Pollut Res 26(1):126–140
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University Jeddah, Saudi Arabia, under grant no. KEP-2-155-42. The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, M.Y.A., ElKashouty, M., Khan, N. et al. Spatio-temporal evaluation of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater, Khulais, Saudi Arabia. Appl Water Sci 13, 123 (2023). https://doi.org/10.1007/s13201-023-01928-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01928-z