Abstract
Lake Bunyonyi is one of the major resources of social-economic potential in the districts of Rubanda and Kabale, South-Western Uganda. The lake’s sub-catchment faces environmental problems because of intensive agriculture, settlement, business and tourism activities, which consequently cause pollution of water in the lake’s system. This study, therefore, intended to determine the processes that govern nitrogen dynamism using a numerical model that takes into account various processes in the system using STELLA® 8.1.1 software. From the model simulation, it was found that mineralization, microbial uptake and nitrification were the major processes governing nitrogen transformation in the water phase, accounting for 47.8% (0.49 g/d m−2), 44.2% (0.45 g/d m−2), and 7.8% (0.05 g/d m−2), respectively. The developed model predicted reasonably well the behaviour of the lake evidenced by the validation results of observed and simulated data that showed good linear regression coefficients (R2) of organic nitrogen (0.48), ammonia–nitrogen (0.68), and nitrate–nitrogen (0.61). The model has proven suitable for application on lakes with characteristics similar to that of Lake Bunyonyi. The study recommended that a compressive investigation that puts into consideration all the possible sources of nutrient and water inflow into the lake system be done on Lake Bunyonyi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide anthropogenic activities are responsible for the excessive nutrient inflow into lake systems. The excessive nutrient loading into freshwater ecosystems causes eutrophication which is linked to oxygen depletion, development of harmful algal blooms, shifts in phytoplankton populations, and reduced water quality (Brooks et al. 2016). Ongore et al. (2013) reported that nutrients in combination with heavy metals from various anthropogenic activities like mining and industries caused the aquatic ecological degradation of the Lake Victoria catchment. In South-Western Uganda, Lake Bunyonyi is very important for the livelihoods of people in Kabale and Rubanda Districts. The wetland around the lake provides materials for thatching houses and handicraft making. In addition, the lake is the major source of water for domestic use, used for small-scale fish farming, and is a popular tourism destination site.
Nevertheless, the Lake is threatened by pollution from hotels, campsites and agricultural activities. Besides, population growth has caused degradation and destruction of hill slopes and wetlands, and nutrients are carried directly into the lake (Van Dam et al. 2007). To formulate deserving sustainable management strategies, a good understanding of nutrient transformation using a dynamic ecological model is considered necessary. Dynamic ecological models developed and applied have been demonstrated to be powerful tools for simulation of nutrient transformation and removal from lakes and wetland ecosystems (Van Dam et al. 2007; Tang et al. 2016; Biswas et al. 2018; Mayo et al. 2018; Radbourne et al. 2019). Nevertheless, no significant efforts have been made to model the processes that govern nutrient transformation and removal processes in small freshwater lakes like Lake Bunyonyi which suffer pollution from diffuse sources. This study, therefore, modelled the processes governing nitrogen transformation using a dynamic simulation model. Ultimately, the findings of the study will be used as the basis for the establishment of the best management strategies for Lake Bunyonyi while maintaining its ecological quality.
Materials and methods
Sample collection and examination
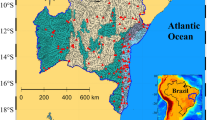
Water samples were collected for six months in both rainy (March–May 2020) and the dry season (June–August 2020). Samples from the inflow and the outflow rivers were collected from Rivers Kagoma and Heissesero (Fig. 1), respectively. Samples were collected into 1L plastic sampling bottles between 9:00 and 11:00 am prepared and then transported in an icebox with ice to the National Water and Sewerage Corporation (NWSC) Central Laboratories in Kampala for analysis within 24 h. While in the laboratory, samples were stored in the refrigerator at 4 °C before analysis. Water temperature, DO levels and pH were measured on-site during sampling. DO and water temperature were measured using the DO meter (DO 5510 M.R.C model). The pH was measured using a water-resistant hand-held pH meter (HI8314 HANNA instruments) following APHA (2017) standards. In the laboratory, samples were analysed for the determination of NH3-N, NO3-N and Org-N following the APHA (2017) standard procedures as described by Saturday et al. (2021). Besides, climatic secondary data such as air temperatures, sunshine, relative humidity, rainfall for a period of six months (March–May 2020) and June–August 2020 were collected from Uganda National Meteorological Authority, Kabale centre. The water inflow into the lake through the Kagoma River and outflow through the Heissesero River were measured onsite using the float-area method as described by Dahal and Dorji (2019).
Model development
Conceptual model of nutrient balance
A conceptual schematic model that encompasses the forcing variables, state variables and the activities governing nitrogen transformation processes is used (Fig. 2). The forcing variables considered in this model are the volume of water in the lake (Q), water temperature (T), pH, volume of water inflow (in) and outflow (out), and DO. The state variables are the major forms of nitrogen; Organic Nitrogen (Org-N), Ammonia Nitrogen (NH3-N) and Nitrate Nitrogen (NO3-N) as indicated by boxes connected by respective processes. The nitrogen transformation considered includes nitrification, denitrification, volatilization, mineralization, microbes’ ammonia and nitrates uptake, algae uptake, sedimentation and decaying processes. A complete materials balance includes lake inflow and outflow, nutrients contained in the inflow and outflow of the lake, precipitation, evaporation, solar radiations, and air temperature; all of which influence nitrogen dynamic processes in the lake system are considered in the model. The nutrient mass balance around state variables was done based on the simplified Eq. (1) (Jørgensen and Bendoricchio 2001).
Here, Input = Nutrient load that enter the lake from diverse sources and through different ways; Output = Nutrient concentration that leaves the lake through different ways; Reaction = the way nutrients leave the lake system by chemical transformation into other substances.
As illustrated in the conceptual model diagram (Fig. 2), nitrogen enters the Lake Bunyonyi system majorly by inflow stream and runoff from agricultural land use activities. Besides nutrient inflow via stream discharges and agricultural runoff, nutrients enter into the lake system through direct rainfall on the lake. While in the lake, nutrients can be taken up by aquatic plants, animals and microbes but are released back into the lake system through excretion and decomposition after death. This precisely means that nutrients are temporarily stored in the biomass. It is generally expected that nutrient fixation is larger than release because organic matter cannot all be decomposed and therefore some nutrients remain fixed in organic matter. Besides, fish catches and water abstraction remove nutrients from the lake and some nutrients are adsorbed to the sediment and may be released when the sediments are suspended by waves.
The following assumptions were considered for the development of the Model.
-
i.
The inlet of water to the lake considered in the model is Kagoma River, while the other non-sources like runoff and precipitation are not included in the Model.
-
ii.
The outlet which has been considered in this model is the Heissesero River; other water loss ways like underground seepage and evaporation are not included in the model.
-
iii.
Similar microorganisms within the lake take up both NO3-N and NH3-N.
-
iv.
The rivers Kagoma (inlet) and Heissesero (outlet) are not seasonal; they continue to bring water into the lake and drain water from the lake throughout the year, respectively.
Based on these assumptions, the following equations for the mass balance of state variables and nitrogen transformation processes were derived.
Ammonia–nitrogen
Based on the mass balance, NH3-N was added in the lake through Kagoma River, and within the lake by mineralization, regeneration from the sediments, while, volatilization, nitrification, uptake by plants and microorganism processes were reducing NH3-N concentration within the lake and some were drained from the lake by Heissesero River as shown in Eq. (2).
where, \(\frac{{\partial Q_{IF} }}{\partial t}\). = Water inflow rate (m3/d), \(\frac{{\partial Q_{OF} }}{\partial t}\) = Water outflow rate (m3/d), \(\frac{{\partial \left[ {{\text{NH}}_{3} - {\text{N}}_{{{\text{in}}}} } \right]}}{\partial A}\) = Ammonia–nitrogen loading in water inflow (g/d m−2), \(r_{m}\) = Rate of mineralization of organic nitrogen (g/d m−2), rreg = Rate of regeneration (g/d m−2), \(\frac{{\partial \left[ {{\text{NH}}_{3} - {\text{N}}_{{{\text{out}}}} } \right]}}{\partial A}\). = Ammonia–nitrogen loading in water outflow (g/d m−2), \(r_{g1}\). = Ammonia uptake by microorganisms, \(r_{v}\) = Rate of volatilization (g/d m−2), \(r_{u1 }\). = Uptake by the plant (g/d m−2), rn = Rate of nitrification of ammonia (g/d m−2).
Nitrate–nitrogen
Based on the mass balance, NO3-N was added to the lake through the Kagoma River. The nitrification, denitrification, and nitrate uptake by microorganisms and algae were the major processes reducing NO3-N concentration within the lake and some were dined through Heissesero River as shown in Eq. (3).
\(\frac{{\partial Q_{{{\text{in}}}} }}{\partial t}\). = Water inflow rate (m3/d), \(\frac{{\partial Q_{{{\text{out}}}} }}{\partial t}\) = Water outflow (m3/sec), \(\frac{{\partial \left[ {{\text{NO}}_{3} - {\text{N}}_{IF} } \right]}}{\partial A} + r_{n}\). = Nitrate-nitrogen inflow (g/d m−2), rg2 = Rate of nitrate uptake by microorganisms, \(\frac{{\partial \left[ {{\text{NO}}_{3} - {\text{N}}_{OF} } \right]}}{\partial A}\) = Nitrate–nitrogen outflow (g/d m−2), \(r_{n}\) = Rate of nitrification (g/d m−2), \(r_{dn}\) = Rate of denitrification (g/d m−2).
Organic-nitrogen
Like NH3-N and NO3-N, Org-N was added into the lake through Kagoma River and within it by decomposition. On the other hand, mineralization, nitrification, denitrification, and nitrate uptake by microorganisms and algae were responsible for Org-N reduction within the lake and some were drained off it via Heissesero River as shown in Eq. (4).
where, \(\frac{{\partial Q_{{{\text{in}}}} }}{\partial t}\) = Water inflow rate (m3/d), \(\frac{{\partial Q_{{{\text{out}}}} }}{\partial t}\) = Water outflow (m3/d), \(\frac{{\partial \left[ {{\text{Org}} - N_{{{\text{in}}}} } \right]}}{\partial A}\) = Organic-nitrogen inflow (g/d m−2), \(\frac{{\partial \left[ {{\text{Org}} - N_{{{\text{out}}}} } \right]}}{\partial A}\) = Organic-nitrogen outflow (g/d m−2), \(r_{g1}\) = Rate of ammonia uptake by microorganisms, \(r_{g2}\) = Rate of nitrate uptake by microorganisms (g/d m−2), \(r_{m}\) = Rate of mineralization (g/m2/day), \(r_{s}\) = Rate of sedimentation (g/d m−2).
Equations for nutrient dynamic processes
Mineralization
The mass balance for mineralization of organic nitrogen in the lake system was modelled using first-order kinetics as presented in Eq. (5).
where, K = Mineralization rate of constant nitrogen (day−1).
Nitrification
According to Fritz et al. (1979), the nitrification process model is based on the assumption that nitrite formation by Nitrosomonas is a rate-limiting step and is inhibited by dissolved oxygen, temperature, and pH. The nitrification process was modelled using Eq. (6). The temperature and pH influence on Nitrosomonas bacteria were explained by the empirical relationship presented by Eqs. (7) and (8), respectively.
where, \(\mu_{n}\) = maximum Nitrosomonas growth rate (d−1), \(Y_{n}\) = Yield coefficient for Nitrosomonas bacteria (mg VSS/mg N), \(K_{1}\) = Ammonia Nitrosomonas half-saturation constant (g/d m−2), \(K_{2}\) = Oxygen Nitrosomonas half-saturation constant (g/d m.−2), \(C_{T }\) = Temperature-dependent factor, \(C_{{{\text{pH}}}}\) = Nitrosomonas growth-limiting factor for pH
where, \(C_{T}\) = Temperature dependence factor, \(T\) = Temperature in °C, \(T_{O}\) = Reference temperature (°C) = 15 °C
Denitrification
To model the rate of denitrification, a combination of denitrifying bacterial activities is applied. The model expression is presented by Eq. (9).
where, \(DR_{ - 20}\) = Denitrification rate constant at 20 °C (d−1), \(\theta\) = Arrhenius constant microorganism growth temperature coefficient.
Ammonia uptake by microorganisms
In the model, it is assumed that ammonium uptake, i.e., MO (NH3-N) would take place as long as is available in the lake since autotrophic bacteria prefer it to nitrates. Equation 10 is used to model NH3-N uptake by microorganisms in the water phase.
where, \(\mu_{{{\text{max}} - 20 }}\) = Maximum growth rate of bacteria at 20 °C (d−1), \(P_{1}\) = Ammonia uptake preference factor, \(K_{3}\) = Ammonia uptake half-saturation constant (g/m3), \(Thita\) = Microorganisms growth temperature coefficient.
Nitrate uptake rate by microorganisms
The nitrate uptake is done by autotrophic bacteria but it is assumed that it takes place after all the NH3-N has been consumed and hence depleted from the system. Equation (11) is used to model the rate of nitrate uptake by microorganisms.
\(\mu_{{{\text{max}} - 20 }}\) = Maximum growth rate of bacteria at 20 °C (d−1), \(P_{1}\) = Nitrate uptake preference factor, \(K_{4}\) = Nitrate uptake half-saturation constant (g/m3), \(Thita\) = Microorganisms growth temperature coefficient.
Sedimentation rate
The transformation processes considered in the modelling of nitrogen in sediments in the lake system are sedimentation and ammonia–nitrogen regeneration. The organic nitrogen in sediments is mineralized by microorganisms, which ultimately regenerate NH3-N to the water column. It is assumed that the rate of regeneration (rr) follows the first-order kinetics presented by Eqs. (12) and (13).
where, \(r_{s} =\) Settling rate of organic-nitrogen to the bed (g/d m−2), \(r_{r}\) = rate of ammonia regeneration (g/d m.−2)
where, \(r_{reg }\). = Regeneration rate constant of ammonia (g/d m−2).
Ammonia volatilization
To model the rate of ammonia volatilization, the mass balance Eq. (14) is used.
where, h = Water depth in m, T = Temperature in °C, KL = Mass transfer coefficient = 0.056 exp (0.13 (T−20).
Model calibration
To run this model, the STELLA software (version 9.0.1) was used. The software has the capabilities to simulate the behaviour of nitrogen transformation in the Lake Bunyonyi system using conservation of mass principles. The mathematical equations presented in subsection 7.5.1 for the mass balance of ammonia–nitrogen, nitrate-nitrogen and organic-nitrogen along with forcing functions were entered in the STELLA software. Data collected from samples obtained from Lake Bunyonyi were used as inputs for the model calibration. These data include monthly averages for NH3-N, NO3-N, Org-N, DO, temperature, and pH (of the le system, inflows and outflows), rainfall, solar radiation, water inflows and outflows.
Nutrient mass balance
Total nutrient inflow was computed as the sum of the total volume of water that flows into the lake system multiplied by the concentration of nutrients in water inflows (Eq. 15). Total nutrient outflow was computed as a sum of the total volume of water outflow multiplied by the concentration of nutrients in the water outflows (Eq. 16).
To obtain the best values of coefficients, model calibration was performed. The model efficiency criterion by Nash and Sutcliffe (1970) was used to establish the efficiency of the model.
Results
Water inflow and outflow rate
The quantity of water inflow (Q_in) recorded varied from 20,044.80 to 73,612.80 m3/d with a mean value of 39,887.90 ± 22,959.58 m3/d (Fig. 3). The volume of water outflow (Q_out) from the lake ranged from 58,406.40 to 139,276.80 m3/d, obtained in July and March 2020 with the mean value of 103,867.20 ± 34,367.59 m3/d. These values of recorded water inflow and outflow volumes were used as input variables in modelling processes that govern nutrient dynamics in the lake system.
Characteristics of physico-chemical parameters
To fully understand the processes governing nitrogen dynamics in Lake Bunyonyi, the physico-chemical parameters water temperature, pH, DO, NO3-N, NH3-N and Org-N were measured both at the inflow and outflow waters. Thereafter, the recorded values were used as inputs in the model. In the inflow waters (Table 1), water temperature varied from 20 to 22.40 °C with a mean value of 20.75 ± 0.94 °C; DO level between 7.10 and 9.70 mg/l/d with a mean value of 7.87 ± 0.93 mg/l/d, and pH values ranged 7.20 and 8.70 with a mean value of 7.90 ± 0.52 was recorded (Table 1). Similarly, nutrients ranged between 0.01 and 0.07 mg/l/d with a mean value of 0.05 ± 0.02 mg/L/d for NH3-N; NO3-N values ranged between 0.00 and 0.02 with a mean value of 0.01 ± 0.01 mg/l/d, while Org-N ranged from 0.10 to 2.80 with a mean value of 1.69 ± 1.16 mg/l/d (Table 1).
In the outflow waters, the mean value for water temperature was 20.74 ± 0.41 °C, DO ranged between 5.90 and 8.50 mg/l/d with a mean value of 7.39 ± 0.99 mg/l; pH values ranged between 7.00 and 8.20 with a mean value of 7.53 ± 0.43 (Table 2). Similarly, values for Org-N ranged from 0.00 to 1.40 mg/l/d with a mean value of 0.38 ± 0.51 mg/l/d, while NH3-N and NO3-N recorded mean values of 0.07 ± 0.03 mg/l/d and 0.02 ± 0.01 mg/l/d, respectively (Table 2).
Modelling process
Model calibration and validation
Results of the calibration are shown in Table 3 together with some literature values for comparison. These calibrated parameters were used for modelling processes that govern nitrogen transformation and removal from the Lake Bunyonyi system. Figures 4, 5 and 6 depict graphs of simulated and observed NH3-N, Org-N and NO3-N, respectively, against time after running a calibrated model with a new data set and the forcing functions to reflect new conditions and to observe how well the model simulations fit the new data set. The simulation results were more or less related to the field data for all nitrogen species. The field data for NH3-N tend to peak in the second month (April 2020) of data collection, while the model shows a gradual increase which peak in the third month (May 2020) of data collection. Both simulated and field data for NO3-N slowdown in the second (April 2020) and third month (May 2020) of data collection and later gradually increased in the fifth month (July 2020) (Fig. 5). Both the simulated and measured Org-N data showed a gradual decline during months of data collection.
The efficiency of the model is demonstrated by Figs. 7, 8 and 9 for the observed and simulated NH3-N, Org-N and NO3-N, respectively. The model output showed a good agreement with observed values. The observed R squared values for NH3-N, Org-N and NO3-N were 0.62, 0.63 and 0.77, respectively, implying a good agreement between observed and simulated data.
Nutrient mass balance
Figure 10 depicts the flow of nutrients from one state variable to another. Organic nitrogen whose inflow value was 0.466 g/d m−2 in the water had 0.000063 g/d m−2 transported into the sediment phase. Nevertheless, out of this amount, 0.0002 g/d m−2 was returned to NH3-N into the water phase through NH3-N regeneration leaving 0.00062 g/d m−2, as the actual Org-N which was removed from the water phases through the sedimentation process. Likewise, algae take up 0.0003677 g/d m−2 of nutrients in form of NO3-N and NH3-N for their cellular growth of which 0.00032 g/d m−2 goes to the sediment through decomposition, leaving 8.85 × 10 −9 g/d m−2as the actual nitrogen contained in algae cells.
In addition, Org-N can as well be converted to NH3-N through mineralization amounting to 0.49 g/d m−2. Nevertheless, nitrification transforms 0.0455 g/d m−2 NH3-N to NO3-N and the denitrification process which removes nitrogen from the lake system transforms 0.0795 g/d m−2 of the total concentration value. The NO3-N and NH3-N inflow into the lake system was 0.00318 g/d m−2 and 0.00012 g/d m−2, respectively. Out of the total NO3-N and NH3-N in the water phase, 0.453 g/d m−2 is taken up by microorganisms which subsequently transforms back to Org-N, leaving 0.44 g/d m−2 and 0.008 g/d m−2 as the actual NH3-N and NO3-N concentrations in the lake, respectively (Fig. 10). Based on results, it is evident that mineralization (45.8%), microbial uptake (42.4%), and denitrification (7.4%) were the three main processes responsible for nutrient transformation in the lake (Fig. 11). Other processes such as volatilization, sedimentation, nitrification and uptake by algae were responsible for 0.2% nutrient dynamism in the lake system (Fig. 11). Nutrients from the lake through outflow waters plus reactions account for 18.2% implying that nutrient accumulation accounts for 81.8%.
Model application
The model developed is suitable for freshwater lakes. Its application requires the knowledge of the inflow and outflow concentrations of Org-N, NH3-N and NO3-N; the physical parameters: pH, temperature and DO level; the depth of lake; the quantity of inflow and outflow waters. Upon establishment of all the desired information, all the forcing functions, equations, parameters and constants values are entered into the model via the conceptual model (Fig. 2). In STELLA software, the selection of estimation for integration of the differential equations is provided using Runge–Kutta fourth-order Equation because of its low truncation error and fast convergence on given initial values. With the menu in the STELLA software, one can choose any state variable or process to be simulated.
Discussion
Based on the outputs of model calibration, the results revealed a good agreement between observed and simulated data. Related results have previously been observed by Mayo et al. (2018) in wetland systems who reported model coefficients (R2) of 0.54, 0.51 and 0.53 for NO3-N, Org-N and NH3-N, respectively. Besides, it can be observed that the model efficiency of the current study was slightly lower than that of Senzia (2003) whose regression value (R2) for NO3-N was 0.82. These observed slight differences in the model efficiencies can be attributed to differences in the ecosystem setup. Besides, the model developed for the constructed wetland, where the flow was controlled while the current model developed is for uncontrolled environment.
The prominence of mineralization and microbial uptake as the main processes responsible for nutrient transformation in the lake is attributed to somewhat high DO, temperatures and pH that favour the said processes in the lake system. Volatilization was responsible for minimal nitrogen transformation and removal from the lake system perhaps due to average pH of less than 9 which does not encourage ammonia stripping.
The volume of water outflow was 61.6% higher than the water inflow implying that the volume of water in Lake Bunyonyi was not a true reflection of water inflows via rivers and streams. Precipitation and groundwater inflow are presumably the major sources of water in Lake Bunyonyi. The observed temperatures were within ideal ranges for the biological nitrogen transformation processes such as mineralization, denitrification, nitrification and volatilization. Similar temperature range values were obtained by Mayo and Hanai (2014) in a study conducted at the Pilot High Rate Pond in Dar es Salaam, Tanzania. Temperature influences the DO content of water, the rate of photosynthesis by aquatic plants and the metabolic rates of aquatic organisms (Bhateria and Jain 2016). The recorded average pH values are conducive for most biological nitrogen transformation processes including volatilization which requires values not greater than 8.0 (Dari et al. 2019). DO in the inflow and outflow waters of the lake that were greater than 7 mg/l are largely attributed to algal photosynthesis which produces oxygen. Besides, DO levels increased by 6.1% from the concentration level obtained from the inflow waters possibly due to photosynthesis taking place in the lake system. Besides, the level of DO contained in the outflow waters suggests sufficient aeration of the lake to support the biological oxidation of both organic and inorganic pollutants in the lake system.
The NH3-N and NO3-N decreased by 25.8% and 50%, respectively, from the inlet to the outlet of the lake system, which indicates high NH3-N and NO3-N in nutrient retention, in the lake. The reduction was attributed to uptake by microorganisms (algae and bacteria) and nitrification processes. The process of volatilization was unlikely since it requires pH above 9.4 (Reddy and Graetz 1988; Mayo and Hanai 2014; Dari et al. 2019). NH3-N uptake by microorganisms was found to be one of the major pathways for NH3-N reduction because it is a preferred source of nitrogen than nitrate (Neel et al. 1961; Fritz et al. 1979; Mayo and Hanai 2014).
Besides, Org-N concentration decreased by 0.9% from the inflow to the outflow of the system. This is equivalent to an accumulation rate of 99.1% of Org-N concentration from the inflow value of 0.466 to 0.00429 g/d m−2. On other hand, low retention of Org-N was reported by Mwanuzi et al. (2003) due to the high export of Org-N from the wetlands fringing Lake Victoria. The study results further indicate that volatilization and nitrification processes were responsible for minimal nitrogen removal from the lake system. While the model emphasizes the importance of Org-N in the lake system, its accumulation lowers dissolved oxygen levels and affects the rate of nitrification. Possibly, this explains the observed low rates of nitrification and volatilization.
Conclusion
Based on the study results, we conclude that mineralization, microbial uptake, and denitrification were found to be the major processes that govern nitrogen dynamics in the lake system. Mineralization transforms 0.49 g/d m−2 to NH3-N, while nitrification transforms 0.05 g/d m−2 to NO3-N, and microbial uptake is responsible for the removal of 0.45 g/d m−2 (NH3-N and NO3-N) from the water phase. Besides, denitrification can transform 0.08 g/d m−2of nutrients in the water phase. The developed model shows sensitive parameters that influence the output are Org-N levels, microbial uptake, mineralization rate and maximum growth rate at 20 °C. In addition, ammonia Nitrosomonas half-saturation constant, oxygen Nitrosomonas half-saturation constant, and ammonia Nitrosomonas half-saturation constant influence the model output. Much attention should be paid to the mentioned parameters during modelling to get results that reflect the reality in a lake system. Besides, the developed model will be useful for the prediction of nutrient dynamisms in lakes that share similar characteristics with Lake Bunyonyi.
Availability of data and materials
All data generated and analysed during this study are included in this published article.
Abbreviations
- APHA:
-
American Public Health Association
- DO:
-
Dissolved oxygen
- DR_20:
-
Denitrification rate at 20 °C
- Miner. Rate:
-
Organic nitrogen mineralization rate
- NH3 Reg rate:
-
NH3 Regeneration rate
- NWSC:
-
National Water and Sewerage Corporation
- Reg_rate:
-
Ammonia regeneration rate constant
- Sed_ rate:
-
Organic nitrogen sedimentation rate
- Thita:
-
Microorganism growth temperature coefficient
- Umax_20:
-
The maximum growth rate of algae and bacteria at 20o C
- Un:
-
Maximum Nitrosomonas growth rate range
- WHO:
-
World Health Organization
References
APHA (2017) Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association. https://www.standardmethods.org
Bhateria R, Jain D (2016) Water quality assessment of lake water: a review. Sustain Water Resour Manag 2(2):161–173
Biswas JK, Bera B, Chanda R, Sarkar SK, Majumdar J, Majumder S, Hazra S (2018) Nutrient modelling of an urban lake using the best subset method. Int J Environ Sci Technol 15(9):1867–1878
Brooks BW, Lazorchak JM, Howard MD, Johnson MV, Morton SL, Perkins DA, Reavie ED, Scott GI, Smith SA, Steevens JA (2016) Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ Toxicol Chem 35(1):6–13
Charley RC, Hooper DG, McLee AG (1980) Nitrification kinetics in activated sludge at various temperatures and dissolved oxygen concentrations. Water Res 14(10):1387–1396
Dahal D, Dorji U (2019) Comparative analysis of water discharge measurement methods and seasonal trend analysis of Taebayrongchu sub-watershed, Punakha. Bhutan J Res Dev 8(2)
Dari B, Rogers CW, Walsh OS (2019) Understanding factors controlling ammonia volatilization from fertilizer nitrogen applications. BUL
Ferrara RA, Harleman DR (1980) Dynamic nutrient cycle for waste stabilization ponds. J Environ Eng Div 106(1):37–54
Fritz JJ, Middleton AC, Meredith DD (1979) Dynamic process modelling of wastewater stabilization ponds. J Water Pollut Control Federation, 2724–2743
Henze M (1991) Capabilities of biological nitrogen removal processes from wastewater. Water Sci Technol 23(4–6):669–679
Jørgensen SE, Bendoricchio G (2001) Fundamentals of ecological modelling (vol 21). Elsevier
Mayo AW, Bigambo T (2005) Nitrogen transformation in horizontal subsurface flow constructed wetlands I: Model development. Phys Chem Earth Parts a/b/c 30(11–16):658–667
Mayo AW, Hanai EE (2014) Dynamics of nitrogen transformation and removal in a pilot high rate pond. J Water Resour Prot 6(05):433
Mayo AW, Muraza M, Norbert J (2018) Modelling nitrogen transformation and removal in mara river basin wetlands upstream of lake Victoria. Phys Chem Earth Parts a/b/c 105:136–146
Metcalf L, Eddy HP, Tchobanoglous G (1991) Wastewater engineering: treatment, disposal, and reuse (vol 4). McGraw-Hill, New York
Mwanuzi F, Aalderink H, Mdamo L (2003) Simulation of pollution buffering capacity of wetlands fringing the Lake Victoria. Environ Int 29(1):95–103
Nash JE, Sutcliffe JV (1970) River flow forecasting through conceptual models part I—A discussion of principles. J Hydrol 10(3):282–290
Neel JK, McDermott JH, Monday CA (1961) Experimental lagooning of raw sewage at Fayette, Missouri. J Water Pollut Control Feder, pp 603–641
Ongore CO, Okuku EO, Mwangi SN, Kiteresi LI, Ohowa BO, Wanjeri VO, Okumu S, Kilonzi J (2013) Characterization of nutrients enrichment in the estuaries and related systems in Kenya coast
Radbourne AD, Elliott JA, Maberly SC, Ryves DB, Anderson NJ (2019) The impacts of changing nutrient load and climate on a deep, eutrophic, Monomictic lake. Freshw Biol 64(6):1169–1182
Reddy KR, Graetz DA (1988) Carbon and nitrogen dynamics in wetland soils. In: The ecology and management of wetlands. Springer, pp 307–318
Saturday A, Lyimo TJ, Machiwa J, Pamba S (2021) Spatio-temporal variations in physicochemical water quality parameters of Lake Bunyonyi, Southwestern Uganda. SN Appl Sci 3(7):1–14
Senzia MA (2003) Modeling of nitrogen transformation and removal in horizontal subsurface flow constructed wetlands during treatment of domestic wastewater [PhD Thesis]. University of Dar es Salaam
Tang C, Li Y, Acharya K (2016) Modeling the effects of external nutrient reductions on algal blooms in hyper-eutrophic Lake Taihu, China. Ecol Eng 94:164–173
Van Dam AA, Dardona A, Kelderman P, Kansiime F (2007) A simulation model for nitrogen retention in a papyrus wetland near Lake Victoria, Uganda (East Africa). Wetlands Ecol Manage 15(6):469–480
Acknowledgements
We would like to thank NWSC, Kampala – Uganda for accepting us to use their laboratory facilities. We also wish to thank the communities living around Lake Bunyonyi for their support during the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AS conceived and designed the study, collected and analysed data, and drafted the manuscript. TJL, JM and SP contributed to the conception and design of the study, assisted in data interpretation and revision of the manuscript for intellectual content. All the authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saturday, A., Lyimo, T.J., Machiwa, J. et al. Modelling nitrogen transformation in the Lake Bunyonyi ecosystem, South-Western Uganda. Appl Water Sci 12, 189 (2022). https://doi.org/10.1007/s13201-022-01709-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01709-0