Abstract
Iran's most important iron ore mine is located in the central region, and because of the water shortage in this area, the need to reuse the effluent from this mine is essential. On the other hand, there are no suitable conditions for treating large effluent volumes in iron mine in central Iran. For this reason, produced effluent should be reduced and returned to the consumption cycle by using appropriate technology. This study aimed to investigate the ozonation/lime effect on polymer consumption reduction and evaluate the treatment and economic efficiency compared to the currently used treatment method (coagulation-flocculation without ozonation/lime).
The use of ozonation along with the coagulation and flocculation process has been an effective factor in reducing all the studied indicators, which has been a much more significant reduction effect for turbidity (95%), decreasing from 374-350NTU in the non-ozonation process to 110-160NTU, and Chemical oxygen demand (37%). In addition to increasing the treatment efficiency, the hybrid ozonation/coagulation and flocculation process reduced operation costs. The ozonation process caused the high-level conversion of Fe2+ and Al2+ to Fe3+ and Al3+ (> 90%), thus it improved wastewater treatment and increased cost benefit. The hybrid process was affected in improving the effluent quality and reducing the produced sludge volume. The ozonation process caused sludge volume reduction or has photocatalytic effect on it. It effected the micro-sized bubbles production reduction in sludge volume unit. However, estimating the cost–benefit of using this method can be beneficial in making the final decision on whether to use it or not.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In quarries, tailings are extracted along with valuable minerals in ores, which are separated during the crushing and purification steps. Tailings are usually piled up more or less near a mine or processing plant. One of the long-term effects of this operation in mining areas is the increase of some elements concentration in the tailings accumulation areas (Ghose and Sen 2001; Badeenezhad and Azhdarpoor 2019; Jalili 2020). Due to the leakage of produced mineral drains throughout the leaching of mineral deposits by precipitation to the surface and groundwater, they caused many destructive environmental effects. These pollutants' environmental impacts can lead to public health deficiencies in human societies (Xu et al. 2009; Badeenezhad et al. 2019). The iron ore content must also be decomposed and purified after extraction. If impurities such as silica and alumina are above the acceptable range and the iron content is less than 60%, the need for mine effluent treatment will be more necessary than ever (Ding et al. 2017). Most effluents in ore mines are concentrated in the crushing, grinding, sieving, washing, using magnetic separators, or flotation stages (Ghose 2003). Iron ore waste after the preparation process may contain coarse and fine particles in the wash water, which forms tailings.
Due to the climatic conditions of Yazd province and water shortage in this area, the water reuse in the iron ore mining process can be a cost-effective option. In central iron ore, refined tailings contain amounts of water that must be dehydrated and dried have the save mien, and their moisture content should be reached 9–10%. For this purpose, filters are used to separate the resulting concentrated water. Then the dewatered concentrate transferred to the dumpsite and stored there. The tailings are also diverted to thickener for dewatering. There, the solids settle, and water overflows from the top of the thickener. The deposited solid particles are directed to the center by the thickener floor collector arm and pumped to the tailings dam.
On the other hand, due to the mineral nature of the produced effluent in this type of mine, it is not possible to completely dehydrate by filter press. Maximum water reduction of such tailings prevent contaminants infiltration into groundwater and other related environmental problems. For this purpose, the combined processes of sludge dehydration, the reduction of annual polymer consumption, and the reduction of the produced sludge by these mines are an option. On the other hand, particle settling rate and water supply with less turbidity can be achieved for water reuse in this process (Nasrollahi et al. 2014; Jalili et al. 2019).
Due to the limited efficiency of organic matter removal in the conventional coagulation process (Hou et al. 2017), Hybrid Ozone-Coagulation (HOC) process for effluent treatment used in this study. This could be effective according to the pre-tests. Based on the pre-test results, the ozonation coagulation- flocculation was effective, due to the nature of the produced sludge by coagulation with alum and its tendency to absorb water, dewatering is expected to be more difficult. Therefore, reducing polymer consumption can be effective in improving dehydration. Finally, the use of ozone to elements removal has been associated with less sludge production compared to polymerization. The innovation of this study was the conversion investigation of Fe2+ to Fe3+ and Al2+ to Al3+ during the ozonation process. This process significantly can lead to Al3+ and Fe3+ coagulants production and ultimately, it exceedingly reduces the need for coagulant consumption.
Organic matter is one of the most troublesome constituents of water and wastewater that interferes with coagulation and flocculation processes. Coagulation and flocculation are complex treatment technologies that aim to maximize the removal of suspended solids, maximize the removal of natural organic matter (NOM), and minimize the coagulant residual in treated water. Coagulant selection, dosing rates and performance are heavily influenced by the nature and concentrations of NOM, which is a mixture of organic compounds that vary greatly in terms of their physical and chemical characteristics (Leopold and Freese 2009). If the coagulant dose is insufficient to overcome this demand, aluminum-NOM complexes will form and remain in dissolved or colloidal form. The coagulant dose must be increased to allow the dissolved/colloidal aluminum-NOM complexes to form particles that are heavy enough to settle. Alternatively, a coagulant aid such as activated silica, bentonite or lime can be added. It is important to note that some NOM fractions cannot be removed by coagulation at any pH or dose (Canada 2019). Finally, this study aimed to evaluate and compare the efficiency of the coagulation and flocculation process with the hybrid process, coagulation and flocculation with ozonation/lime in crushing lines effluent treatment of Iran's Iron Ore Company.
Materials and methods
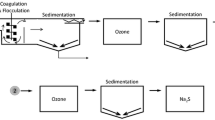
The current study was done to determine the efficiency of the combined process of coagulation and flocculation-ozonation/lime in the effluent treatment of Iran's Central Iron Ore crushing line. The laboratory analyzes were performed by using the jar test method. The experimental parameters in this study included the contact time of ozonation, the polymer concentration, settling time, and coagulation and flocculation time.
Sampling
In this study, Iran's Central Iron Ore Refinery crushing line's raw effluent was investigated. For sampling, the specimen was collected manually through the effluent passage and before polymerization (Alum-based Cationic polymers).
Experimental analysis
All experiments were performed at pH = 7–8 (because the pH of the effluent leaving the crusher line and before adding the polymer in the effluent transfer line to the treatment unit was 7–8 on average) and it was at an average ambient temperature of 22–27 °C. The independent parameters were determined step by step, and the range of anyone is expressed in Table 1.
To determine the efficiency of the investigated method in this subject, the chemical oxygen demand (COD), total suspended solids (TSS), sludge volume index (SVI), suspended solids (SS), and turbidity and finally were measured as a response and compared to the initial effluent.
In the first step, the optimal contact time with ozone was investigated. First, after 5 min of effluent contact with ozone (gO3/hr.5 = nominal capacity of the ozonation device), the jar test (Jar test technical specifications: with timer, distance meter and color touch screen microprocessor speed control, adjustable in 3 different steps, adjustable speed from 10 to 250 rpm and unlimited time adjustment, propeller and rod made of 316 steel, direct current motor without round swing with dimensions 230 × 450 × 1000 (mm)) was run by keeping the polymer/lime concentration and settling time constant, COD, turbidity, and SVI removal percentage was determined. These steps were done for a contact time of 5 min (0.42 gr.O3), 10 min (0.83 gr.O3), and 15 min (1.25 gr.O3) to determine the optimal contact time with O3.
The jar test included ozonation contact time (5, 10, and 15 min) and polymer concentration (three concentrations of 10, 20, and 30 g per ton of dry sludge). Also, three settling times (5, 10, and 20 min), coagulation times (30, 60, and 90 s), and flocculation times (10, 15, and 20 min) were examined. In each step, the optimal value for each parameter was checked. In total, polymer/lime concentration and ozonation time were investigated. So, nine enforcements were performed. In each step, a control sample was considered. After determining the optimal conditions for both processes (coagulation and flocculation process and coagulation and flocculation process with ozonation/lime), two samples were considered for scanning electron microscope (SEM) analysis. Three samples were taken after three ozonation times (5, 10, and 15 min) to determine the conversion of Fe2+ and Al2+ to Fe3+ and Al3+, respectively, by Inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis. Two samples were taken from the iron ore refinery crushing line for ICP-OES analysis to determine some elements concentration in the effluent. The total number of required samples was equal to 63 (56 + 7 = 63).
Instrumental analysis
Due to the oxidation of Fe2+ to Fe3+ and Al2+ to Al3+ during the ozonation, the oxidized particles were deposited into the sludge. In the case of aluminum-based coagulants, the form of aluminum (e.g., particulate or dissolved) that will be present depends on a wide variety of environmental parameters, including pH, temperature, NOM and the presence of inorganic ligands such as fluoride, sulfate, silicate and phosphorus (Canada 2021). When aluminum-based coagulants are added to water, chemical reactions occur with the organic and inorganic particles in that water. NOM acts as a ligand that complexes the positively-charged aluminum ions, exerting a coagulant demand that must be overcome before flocculation can occur (Haarhoff and Edzwald 2013) but NOM amount in iron ore effluent is low. Thus, the amount of dissolved Fe and Al in the supernatant and sedimentation was measured separately by ICP-OES (Élan 6100 DRC-e). Then it was compared with the initial concentration of iron in the effluent. In addition, the other elements concentrations were determined using ICP-OES. The SEM image (SEM FEI Quanta 200) was used to study the three-dimensional image of the formed sludge. Turbidity was measured by the HACH portable turbidity meter. COD was measured by the COD vial according to the Standard Methods for the Examination of Water and Waste Water 2005-B5220 (Palácio et al. 2016). After each experiment, the COD amount relative to the raw sample was evaluated, and its removal percentage was determined. The amount of SVI was also assessed at each stage of the experiments. SEM and ICP-OES analyses were performed by sending samples to the laboratory.
Methods of data analysis
Statistical analysis
In this study, descriptive statistical indicators such as mean, standard deviation, and percentage were used to express the parameters concentrations and process efficiency. The difference between the parameters was determined by T-test. The significance level was considered 0.05. The correlation of the removal efficiency to each variable was determined using Pearson correlation. All statistical analyzes were performed in SPSS 2018. The figures were also drawn using the Origin-pro software 2020.
Cost–benefit analysis
Since this method was used on an industrial scale, the cost–benefit estimation of the process is very valuable. Therefore, in the present study, the cost–benefit analysis of each procedure was determined using EQ. (1) (Gaziano et al. 2006):
Which:
C0: the initial COD concentration, C1: the final COD concentration, E0: process cost, and E1: the coagulation-flocculation cost.
Results
At first, the characteristics of the studied raw effluent and the environmental discharge standard are presented in Table 2.
According to Table 2, the achieved results for all parameters significantly were higher than Iran's standard, as COD = 305 ± 21.06 mg/L (> 60 mg/L), turbidity = 312 ± 12.71 NTU (> 50NTU), and SS = 161 ± 51.34 ml/l (> 0 ml/l). So, the evaluation of advanced oxidation processes such as ozonation is essential. To reach this goal, ozonation process's effect on coagulation and flocculation of effluent from Iran's iron ore was investigated. Finally, the effect of the operative parameters on process efficiency was evaluated. The effluent quality in each of the processes was assessed and measured.
Figure 1 shows the ozonation effect on the amount of COD, turbidity, SVI, and SS remaining in the effluent at different polymer concentrations use.
As shown in Fig. 1, with increasing the polymer dose COD, the turbidity, SVI, and SS removal efficiency increased. But this removal rate was not cost-effective (see Fig. 8: Cost-effectiveness of each process). Thus, without ozonation, parameters including turbidity, the concentrations of COD, and SS at 30 mg/L were lower than other concentrations. SVI is the only indicator that has reached its minimum at a concentration of 30 mg/L (73.68 ml/g). In the presence of 15 min of ozonation, with increasing polymer concentration, the turbidity (59%), COD (37%), and SS (22.22%) decreased. Only the SVI index did not change much (7.38%) with increasing polymer concentration in the presence of ozonation.
In addition, the amount of these indicators in the treated effluent in the presence of the ozonation process was much less than the process without ozonation (p < 0.05). In other words, the elimination rate of the studied parameters in the ozonation process was higher than in the free of ozonation process. According to the results, the COD removal (p = 0.011), turbidity (p = 0.023), SVI (p = 0.028), and SS (p = 0.031) in ozonation process was higher than that in process without ozonation. The effect of ozonation at different coagulation times on COD, turbidity, SVI, and the remaining amount of SS in the effluent is shown in Fig. 2.
When the coagulation time increased, SVI and SS decreased, and turbidity and COD increased significantly (p < 0.05) (Fig. 2a). Moreover, with increasing the coagulation time in the absence of ozonation, COD, turbidity, and SVI increased (Fig. 2b), but in the presence of the ozonation process, COD, SVI, and SS were less compared to the process without ozonation in the effluent (p < 0.023). However, the maximum effect of ozonation during coagulation time variation was related to the turbidity in the effluent, and it decreased from 374 to 350 NTU in the process without ozonation to 160–110 NTU (59%). Thus, ozonation was a significant factor in reducing the turbidity of iron ore effluent. Figure 3 shows the effect of ozonation on COD, turbidity, SVI, and SS indices during flocculation time alterations.
According to Fig. 3, with increasing flocculation time, COD, turbidity, SVI, and SS decreased in the ozonation and in the process without ozonation (p > 0.05). Thus, increasing the flocculation time can be suitable for effluent treatment, and the optimal amount of which was obtained in 20 min. However, cost estimation studies resulting from this increase in flocculation time are essential for real use.
However, the use of ozonation with the coagulation and flocculation process has been an effective factor in reducing all the studied indicators, which has a very significant reduction in turbidity. The ozonation effect on COD, turbidity, SVI, and SS removal in different settling times is shown in Fig. 4.
As shown in Fig. 4, with increasing sedimentation time, the studied indices decreased. The optimal sedimentation time was 30 min. It should be noted that the cost-effective of the process is an influential factor in reducing the COD and SS indices which should be considered. However, these differences were more significant at different sedimentation times in the ozonation process, especially turbidity (p < 0.05). Finally, according to the optimal conditions obtained from the above experiments, the effect of ozonation time on COD, turbidity, SVI, and SS is shown in Fig. 5.
In Fig. 5, each studied parameter, including SVI, COD, SS, SVI, and turbidity, was measured ten minutes after each ozonation step. The results showed that with increasing ozonation time, a significant decrease was observed in COD, turbidity, and SVI (p < 0.05), which was COD (5 min: 12%, 10 min: 24%, and 15 min: 34.42%), turbidity (5 min: 22.43%, 10 min: 32.69%, and 15 min: 37.5%), SVI (5 min: 24.96%, 10 min: 27.64%, and 15 min: 44.7%), and SS (5 min: 13.04%, 10 min: 16.14%, and 15 min: 19.25%).
The SS variations were not significant with changes in ozonation time (p < 0.02). So, SS decreased from 160 mg/L in process without ozonation to 140 mg/L (ozonation time = 15 min). Besides, the lime adding effect in the polymerization and ozonation process on the COD, turbidity, SVI, and SS in the effluent is shown in Fig. 6.
According to Fig. 6, the amount of COD after lime addition in the coagulation and flocculation process and the combined coagulation and flocculation-ozonation process were 145 mg/L and 105 mg/l, respectively. The addition of lime in the combined coagulation and flocculation-ozonation process at 40 mg/l has been more effective in removing COD in the coagulation and flocculation process. Also, the turbidity after lime addition in the coagulation and flocculation process and the combined process were 230 and 28 NTU, respectively. The lime addition in the combined coagulation and flocculation-ozonation process were more effective than the coagulation and flocculation-ozonation process at 81.82 ml/g. In the case of SS, it was more effective in removing turbidity than the coagulation and flocculation process. The reduction reached 150 ml/l in the coagulation and flocculation process, and it reached 120 ml/l in the hybrid process.
Despite the effects and proper efficiency of ozonation in iron ore effluent treatment and reducing the produced sludge volume, it is necessary to study the characteristics, elemental analysis, and microscopic changes of sludge. The sludge flocs from both coagulation and flocculation and the combined process with ozonation were examined by SEM. Figure 7 shows the SEM of iron ore effluent sludge under optimal conditions of two coagulation and flocculation processes and coagulation and flocculation-ozonation.
The sludge structure from the combined process had a smooth and dense surface (Fig. 7B). Besides, Fe2O3 particles with white rod-shaped and cylindrical clusters were observed on the sludge surface (Fig. 7 B-2). According to Fig. 7 (B-1), the deposited particles size on the sludge was less than 1 μm, while the sludge particle size in 7A was less. Table 3 shows the elements and metals concentration in the raw effluent in comparison to obtained effluent from ozonation, polymerization, and simultaneous ozonation and polymerization processes.
The simultaneous use of ozonation and polymerization process was an effective factor in removing elements from the effluent (> 90%). Hence hazardous elements' concentration such as arsenic, silver, cadmium, cobalt, chromium, mercury, manganese, molybdenum, nickel, and lead are below the Iran's standard level. In addition, the concentration of manganese decreased by about 75% during the study process (Table 3) and reached a concentration lower than the standard. In addition, the ozonation process combined with coagulation was able to remove 99.9% of the iron from the iron ore effluent.
Cost-effectiveness
According to the importance of investigating the economic aspects in choosing the application of a method in the industry, estimating the effluent treatment cost from the crusher line by using the current method in Iran's central iron ore (using 30 g polymer/ton of dry SL, an expression of 1.15 g/m3 effluent) was investigated. The results obtained from EQ. 3 for estimating the cost-effectiveness are given in Fig. 8.
According to Fig. 8, the highest cost-effectiveness is related to the process P (30gr/dry SL) + O3 (5 min) (83.49) and then the highest cost-effectiveness is related to P (30gr/dry SL) + O3 (10 min) (63.23, (10 min) (23.63), P (30gr/dry SL) + O3 (15 min) (55.4), P (30gr/dry SL) (16.42), and P (10gr/dry SL) (14.99), respectively. The lowest cost-effectiveness rate was related to P (20gr/dry SL) + O3 (15 min), with a cost-effectiveness rate of -65.23. The cost and efficiency of each process are shown in Fig. 9.
The highest and lowest cost was related to P (30gr/dry SL) + O3 (15 min) and P (10gr/dry SL) + O3 (5 min) with the cost of 38 × 10–4 and 12 × 10–4 Dollar/m3 effluent, respectively. In contrast, the highest and lowest efficiencies were related to P (30gr/dry SL) + O3 (15 min) and P (10gr/dry SL) + O3 (5 min) with 63.8% and 30.5% efficiency, respectively.
Discussion
In this study, the effectiveness of the ozonation process on the current treatment of iron ore mine effluent (coagulation and flocculation by polymerization) in Iran central iron ore with a focus on SVI, COD, SS, and turbidity indices was investigated. To determine the optimal conditions, the effect of changes in design and operation variables including coagulation, flocculation, sedimentation time, polymer concentration, and ozonation time, at a constant speed on the process performance was determined. At first, the treated effluent with the current used process in the mine was examined. It was found that all parameters except SVI in the prototype were higher than the standards, while it has not had a significant effect on other indicators elimination. Thus, the ozonation process efficiency in comparison with the current polymerization was evaluated during the change of effective parameters. In general, all indicators during the combined process with ozonation were less than the polymerization process (p < 0.05). Further, during the ozonation process (Fig. 5), with increasing ozonation time, the number of studied indicators significantly decreased (p < 0.05).
In the study of (Jin et al. 2019), the ozonation-coagulation hybrid process has been effective in improving the purification process. Because with increasing ozonation time, the concentration of available ozone to produce hydroxyl and superoxide radicals and oxidation processes increase. Hence, ozonation changes particle charge, improves hydration, and increases the surface activity of coagulants and coagulants aid. In addition, metal coagulants such as aluminum and iron in the presence of ozone act as a catalyst that produces higher levels of hydroxyl radicals compared to the absence of coagulants (Fe-HOC) and modified precipitation process (Jin et al. 2019; López-Vinent et al. 2021). Although less attention has been paid to ozone use in improving the iron ore effluent treatment, but similar studies have shown a significant reduction in the sludge volume of other industrial wastewaters using combined ozonation processes (Solís et al. 2021; Hashimoto et al. 2021; Yang et al. 2021; Wu et al. 2020).
According to the results, with increasing the polymer concentration and settling time and coagulation time decreasing, the level of all four indicators decreased (Fig. 1). While such a trend is not observed for the current non-integration process. As the polymer concentration increases, the turbidity and COD increase, which in addition to the inorganic nature of the carbon content, it can be due to the re-suspension of flocs at high polymer concentrations. In contrast, in the hybrid process, the additional flocs become unstable by ozonation, and the settling capacity was increased.
In the study by (Setareh et al. 2020), the hybrid ozonation-ultrasonic process removed 33—50% of the turbidity. In other study, in 60 min, the COD and turbidity removal rate was 59% and 76%, respectively (Ashraf et al. 2016). Although no results have been reported on the effect of ozonation on mineral effluents, but with increasing flocculation time, the studied indices have decreased. However, the variation trend in the hybrid process has been proportional to the increase in time (R = 0.63). The SVI index did not change much. However, it was within the recommended standard range.
In a study by (Zhang et al. 2018), the ozonation with the micro coagulation and precipitation process was an effective factor in improving the treatment process due to organic pollutants removal. In the study of (Kato et al. 2018), the virus removal efficiency increase in the combined process of ozonation and coagulation-settling. Because during the increase of flocculation time, sufficient time was provided for large and sedimentable flocs formation, which increased the sedimentation time in the hybrid process, its effects were intensified (Fig. 4).
Additionally, the radicals from the ozonation process can neutralize the stable particles that precipitate the colloids (Canada 2019, 2021). However, with the increasing coagulation time, the indicators amount has increased (Fig. 2), which creates unfavorable conditions for the instability of colloids. Thus, reducing the coagulation time in this process is recommended.
In the study of (Forsido et al. 2020), the use of lime increases the removal rates of Al (98%), Ba (95.8%), and Mg (99.7%). Moreover, elements and metals concentration, turbidity, and SS decreases significantly (Sun et al. 2020; Salmani et al. 2021; Salehi et al. 2020). Because in the coagulation-flocculation process, the amount of acidity increased, so lime can provide alkalinity as a simple, cheap, available, and effective method (pH > 9.5) and is effective in removing metal oxides. For example, the study by (Zheng et al. 2020) showed that the addition of lime increases the turbidity removal rate by 99.99% and SS by 99.84%.
Based on ICP-OES analysis results in raw effluent, the combined ozonation-polymerization process was an important factor in removing elements from the effluent. The concentration of hazardous elements such as arsenic, silver, cadmium, cobalt, chromium, mercury, manganese, molybdenum, nickel, and lead was less than the standard (p < 0.001). The results of other study in 2020 also showed that the combination of ozonation and coagulation process results in Cu removal (99.7%) (Salmani et al. 2021; Nguyen et al. 2021; Abbasia et al. 2020).
Manganese is an insoluble compound of Mn2O3 in the face of ozone-producing radicals (Ryu et al. 2019). The combined ozonation process with coagulation can remove 99.9% of iron from iron ore effluent, which is less efficient for the polymer at a concentration of 30 mg/L.
In the SEM image of the sludge under optimal conditions, spherical particles due to the presence of aluminum compounds and nanometer particles of iron oxide on the surface of the it were observed. The structure of the sludge layers from the coagulation and flocculation-ozonation process is discernible. The presence of white clusters of rods and cylinders cavities with micro and nano-size in the precipitated sludge has also been confirmed, which may be due to the presence of calcium oxide or other precipitated compounds (Seifan et al. 2020; Sibiya 2020; Smoczynski et al. 2019; Smoczyński et al. 2014; Lange 1994). However, the cavities dimensions in the coagulation and flocculation process were bigger than in hybrid process. In the study of iron oxide compounds produced during the oxidation process, their size was less than 100 μm (Mari et al. 2020). Thus, in the current process, only iron particles with larger sizes have been removed. While in the hybrid process, fine iron particles, which are usually in the form of colloids, were also removed.
Based on previous studies, the use of ozonation in combination with other processes, including Fenton, accounts for about 85% of the total operating cost (Wu et al. 2020), and the costs of sludge management were reduced (Fig. 9). It requires further studies in the target industries. Besides, the modification of the ozonation process, such as micro-sized bubbles for volume reduction or photocatalytic effect on sludge volume reduction, is recommended.
Conclusion
The combined ozonation-coagulation and flocculation process was effective in improving the quality of central iron ore crusher line effluent. Adding lime reduces SS, SVI, and turbidity. By using the ozonation process, the elements in the effluent, such as iron, aluminum, and others, were decreased more than the use of coagulant alone, which was statistically significant (p < 0.005). Also, the lime by increasing the effluent alkalinity reduced toxic elements and elements in the effluent. Other parameters such as final sludge volume and turbidity have also improved after the lime addition. However, lime is a cheaper and more accessible material than polymers used in the Bafgh iron ore refinery. Finally, the simultaneous use of the ozonation/calcination process with coagulation and flocculation was effective than coagulation and flocculation alone for effluent treatment.
References
Abbasia F, Samaeia MR, Azhdarpoora A, Jalilib M, Malekniaa H, Mehdizadeha A (2020) Removal, optimization and kinetic modeling of high concentration of methyl tertiary butyl ether from aqueous solutions using copper oxide nanoparticles and hydrogen peroxide. Desalin Water Treat 181:278–288
Ashraf MI, Ateeb M, Khan MH, Ahmed N, Mahmood QJS, Technology P (2016) Integrated treatment of pharmaceutical effluents by chemical coagulation and Ozonation. Sep Purif Technol 158:383–386
Badeenezhad A, Azhdarpoor A (2019) Efficiency of the activated carbon and clinoptilolite particles coated with iron oxide magnetic nanoparticles in removal of methylene blue. Desalin Water Treat 154:347–355
Badeenezhad A, Azhdarpoor A, Bahrami S, Yousefinejad S (2019) Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol Simul 45(7):564–571
Canada H. 2019 Guidance on Natural Organic Matter in Drinking Water. Document released for public consultation
Canada H. 2021 Guidelines for Canadian Drinking Water Quality: Guideline Technical Document. G - Aluminum Published by Health Canada
Ding J, Ma S, Shen S, Xie Z, Zheng S, Zhang Y (2017) Research and industrialization progress of recovering alumina from fly ash: a concise review. Waste Manage 60:375–387
Forsido TT, McCrindle RI, Maree J, Monyatsi L (2020) Removal of Al, Ba and Mg from industrial wastewater using EAFDS and lime. Appl Water Sci 10(6):1–7
Gaziano TA, Opie LH, Weinstein MC (2006) Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. The Lancet 368(9536):679–686
Ghose M. 2003 Environmental impacts of Indian small-scale mining industry-an overview
Ghose M, Sen P (2001) Characteristics of iron ore tailing slime in India and its test for required pond size. Environ Monit Assess 68(1):51–61
Haarhoff J, Edzwald JK (2013) Adapting dissolved air flotation for the clarification of seawater. Desalination 311:90–94
Hashimoto K, Kubota N, Okuda T, Nakai S, Nishijima W, Motoshige HJC (2021) Reduction of ozone dosage by using ozone in ultrafine bubbles to reduce sludge volume. Chemosphere 274:129922
Hou R, Jin X, Jin P, Wang X (2017) Characteristics and mechanism of hybrid ozonation-coagulation process in wastewater reclamation. JHjkxHk 38(2):640–646
Jalili M (2020) Environmental burden of disease from municipal solid waste incinerator. J Environ Health Sustain Dev 5(1):922–924
Jalili M, Hosseini MS, Ehrampoush MH, Sarlak M, Abbasi F, Fallahzadeh RA (2019) Use of water quality index and spatial analysis to assess groundwater quality for drinking purpose in Ardakan Iran. J Environ Health Sustain Dev. 4(3):834–842
Jin X, Jin P, Hou R, Yang L, Wang XCJ (2017) Enhanced WWTP effluent organic matter removal in hybrid ozonation-coagulation (HOC) process catalyzed by Al-based coagulant. J Hazard Mater 327:216–224
Jin X, Wang Y, Zhang W, Jin P, Wang XC, Wen LJC (2019) Mechanism of the hybrid ozonation-coagulation (HOC) process: comparison of preformed Al13 polymer and in situ formed Al species. Chemosphere 229:262–272
Kato R, Asami T, Utagawa E, Furumai H, Katayama HJ (2018) Pepper mild mottle virus as a process indicator at drinking water treatment plants employing coagulation-sedimentation, rapid sand filtration, ozonation, and biological activated carbon treatments in Japan. Water Res 132:61–70
Lange FF. Effect of Surface Forces on the Rheology of Particle-Liquid Systems and the Consolidation of Ceramic Powders. 1994.
Leopold P, Freese S. 2009 A simple guide to the chemistry, selection and use of chemicals for water and wastewater treatment: Water Research Commission Pretoria
López-Vinent N, Cruz-Alcalde A, Ganiyu SO, Sable S, Messele SA, Lillico D et al (2021) Coagulation-flocculation followed by catalytic ozonation processes for enhanced primary treatment during wet weather conditions. J Environ Manag 283:111975
Mari E, Tsai P-C, Eswaran M, Ponnusamy VK (2020) Efficient electro-catalytic oxidation of ethylene glycol using flower-like graphitic carbon nitride/iron oxide/palladium nanocomposite for fuel cell application. Fuel 280:118646
Nasrollahi Z, VasfiEsfastani S, Norizadeh S (2014) Environmental Assessment Of Economic Activity By Using I-O Table (Yazd). Econ Model 8(26):75–89
Nguyen MK, Pham TT, Pham HG, Hoang BL, Nguyen TH, Nguyen TH et al (2021) Fenton/ozone-based oxidation and coagulation processes for removing metals (Cu, Ni)-EDTA from plating wastewater. J of Water Process Engineering 39:101836
Palácio SM, Espinoza-Quiñones FR, de Pauli AR, Piana PA, Queiroz CB, Fabris SC et al (2016) Assessment of anthropogenic impacts on the water quality of Marreco River, Brazil, based on principal component analysis and toxicological assays. Water Air Soil Pollut 227(9):1–11
Pourang N, Noori A (2014) Heavy metals contamination in soil, surface water and groundwater of an agricultural area adjacent to Tehran oil refinery Iran. Int J Environ Res 8(4):871–886
Ryu HW, Song MY, Park JS, Kim JM, Jung S-C, Song J et al (2019) Removal of toluene using ozone at room temperature over mesoporous Mn/Al2O3 catalysts. Environ Res 172:649–657
Salehi H, Ebrahimi AA, Ehrampoush MH, Salmani MH, Fard RF, Jalili M et al (2020) Integration of photo-oxidation based on UV/Persulfate and adsorption processes for arsenic removal from aqueous solutions. Groundw Sustain Dev 10:100338
Salmani MH, Abedi M, Mozaffari SA, Mahvi AH, Sheibani A, Jalili M (2021) Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract. J Environ Health Sci Eng 19(1):603–612
Seifan M, Sarabadani Z, Berenjian A (2020) Microbially induced calcium carbonate precipitation to design a new type of bio self-healing dental composite. Appl Microbiol Biotechnol 104(5):2029–2037
Setareh P, Khezri SM, Hossaini H, Pirsaheb MJ (2020) Coupling effect of ozone/ultrasound with coagulation for improving NOM and turbidity removal from surface water. J Water Process Eng 37:101340
Sibiya SM. 2020 An investigation into the effect of inline coagulation and air scouring to minimize ultrafiltration membrane fouling: Stellenbosch: Stellenbosch University
Smoczyński L, Ratnaweera H, Kosobucka M, Smoczyński M (2014) Image analysis of sludge aggregates. Sep Purif Technol 122:412–420
Smoczynski L, Kalinowski S, Cretescu I, Smoczynski M, Ratnaweera H, Trifescu M et al (2019) Study of sludge particles formed during coagulation of synthetic and municipal wastewater for increasing the sludge dewatering efficiency. Water 11(1):101
Solís RR, Chávez AM, Monago-Maraña O, de la Peña AM, Beltrán FJJ (2021) Photo-assisted ozonation of cefuroxime with solar radiation in a CPC pilot plant Kinetic parameters determination. Sep Purif Technol 266:118514
Sun Y, Zhou S, Pan S-Y, Zhu S, Yu Y, Zheng H (2020) Performance evaluation and optimization of flocculation process for removing heavy metal. Chem Eng J 385:123911
Wu C, Chen W, Gu Z, Li QJ (2020) A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci Total Environ 762:143131
Xu J-C, Chen G, Huang X-F, Li G-M, Liu J, Yang N et al (2009) Iron and manganese removal by using manganese ore constructed wetlands in the reclamation of steel wastewater. J Hazard Mater 169(1–3):309–317
Yang Y, Liu Z, Demeestere K, Van Hulle SJ (2021) Ozonation in view of micropollutant removal from biologically treated landfill leachate: removal efficiency OH exposure and surrogate-based monitoring. Chem Eng J 410:128413
Zhang Q, Liu B, Liu Y, Zha X (2018) Optimization of Coagulation and Ozonation Processes for Disinfection by–Products Formation Potential Reduction. J Water Chem Technol 40(4):246–252
Zheng L, Jiao Y, Zhong H, Zhang C, Wang J, Wei Y (2020) Insight into the magnetic lime coagulation-membrane distillation process for desulfurization wastewater treatment: From pollutant removal feature to membrane fouling. J Hazard Mater 391:122202
Acknowledgements
The present article is the result of a research project on the relationship between industry and School of Health, Environmental Science and Technology Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, No. 8021. Finally, the authors of this article are very grateful for the cooperation of the Iran's Central Iron Ore Company.
Funding
The authors received no specific funding for this work
Author information
Authors and Affiliations
Contributions
MJ and FA collaborated in data collection, effluent testing, and statistical analysis. AD, HFB, and AAE collaborated in writing the initial draft of the article, leading the experiments, and preparing the materials and prototype of the effluent. Also, all authors have read and approved the final file of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jalili, M., Abbasi, F., Dalvand, A. et al. The ozonation effect on flocculation and polymer consumption reduction in the hybrid treatment of Iran Central iron ore companies' effluent: a cost–benefit study. Appl Water Sci 13, 51 (2023). https://doi.org/10.1007/s13201-022-01853-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01853-7