Abstract
Hydrogeochemical and multivariate assessment of groundwater samples of the Churachandpur sub-division of the state of Manipur was carried out to study its suitability for potable and irrigational uses. The study extensively discusses the underlying hydro-geochemistry using multivariate geostatistical tools for physicochemical parameters of 35 spatially distributed groundwater samples during pre- and post-monsoon seasons. In situ parameters (pH, TDS, DO, EC, salinity, ORP) were assessed in field conditions, and it was found that few samples exceed concentration than the prescribed standards. Both positive and negative ORP values and low DO levels of the samples indicate oxidizing and reducing aquifers due to recent unstable geologic formations. Although the concentration of Cl−, SO42−, and NO3− is well within desirable levels, the concentration of F− and As3+ of a few samples exceeds the prescribed standard. The water quality index of the groundwater samples signifies that more than 50% of samples are unsuitable for potable uses for both seasons. The geochemical analysis indicates that most of the water is Ca–Mg–Cl–HCO3 type followed by Mg–Ca–Cl–HCO3 types in both the seasons and dominated by the rock-weathering geochemical process. Multivariate analysis and geostatistical mapping highlight the spatial variability of water quality parameters and possibilities of the occurrence of geogenic contaminants in the aquifers of the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
India is a major groundwater-dependent country, and around 90% of groundwater is extracted for irrigation use (Coyte et al. 2018). The usefulness of groundwater resources is dependent on their availability, accessibility, and quality (Kresic 1997). Accessibility to quality groundwater determines the human health, socioeconomic condition, and overall development of a region and is influenced by several natural and anthropocentric factors (Alam 2019, 2017; Choudhury et al. 2011; Mahanta et al. 2009, 2011). Anthropogenic activities, such as over-exploitation of groundwater resources, excessive use of chemical fertilizer, pesticides, and dumping of solid and industrial waste, can deteriorate the groundwater quality (Todd 2003). Recent studies have revealed geogenic contaminants in the region’s aquifers and their dissolution to groundwater due to hydrogeochemical interactions (Alam et al. 2020a; Mukherjee et al. 2015; Mahanta et al. 2015, 2011; Bhattacharya et al. 2012. The hydrogeochemical interactions are influenced by various geochemical reactions and dissolution processes such as oxidation–reduction reactions, precipitation, sorption, hydrogeological conditions, and climatic factors (McSween et al. 2003; Drever 1997). Thus, assessing basic ions in groundwater can extensively explain the hydrogeochemical interaction in the aquifer formations, dissolution of contaminants, and water types of a region (Prusty et al. 2018; Mukherjee et al. 2012). Similarly, these basic ions can also be used to assess the irrigation water quality that can cause severe impacts on the fertility and productivity of the soil (O’Geen 2015; Fetter 2007). The present study attempts to assess spatiotemporal groundwater quality and geochemical properties of groundwater sources in the region using various water quality indices multivariate and geochemical tools to ensure sustainable groundwater resources for the region’s overall development.
Study area
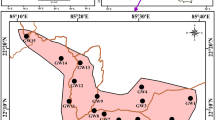
The study was carried out in the Churachandpur sub-division, located in the hilly terrain of the south-western part of the state of Manipur (India) between the coordinates 93° 35′–93° 55′ E and 24° 15′–24° 40′ N (Fig. 1). The region is part of hilly terrain and Khuga river valley, which is a southern extension of Manipur valley with higher population density. Agriculture and rice cultivation is the primary source of livelihood of the people in the region. More than 90% of the total geographical location of the region is covered by the forest and very rich in forest biodiversity with endemic, rare floral species (MSAPCC 2013). The majority of the people in the study area depend on the spring water, dug wells, tube wells as a source of their potable water apart from limited public water supply from rivers and streams (CGWB 2013).
Materials and methods
Assessment of in situ parameters
As per the standard procedures, the groundwater samples were collected from 35 spatially distributed locations, including dug wells, shallow tube wells, and natural springs. Samples were collected in properly rinsed HDPE bottles from different sampling sites after sufficiently flushing the source and preserved with a few drops of 0.2 N HNO3 for further laboratory analysis of ions (APHA 2005). In situ parameters including temperature, pH, EC, DO, Salinity, TDS, ORP were assessed with the help of field testing electrodes and sensors (Eutech, Extech, Hanna). Field testing kit Arsenator (Wagtech) and ion meter (Thermo) were used to determine As3+ and F− concentration, respectively, in the field condition. The geographic coordinates of the sampling locations were recorded using a GPS handset (Garmin, Etrex 30).
Assessment of basic parameters and ions
The preserved samples were further brought to the laboratory and analysed to determine the basic ions and confirm the presence of geogenic contaminants (such as As3+ and F−). The samples were analysed using standards procedures of titrimetric, gravimetric, spectrophotometric, and ion chromatographic (IC) methods as described in standard procedures of APHA (2005).
Water quality index
Based on WHO standards, potability uses of groundwater sources were appraised using water quality indices (WQI) (WHO 2011). WQI is a combined mathematical water quality rating determined based on the overall influences of water quality parameters for specific uses and purposes. WQI was determined by assigning different weights (Wi) to water quality parameters according to their significant impacts on the overall water quality, and relative weights (Wr) are calculated as per the given equation (Ramakrishnaiah et al. 2009):
where i is the number of water quality parameters under consideration for evaluation of WQI. As per the WHO guidelines and standards, the water quality rating scales (Qi) of each parameter are determined using the following equation:
where Ci represents the measured concentration of parameters and Si represents the drinking water quality standards according to WHO (2011). Then, the sub-indices (SIi) of each parameter are evaluated using the following equation:
The cumulative WQI is then calculated using the following summation of sub-indices (SIi)
The calculated WQI is then used for classification for status drinking water as unsuitable (WQI > 300); very poor (200 < WQI < 300); poor (100 < WQI < 200); good (50 < WQI < 100) and excellent (WQI < 50) (Alam et al. 2020b).
Geochemical assessment
Geochemical classification and water type of the groundwater samples can be determined through graphical plots between major cations and anions in the form of a trilinear diagram (Piper 1953). This is a prominent geochemical tool that categorizes the groundwater types or geochemical facies of water samples. Further, Gibb’s plots between cationic and anionic ratios as a function of TDS are widely used to assess and identify the dominant geochemical processes (precipitation, rock, and evaporation dominance) that govern the dissolution of ions in groundwater (Gibbs 1970).
Multivariate analysis
Multivariate statistical analysis of groundwater quality parameters was carried out to simplify and identify complex hydrogeochemical relationships between parameters. The Pearson’s correlation (r) coefficient matrix represents the co-variability/correlation between the water quality parameters. The t-test was applied to observe the seasonal variability between the water quality parameters considering the assumption of normality at the significance level of p-value smaller than 0.05 (p < 0.05). In addition, the principal component analysis (PCA) and hierarchical cluster analysis (CA) were carried out to establish the geochemical interactions and grouping of water quality parameters based on their similar characteristics (Alam et al. 2020b).
Irrigation water quality
The concentration of EC, TDS, and irrigation water quality indices such as sodium absorption ratio (SAR), residual sodium carbonate (RSC), sodium percentage (%Na), Wilcox diagram, USDA classification, and permeability index (PI) is generally used for assessing salinity and alkali hazards of irrigation water. An increase in the percentage of sodium increases the SAR of the soil that represents the alkali hazards. These values can be evaluated using the following equations (concentrations of ionic species in meq/l)
The RSC is the factor that regulates the impacts of carbonate (CO32−) and bicarbonate (HCO3−) of the water on soil. The higher RSC value signifies more scope for sodium absorbability by soil. Mathematically, RSC can be expressed as
Similarly, the permeability index determines soil’s ability to transmit water and air (Doneen 1964), and mathematically, it can be defined as given below:
where the ionic concentrations are expressed in meq/l. The US Salinity Laboratory’s (USSL 1954) diagram is widely used to classify and rate the irrigation water into 16 classes based on plotting between alkali hazards (SAR) and salinity hazards (EC) (Wilcox 1955).
Results and discussion
Statistical summary and descriptive statistics of twenty (20) physicochemical parameters of 34 spatially distributed groundwater samples are presented in Table 1. The table describes the minimum, maximum, average, standard deviation, and coefficient of variation (CV) to show the variability of groundwater physicochemical parameters, including in situ parameters and significant ions such as F− and As3+.
Evaluation of in situ parameters
The majority of water quality parameters for both PM and PoM were found within the acceptable range except few samples that exceed WHO the prescribed standards (Table 1). During the PoM season, some samples show acidic characteristics due to the infiltration of overland water from heavy precipitation. TDS values in both seasons show exceeding amounts with an average of 300.3 ppm and 272.9 ppm during PM and PoM, respectively. High EC concentration in both the seasons and their strong correlation with TDS (r > 0.97 at p < 0.05) signifies the dissolution of inorganic lithophilic ions from the aquifer matrix of the study area. This is further ascertained by the high degree of a strong correlation between EC, TDS, and salinity concentrations in both seasons (r > 0.95 at p < 0.05). The groundwater quality parameters also exhibit significant spatiotemporal variability in concentrations in both seasons, indicating the seasonal influence of subsurface runoff. Low DO levels and negative ORP values of the samples indicate reducing aquifer conditions or may be due to microbial activity and shallowness of the aquifer (CGWB 2013). The ORP values, which range from − 94 to 215 mV and − 68 to 211 mV during PM and PoM, respectively, justify the existence of both oxidizing ad reducing aquifers in the region. The 70.7% and 66.7% samples show hard to very hard category hardness due to the presence of both carbonate and non-carbonate hardness (Table 2). Arsenic (As3+) and fluoride (F−) in a few groundwater samples indicate that the aquifers in the study area are not free from arsenic and fluoride-bearing aquifers. The geostatistically interpolated map (Fig. 2) shows the possible spatial distribution of fluoride in the aquifer of the study area for both PM and PoM. The interpolated maps indicate the presence of potential Fluoride bearing aquifers in the central and southern portion of the study area during both seasons.
Water quality index (WQI)
The WQI values evaluated for the groundwater samples of the study area indicate that most of the samples fall in the category of poor to unsuitable during both PM (64.7%) and PoM (52.9%) seasons (Table 3). It was observed that WQI is mainly influenced by the presence of geogenic contaminants like Fluoride and other water quality parameters like EC, TDS, TH. The geostatistical interpolation maps for WQI (Fig. 3) for the study area indicate poor WQI in the central portion of Churachandpur town mainly due to rapid urbanization, overexploitation of groundwater, and other anthropogenic influences. Poor and unsuitable WQI indicates possibilities of public health threats to the region if the population is more inclined to use groundwater resources in the future.
Geochemical analysis
The normalized inorganic charge balance (NICB) (ƩZ+-ƩZ−)/ƩZ+ + ƩZ−), i.e. the extent of deviation between the total cations (Ca2+, Mg2+, Na+, K+) and anions (SO42−, NO3−, Cl−, HCO3−) expressed in meq/l for each set of samples, was assessed and found in the accepted range of ± 10% (Domenico and Schwartz 1990). The Piper (1953) trilinear diagrams and Durov’s diagram were used to evaluate the geochemical facies of groundwater considering significant cations and anions (Figs. 4 and 5). These graphical plots represent the major water types of the study area that are distinctly categorized as Ca–Mg–Cl–HCO3 type followed by Mg–Ca–Cl–HCO3 type water where concentrations of F− were found to be higher due to co-precipitation process of F− with Mg-rich calcite resulting in increased concentration of Mg2+. Some samples with geochemical facies of Ca–Mg–Cl–SO42− types of water represent younger alluvial sediments of fluvio-lacustrine origin (Fig. 5). The Gibbs diagram (Fig. 6) indicates the chemical weathering of rock-forming minerals as a significant geochemical process that governs the dissolution of ions in groundwater.
Multivariate analysis
Correlation coefficients (r)
Pearson’s correlation coefficients (r) between the water qualities parameters and WQI are presented in Table 4. The high degree of significant correlation between various parameters such as EC, TDS, and Salinity shows very high co-variability (r at p < 0.05) within them, indicating typical inorganic ion load in the groundwater samples during both seasons. Similarly, Salinity, TDS, Na+, Ca2+, and Cl− show a high degree of correlation, indicating the probable presence of halite-aquifer and Na and Ca-hardness of water. A strong and moderate correlation between TH, Cl− and salinity suggests the presence of non-carbonate hardness in the groundwater of the study area. The WQI exhibits moderate correlation with F− indicative of the higher concentration of F− influencing potability of the groundwater samples. The positive correlation of WQI with EC, salinity, TDS, Cl−, TH, Ca2+, Mg2+ during both seasons signifies that these parameters deteriorate the drinking water quality.
Principal component analysis (PCA)
The three principal components (PCs) for both PM and PoM samples are presented in Table 5 (with eigenvalue > 1), which accounts for 61.24% (PM) and 62.4% (PoM) of variances in the hydrogeochemical variables and interactions. During pre-monsoon PC-1 (37.4%), PC-2 (50.5%), PC-3 (61.24%) account for variances within the samples. Similarly, during post-monsoon season PC-1 (35.4%), PC-2 (53.4%), PC-3 (62.4%) account for variances within the variables. It is fairly distinct that during both PM and PoM seasons, about 35.7% of variances of the data are highly correlated with EC, salinity, TDS, Ca2+, Na+, TH, and Cl−. Similarly, PC-2 indicates 24.6% of variances in the variables are correlated with pH, DO, and F− during PM and F−, Cl−, during PoM. These variations can be attributed to heavy precipitation and the shallow aquifer of the region (CGWB 2013).
Cluster analysis (CA)
The water quality parameter that determines the hydrogeochemical characteristics of the study area was analysed using hierarchical cluster analysis (CA). The CA indicates three major water quality parameters that determine the WQI and hydrogeochemical characteristics of the groundwater samples, i.e. groups I, II, and III (Fig. 7 and Table 6). The group exhibits considerable spatial variability of water quality parameters due to the inconsistency and variability of the aquifers formations. A higher concentration of Ca2+, EC, TDS, ORP, and TH indicates the presence of inorganic ions and non-carbonates hardness of water due to the possibility of dissolution from halite-dominated aquifers. Groups II and III represent more non-carbonate types of hardness as HCO3− concentrations are comparatively lesser. Moreover, the WQI of the samples indicates the influence of higher concentrations of EC, TDS, TH and the presence of alkaline earth metals ions.
Irrigational water quality
Based on the concentration of EC, TDS, sodium percentage (%Na), and irrigational water quality indices (Table 7), the suitability of groundwater was assessed for agricultural use. Although the concentration of EC and salinity exhibits higher values, all the groundwater samples were found within permissible range to the excellent irrigational water quality category. The TDS concentration of the samples was found in the freshwater range (< 1000 mg/l). Similarly, the sodium percentages (%Na), SAR, PI, and RSC of all groundwater samples exhibit excellent to good category irrigational water and signify safe irrigation water in both seasons (PM and PoM). The plots between salinity (EC) and alkali (Na) hazards (Fig. 8) illustrate that the samples are in the category of excellent to good followed by good to permissible, respectively. The USSLS diagram (Fig. 9) demonstrates the plots between alkali (sodium) and salinity (EC) hazards. It reveals that around 15.0% (PM) and 26.4% (PoM) samples fall in the category of C1-S1 (low-salinity and low-alkali hazards), followed by 35.3% (PM) and 32.4% (PoM) samples in the category of C2-S1 (medium-salinity and low-alkali hazards). The remaining 49.7% (PM) and 31.0% (PoM) samples fall in the category of C3-S1 (high-salinity and low-alkali hazard), respectively.
Conclusion
The study highlights significant spatial variability of WQI and hydrogeochemical characteristics of groundwater resources of the study area. The presence of geogenic contaminants (F−, As3+) in some groundwater samples in exceeding concentration indicates the possibility of contaminant aquifers. Moreover, exceeding EC, TDS, salinity, TH, Na+, and Cl− in groundwater samples signifies their influence on the overall groundwater WQI. It was found that more than 50% of samples of the region are in the category of poor to unsuitable potable water as per the WHO standard. The presence of F− concentration in 8.8% (PM) and 17.6% (PoM) samples exceeds the WHO permissible limit. The geostatistically interpolated map indicates possible fluoride-affected zones that contribute to unsuitable WQI in the habitations of the region. The geochemical evaluation demonstrates that the rock-weathering processes dominate the aquifers, resulting in Ca–Mg–Cl−–HCO3− type water followed by Mg–Ca–Cl–HCO3 types water. The principal component analyses indicate more than 60% variance in the hydrogeochemical variables of the study area and signify that the underlying geochemical process is highly correlated with EC, TDS, TH, Ca2+. But the irrigational water qualities were found to be suitable according to irrigational water quality indices indicating that groundwater is safe for agricultural uses.
References
Alam W, Yumnam G (2017) Hydrogeochemical assessment of groundwater resources of Imphal city. J Ind Water Works Assoc 39(3):197–204
Alam W, Gyanendra Y, Neihsial MT, Nesa N (2019) Hydrogeochemical assessment of groundwater arsenic and fluoride contamination in Thoubal district, Manipur. India J Earth Sci India 12(1):38–52
Alam W, Gyanendra Y, Chanda R, Laishram RJ, Nesa N (2020a) Hydrogeochemical assessment and evaluation of groundwater quality in selected areas of Bishnupur district. Manipur J Geol Soc of India 96(3):272–278
Alam W, Singh KS, Gyanendra Y, Laishram RJ, Nesa N (2020b) Hydrogeochemical assessment of groundwater quality for few habitations of Chandel district, Manipur (India). App Water Sci 10(5):1–13
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. America Public Health Association, Washington DC
Bhattacharya P, Sracek O, Eldvall B, Asklund R, Barmen G, Jacks G, Koku J, Gustafson JE, Singh N, Balfors BB (2012) Hydrogeochemical study on the contamination of water resources in a part of Tarkwa mining area Western Ghana. J Afr Earth Sci 66(67):72–84
Bouwer H (1978) Groundwater Hydrology. McGraw-Hill Book, New York, p 480
Census (2011) District Census Handbook, Bishnupur, Census of India, Series 15, Part XII-B, Directorate of Census Operation, Govt of Manipur
Central Groundwater Board (CGWB) (2013) Groundwater information booklet of Churachandpur district, Manipur, technical report series: D, No: 26/2013-14, central groundwater board, north eastern region, ministry of water resources. Govt. of India, New Delhi
Choudhury R, Mahanta C, Borah PJ, Saikia L, Alam W (2011) Screening of groundwater arsenic contamination in unexplored parts of brahmaputra floodplains in Assam. In: Proceedings of conference on arsenic in groundwater in southern Asia. Hanoi, Vietnam
Coyte RM, Jain RC, Srivastava SK, Sharma KC, Khalil A, Ma L, Vengosh A (2018) Large-scale uranium contamination of groundwater resources in India. Env Sci Tech Lett 5(6):341–347
Domenico PA, Schwartz FW (1990) Physical and chemical. John Wiley, New York, pp 410–420
Doneen ID (1964) Notes on water quality in agriculture, published as water science and engineering. Department of Water Sciences and Engineering, University of California, California
Drever JJ (1997) The geochemistry of natural water, 3rd edn. Prentice-Hall, Upper Saddle River, New Jersey, p 38
Eaton FM (1950) Significance of carbonate in irrigation water. Soil Sci 69:123–133
Fetter FW (2007) Applied hydrogeology. CBS Publishers, New Delhi
Freeze RA and Cherry JA (1979) Groundwater. Englewood Cliffs, NJ, Prentice-Hall, p 604
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170:1088–1090
Kresic N (1997) Hydrogeology and groundwater modeling. Lewis Publishers, pp 35
Mahanta C, Pathak N, Choudhury R, Borah P, Alam W (2009) Quantifying the spread of arsenic contamination in groundwater of the brahmaputra floodplains, Assam, India: a threat to public health of the region, great rivers: proc of world environmental and water resources congress 2009. ASCE, Kansas City, Missouri
Mahanta C, Choudhury R, Borah P, Sailo L, Mondal S, Saikia L, Alam W (2011) Monitoring and surveillance of groundwater arsenic contamination in the brahmaputra floodplain in Assam. Proceeding in world environmental and water resources congress 2011: bearing knowledge for sustainability. ASCE, Reston, pp 4255–4266
Mahanta C, Choudhury R, Basu S, Hemani R, Dutta A, Barua PP, Borah PJ, Bhattacharya M, Bhattacharya K, Alam W, Saikia L, Mukherjee A, Bhattacharya P (2015) Preliminary assessment of arsenic distribution in brahmaputra river basin of India based examination of 56180 public groundwater wells; safe and sustainable use of arsenic-contaminated aquifers in the gangetic plain. Springer, USA, pp 57–64
McSween HY, Uhle M, Richardson SM (2003) Geochemistry: pathways and processes, 2nd edn. Columbia University Press, USA
MSAPCC (2013) Manipur state action plan for climate change, directorate of environment. Govt. of Manipur, India
Mukherjee A, Scanlon B, Fryar A, Saha D, Ghose A, Chowdhuri S, Mishra R (2012) Solute chemistry and arsenic fate in aquifers between the himalayan foothills and Indian craton (including gangetic plain): influence of geology and geomorphology. Geochim Cosmochim Acta 90:283–302
Mukherjee A, Saha D, Harvey CF, Taylor RG, Ahmed KM, Bhanja SN (2015) Groundwater systems of the Indian sub-continent. J of Hydrol Reg Stud 4:1–14
O’Geen A (2015) Drought tip: reclaiming saline, sodic, and saline-sodic soils. Agriculture and Natural Resources Publication 8519, University of California
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. United States Geological Survey, Washington DC
Prusty P, Farooq SH, Zimik HV, Barik SS (2018) Assessment of the factors controlling groundwater quality in a coastal aquifer adjacent to the Bay of Bengal, India. Environ Earth Sci 77:762
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka state, India. J Chem 7:428–432
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agriculture Handbook, US Salinity Laboratory, Department of Agriculture, Washington DC, p 60
Todd DK (2003) Groundwater hydrology, 2nd edn. John Wiley, New York, p 535
United States Salinity Laboratory Staff (USSLS) (1954) Diagnosis and improvement of saline and alkaline soils. Agriculture Handbook, US Department of Agriculture, Washington DC
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva, pp 224–334
Wilcox LV (1955) Classification and use of irrigation water. US Department of Agriculture, Circular 969, Washington DC, p 19
Acknowledgements
The authors would like to acknowledge the Department of Earth Science, Manipur University, for allowing ArcGIS software to prepare study area map geospatial mapping.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
WA was involved in the conceptualization, methodology, original draft preparation, reviewing and editing, and project administration; KZ contributed to the sampling, samples analysis, and investigation; YG was involved in the sampling, samples analysis, and investigation; RJL contributed to the resources, reviewing, and editing; NN helped in the project administration, initial draft preparation, resources, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
This statement is to certify that there is no conflict of interest to declare. All Authors have seen and approved the manuscript being submitted. The article is the Authors’ original work, and it is not under consideration for publication or submitted for publication, nor has it been published in whole or in part elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alam, W., Zamminsion, K., Gyanendra, Y. et al. Geochemical and multivariate assessment of groundwater resources of churachandpur sub-division of Manipur, India. Appl Water Sci 12, 111 (2022). https://doi.org/10.1007/s13201-022-01638-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01638-y