Abstract
Removal of heavy metals and dyes from wastewater has received great attention due to scarcity of clean water worldwide. Herein an approach was introduced to attain this goal by employing a single material. Chitosan films were prepared, cross-linked it and utilized for the adsorption of cobalt from aqueous medium. The cobalt adsorbed chitosan was then reutilized as a photocatalyst for the photodegradation of methyl violet dyes. The prepared chitosan, cross-linked chitosan and cobalt adsorbed cross-linked chitosan were characterized through scanning electron microscopy (SEM), energy-dispersive X-ray (EDX), thermogravimetric analysis (TGA) and Fourier transform infrared spectroscopy (FTIR). SEM and EDX confirmed the adsorption of cobalt on the cross-linked chitosan. TGA analysis proved the increase in thermal stability with cross-linking while FTIR confirmed the cross-linking of chitosan. Maximum cobalt adsorption of 144 mg/g occurs at 600 ppm salt concentration. The photodegradation study shows that the fresh cobalt adsorbed cross-linked chitosan degraded about 97.78% methyl violet dye within 180 min under UV light, while the recovered degraded about 86.97% within the same time. Efficient degradation was observed at low initial dye concentration and at 30 ppm about 92.16% dye degraded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contaminants such as heavy metals and toxic dyes get discharged daily into water sources and constitutes a serious environmental hazard (Ghorai et al. 2014; Corda and Kini 2020). Heavy metals are naturally occurring metallic elements that are characterized by relatively high atomic density (4 g/cm3) and high relative atomic weight with an atomic number greater than 20 (Chibuike and Obiora 2014; Mahey et al. 2020). Heavy metals have been extensively used in agriculture, industry, medicine and other fields for a long history (Shi and Cai 2020). Heavy metals are serious environmental problems due to their persistence, non-degradability, biological toxicity and capability of entering the food chain (Hong et al. 2020). Heavy metals penetrate to human body through food, water and air and cause various disorders (Aprile and De Bellis 2020). High-level exposure to heavy metals may cause organ damage, cancer and joint diseases, and in extreme cases, it may cause death (Swelam et al. 2018). Among heavy metals cobalt (Co) is considered as a strategic metal due to their increase consumption (Bernabé et al. 2019) and wide range applications in various fields and industries such as solar cells (Marand et al. 2020), super-capacitors (Yang et al. 2020), catalysis (Senthamarai et al. 2020), alloying (Ivas’kevych 2020) and textiles (Ding et al. 2019). These industries contaminate water with cobalt which is considered as a major toxic heavy metal pollutant (Al-Jlil 2017). Cobalt adverse effects occur at levels of 7 μg/L or more and in excess can affect multiple organ system (Garcia et al. 2020). It readily adsorbed and moved into plants due to its high mobility and causing various toxicities (Lwalaba et al. 2020). In human, the ingestion of soluble Co salts at elevated amount produce effects on the cardiovascular, hematologic, nervous and endocrine system (Rodrigues et al. 2020; Danzeisen et al. 2020). The standard level of cobalt in drinking water is 2 μg/L (Al-Shahrani 2014). Different methods reported for cobalt removal are reverse osmosis (Imiete and Viacheslovovna Alekseeva 2018), ion exchange method (Aşçi and Kaya 2014), leaching (Khosravirad et al. 2020), solvometallurgical recovery (Peeters et al. 2020), solvent extraction (Yamina Boukraa 2020), adsorption (Bhatnagar et al. 2010; Elmorsy et al. 2019; Dehghani et al. 2020), etc. Among these methods adsorption of cobalt is an effective technique and has many advantages such as environmentally friendly, high efficiency and cost-effective process (Zhang et al. 2017).
Dyes are the organic soluble compounds which impart color to a given substrate due to the presence of chromophoric groups in its structures (Lellis et al. 2019). Dyes are widely used in plastic, textiles and printing industries, result in severe water pollution (Khan et al. 2016; Wu et al. 2020) and create esthetic and environmental issues (Routoula and Patwardhan 2020). Most of these dyes are toxic, carcinogenic and non-biodegradable due to their large size and complex structures, and their accumulation could create potential risks and threats to human and aquatic life (Khan et al. 2020a). Among these dyes, methyl violet is a toxic dye, which is harmful to aquatic life and human beings. In human, it causes severe eye and skin irritation, and gastrointestinal tract irritation if swallowed (Chandraboss et al. 2015; Saeed et al. 2017). Various strategies are reported for the removal of methyl violet such as adsorption (Esmaeili et al. 2018), biosorption (Hamitouche et al. 2016), biodegradation (Parshetti et al. 2009), ion exchange method (Wu et al. 2008) and photocatalytic degradation (Yang et al. 2013; Wu et al. 2017; Dewangan et al. 2020). Among these methods photodegradation has advantages over other conventional methods due to simplicity, pollutants complete mineralization, cost-effectiveness, performed at ambient pressure and temperature, no harmful by-products and reducing pollutants to ppm and ppb concentration (Khan et al. 2020b).
In the present study, cobalt was adsorbed on the cross-linked chitosan film and then cobalt adsorbed cross-linked chitosan (Co/cross-linked chitosan) were utilized as photocatalyst for the photodegradation of methyl violet dye (MV). Chitosan is a polysaccharide mainly composed of D-glucosamine and, in a lower proportion, N-acetyl-D-glucosamine units randomly β-(1-4)-linked (Ojeda-Hernández et al. 2020). Chitosan were first cross-linked through glutaraldehyde which could cross-link the amino groups. The cross-linking improves its heat resistance, mechanical strength, chemical stability and reduce its solubility in acidic medium (Szeto et al. 2014). Chitosan is a low-cost polymer (Upadhyay et al. 2020), widely reported for adsorption of heavy metals in pure and modified forms (Zhuang et al. 2018; Pietrelli et al. 2020; Velasco-Garduño et al. 2020). Chitosan materials are reported as photocatalyst for the photodegradation of dyes (Khan et al. 2019, Khan et al. 2021; Aziz et al. 2020; Ahmad et al. 2020). The effect of irradiation time, photocatalysts dosage, initial dye concentration, pH of the medium and photocatalyst sustainability on photodegradation of MV dye was evaluated.
Experimental works
Materials
Chitosan, acetic acid, glutaraldehyde and cobalt chloride hexahydrate (CoCl2·6H2O) were purchased from Sigma-Aldrich. Methyl violet dye was purchased from Scharlau.
Preparation of chitosan film
The known amount of chitosan was dissolved in 250 mL 5% (v/v) acetic acid to prepare viscous solution of chitosan polymer. The gel form of chitosan was stirred at room temperature to become more viscous and clear solution. The clear and viscous solution of chitosan was poured in petri dish and place on smooth surface. The petri dish was allowed to stand at room temperature to evaporate solvent and form chitosan film. The obtained transparent film of chitosan was dried in oven at 45 °C to evaporate all solvent molecules and avoid its thermal degradation.
Cross-linking of chitosan
Small strips of chitosan were added to 100 ml clutaraldehyde. The solution is allowed for 3 h at room temperature which forms hydrogels like structure. The chitosan strips is then separated and washed several times with distilled water to remove unreacted species. The obtained cross-linked chitosan samples were then oven-dried at 45 °C for overnight.
Adsorption of cobalt on cross-linked chitosan
To study the adsorption capacity of cross-linked chitosan film, 0.02 g chitosan film was shacked in 10 mL CoCl2. 6H2O aqueous solutions of different concentrations (150 ppm, 300 ppm, 450 ppm, 600 ppm, 750 ppm and 900 ppm) for 120 min using mechanical shaker (Wrist action shaker model 75-Burrel, Scientific Pittsburgh, PA, USA). After completion the adsorption times, the Co adsorbed cross-linked chitosan (Co/cross-linked chitosan) strips were separated and the amount of Co adsorbed from aqueous solutions was investigated using atomic adsorption spectrometer (AAS). The sample having maximum Co adsorbed was then utilized for dye degradation study.
The amount of Co adsorbed on cross-linked chitosan was calculated using the adsorption capacity Eq. 1.
where Co is the initial concentration of Co ions in ppm, Ce is the concentration Co ions at equilibrium, m is the mass of the adsorbent and V is the volume of solution containing solute.
Photocatalytic degradation of methyl violet dye
The Co/cross-linked chitosan strips were used as photocatalysts for the photocatalytic degradation of MV dye under UV light irradiation (254 nm, 15 W) as a function of several parameters. In irradiation time study, 0.02 g Co/cross-linked chitosan strips was added to 10 mL MV solution (50 ppm) in 50-mL beakers. The samples were covered with transparent sheet in order to allow UV light radiation and to prevent dehydration. The samples were placed in dark for 30 min to attain adsorption–desorption equilibrium. The dye solutions were then irradiated under UV light as a function of time (30, 60, 90, 120, 150 and 180 min) with constant stirring. After specific irradiation time, Co/cross-linked chitosan strips were removed from dye solution.
The recovered Co/cross-linked chitosan strips were washed properly for several times with distilled water in order to remove the adsorbed dye and dried at 45 °C (to avoid thermal decomposition of chitosan) in oven. The recovered catalysts were used under the same experimental conditions in order to check its sustainability. The effect on the photocatalyst dosage (0.01, 0.02, 0.04 and 0.6 g), initial dye concentration (30, 40, 50, 60 and 70 ppm) and pH of the medium (2, 4, 6, 8 and 12) on photodegradation were also investigated keeping other parameters constant. The %degradation of MV dye is calculated by using Eq. 2 and 3.
where C0 represents the initial concentration of dye, C stands for dye concentration after the reaction, Ao symbolize initial absorbance and A shows the absorbance of dye after the reaction.
Instrumentation
The morphological study of chitosan, cross-linked chitosan and Co/cross-linked chitosan were analyzed using JEOL, JSM-5910 SEM. The EDX spectrometric study analysis of samples was performed on EDX (model INCA 200/Oxford Instruments, UK, company oxford), in order to investigate the elemental composition of the samples. The functional group analysis of the samples was performed through FTIR spectrometer (PerkinElmer, serial number 95120). The TGA thermograms of all samples were obtained in a nitrogen atmosphere at a heating rate of 10 ºC/min from room temperature to 600 °C using a TGA (TGA-50 Shimadzu). The photodegradation study of methyl violet was performed using UV–visible spectrophotometer (UV-1800, Shimadzu, Japan).
Results and discussion
Cobalt adsorption and equilibrium isotherms
Table 1 shows the adsorption of Co on chitosan at different Co salt concentrations. Table 1 shows that adsorption increases as its Co salt concentration increases. The table demonstrates that as maximum adsorption of Co ions on F-chitosan is achieved in the solution sample having 600 ppm concentration. Increasing Co salt concentration beyond 600 ppm, adsorption of Co decreases. The F-chitosan with maximum Co adsorption, i.e., obtained from 600 ppm solution were utilized as photocatalyst for the photodegradation of dye.
Figure 1 shows the equilibrium adsorption quantity of Co (II) at 2 h under various equilibrium concentrations. It was found that initially the adsorption increased as increased the Co (II) concentration and then leveled off. The initial increase in Co (II) adsorption with the increase in its concentration might be due to the availability of active sites on cross-linked chitosan film, and then, the adsorption equilibrium is reached to maximum because of saturation of cross-linked chitosan film active sites. The adsorption equilibrium data of Co (II) were also analyzed by Langmuir and Freundlich isotherm models. These models provided the relationship between the amount of Co (II) adsorbed on surface of adsorbent and the concentration of Co (II) in solution at equilibrium. The linear form of Langmuir equation is represented in Eq. 4
where Ce (mg L–1) is the equilibrium concentration, qe (mg g–1) is the amount adsorbed per gram of adsorbent, and KL and qm are the Langmuir adsorption isotherm constants, respectively. The linear form of Freundlich equation is represented in Eq. 5:
where KF is the Freundlich sorption isotherm constant (mg g–1) and 1/n (g L–1) is a measure of the adsorption intensity of heterogeneity factor. qe (mg g–1) is the amount adsorbed per gram of adsorbent and Ce (mg L–1) is the equilibrium metal ion concentration. The R2 values are calculated for both Freundlich and Langmuir isotherms. The R2 value for Freundlich isotherm was 0.9747 while the R2 value for Langmuir isotherm was 0.6357. It means that the values are best fitted to Freundlich isotherm as compared with Langmuir isotherm.
Morphological and elemental composition study
Morphological study was performed to analyze the surface of chitosan and investigate the adsorbed cobalt particles on its surface. Figure 2 shows the SEM images of chitosan surface of different areas. Images show that chitosan has corrugated surface which is very suitable for metal ions adsorption. Figure 3 represents the SEM images of Co/cross-linked chitosan at different magnifications. Images illustrate that Co ions coagulate over each other on chitosan surface and form bulky particles. The images also revealed that Co particles are uniformly adsorbed on the surface of chitosan.
Figure 4a, b illustrates the EDX spectra of cross-linked chitosan and Co/cross-linked chitosan, which confirmed (4b) the presence of Co on the surface of chitosan. The elemental analysis presented that functionalized chitosan had only carbon and oxygen while in Co adsorbed chitosan, Co and minute quantity of chlorine were also found along with carbon and oxygen. The presence of Cl ion is due to the use of chloride salt for metal adsorption. Figure 4a, b also contains % elemental composition of respective elements in tabular form.
FTIR analysis
Figure 5 shows the FTIR spectra of chitosan, cross-linked chitosan and Co/cross-linked chitosan. The broad absorption peak that became strong after functionalization at 3350 cm−1 was an overlap of N–H and O–H stretching vibration absorption peaks. The absorption bands at around 2921 and 2877 cm−1 can be attributed to C–H symmetric and asymmetric stretching, respectively. The peaks observed at 1634, 1529 and 1378 cm−1, corresponding to characteristic absorption peaks of amide I, amide II and amide III bands. The absorption peak at 1073 cm−1 was the stretching vibration of C–O in the C-6 position. The decrease in the peaks after cobalt adsorption suggests that these functional groups are responsible for cobalt adsorption.
Thermogravimetric analysis
Figure 6 shows the thermal degradation study of chitosan, cross-linked chitosan and Co/cross-linked chitosan. The 1st stage degradation was observed at about 60–155 °C, which might be attributed to the evaporation of absorbed and bound water. The 2nd stage degradation started at 245 °C and continues up to 400 °C, which might be due to the degradation of chitosan main chain. The 3rd stage degradation started at about 450 °C, which might be due to the remaining residue in all samples.
Photodegradation of methyl violet
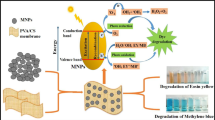
The Co/cross-linked chitosan were utilized as photocatalyst for the photodegradation of methyl violet (MV) dye in aqueous medium under UV light. In a time study, a constant amount of Co/cross-linked chitosan was added to 10 ml MV dye and kept in dark for 20 min to attain adsorption–desorption equilibrium and then kept under UV light for specific irradiation time. Figure 7a shows the UV–visible spectra of MV dye before and after UV light irradiation in the presence of Co/cross-linked chitosan. The figure shows that a decrease in dye absorption spectra is observed with increasing irradiation time. Figure 7b shows %degradation of MV dye photodegraded by Co/cross-linked chitosan. Initially Co/cross-linked chitosan degraded about 70.86% within 30 min which increases up to 97.78% by increasing UV light irradiation time to 180 min.
The used Co/C is washed with distilled water and again used as photocatalyst for MV dye under the same experimental conditions. The photocatalytic activity of recovered Co/cross-linked chitosan is compared with the fresh Co/cross-linked chitosan in order to check its photocatalytic sustainability. Figure 8 shows the comparison of % degradation of MV dye photodegraded by fresh and recycled Co/cross-linked chitosan under UV light. The results show that fresh Co/cross-linked chitosan degraded about 97.78% MV dye within 180 min while the recycled Co/cross-linked chitosan degraded about 86.97% within the same irradiation time. The result shows that recycled Co/cross-linked chitosan is much sustainable and degraded MV dye significantly.
Effect of photocatalyst dosage
The effect of photocatalyst dosage on the rate of photodegradation of MV dye was also studied by adding different amounts of Co/cross-linked chitosan ranging from 0.01 to 0.06 g keeping other parameters constant. Figure 9a represents the UV–Vis spectra of MV before and after UV light irradiation time employing different amounts of Co/cross-linked chitosan. Figure 9b shows the %degradation of MV dye photodegraded by different amounts of Co/cross-linked chitosan. Both the figures revealed that increasing photocatalyst dosage increases the rate of photodegradation of MV dye. It was found that 0.01 g of Co/cross-linked chitosan degraded about 74.07% dye which increases to 97.19% by increasing photocatalyst dosage to 0.06 g.
Effect of initial dye concentration
The effect of initial concentration of MV dye on the photodegradation rate was also evaluated and it is observed that increasing initial dye concentration of MV decrease the rate of photodegradation. Figure 10 shows the %degradation of MV dye potodegraded by constant amount of Co/cross-linked chitosan at various initial concentrations ranging from 30 to 70 ppm. The figure shows that initially at 30 ppm Co/cross-linked chitosan degraded maximally 92.16% which then decreases to about 60% by increasing initial concentration of MV dye to 70 ppm. The lower photodegradation rate at high concentration is due to adsorption of dye molecules on the active sites of photocatalyst surface and also leads to low photon penetration in dye solution.
Effect of pH of the medium
As different industries discharge their effluents at different pH, so its effect on photodegradation of MV dye was also investigated. The pH of the medium plays a significant role in degradation of textile wastes and generation of hydroxyl radicals. Figure 11 represents the %degradation of MV dye photodegraded by Co/cross-linked chitosan at various pH ranges from 2 to 10. The figure shows that rate of dye degradation is faster in acidic media and decreases as pH of the medium increases. The %degradation results revealed that at pH 2 about 92.72% MV dye degraded which increases gradually to 94.11% by increasing pH to 4. After pH 4, the rate of photodergadation of MV dye decreases to 69.23% by increasing pH of the medium to 10.
Conclusion
Cross-linking of chitosan not only increases its mechanical and thermal stability but also increases its metals adsorption capability. The cross-linked chitosan were not only utilized for cobalt adsorption but also reutilized (Co/cross-linked chitosan) for the photodegradation of MV dye in aqueous medium. The photodcatalytic activity of Co/cross-linked chitosan might be due to the presence of Co present on the surface of cross-linked chitosan. The photodegradation study revealed that the rate of photodegradation of MV increases by increasing irradiation time and photocatalysts dosage and decreases by increasing initial dye concentration and pH of the medium. The increase in the dye degradation with increase in photocatalysts dosage is due to the availability of more active sites for dye adsorption followed by photodegradation. The lower efficiency in higher initial dye concentration is due to higher adsorption of dye molecules on the photocatalyst surface which block its active sites and the lower penetration of UV light. The efficiency of recycled photocatalyst shows that cobalt adsorbed chitosan is highly sustainable.
References
Ahmad W, Khan A, Ali N et al (2020) Photocatalytic degradation of crystal violet dye under sunlight by chitosan-encapsulated ternary metal selenide microspheres. Environ Sci Pollut Res 287(28):8074–8087. https://doi.org/10.1007/S11356-020-10898-7
Al-Jlil SA (2017) Adsorption of cobalt ions from waste water on activated Saudi clays. Appl Water Sci 7:383–391. https://doi.org/10.1007/s13201-014-0253-z
Al-Shahrani SS (2014) Treatment of wastewater contaminated with cobalt using Saudi activated bentonite. Alexandria Eng J 53:205–211
Aprile A, De Bellis L (2020) Editorial for special issue “heavy metals accumulation, toxicity, and detoxification in plants.” Int J Mol Sci 21:1–5
Aşçi Y, Kaya Ş (2014) Removal of cobalt ions from water by ion-exchange method. Desalin Water Treat 52:267–273. https://doi.org/10.1080/19443994.2013.781544
Aziz A, Ali N, Khan A et al (2020) Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes. Int J Biol Macromol 153:502–512. https://doi.org/10.1016/J.IJBIOMAC.2020.02.310
Bernabé I, Gómez JM, Díez E et al (2019) Optimization and adsorption-based recovery of cobalt using activated disordered mesoporous carbons. Adv Mater Sci Eng. https://doi.org/10.1155/2019/3430176
Bhatnagar A, Minocha AK, Sillanpää M (2010) Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J 48:181–186. https://doi.org/10.1016/j.bej.2009.10.005
Boukraa Y (2020) Extraction of cobalt and lithium from sulfate solution using Di(2-ethylhexyl)phosphoric acid/kerosene mixed extractant. Russ J Phys Chem A 94:1136–1142. https://doi.org/10.1134/S0036024420060321
Chandraboss VL, Kamalakkannan J, Prabha S, Senthilvelan S (2015) An efficient removal of methyl violet from aqueous solution by an AC-Bi/ZnO nanocomposite material. RSC Adv 5:25857–25869. https://doi.org/10.1039/c4ra14463e
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci. https://doi.org/10.1155/2014/752708
Corda N, Kini MS (2020) Recent studies in adsorption of Pb(II), Zn(II) and Co(II) using conventional and modified materials: a review. Sep Sci Technol 55:2679–2698
Danzeisen R, Williams DL, Viegas V et al (2020) Bioelution, bioavailability, and toxicity of cobalt compounds correlate. Toxicol Sci 174:311–325. https://doi.org/10.1093/toxsci/kfz249
Dehghani MH, Yetilmezsoy K, Salari M et al (2020) Adsorptive removal of cobalt(II) from aqueous solutions using multi-walled carbon nanotubes and γ-alumina as novel adsorbents: Modelling and optimization based on response surface methodology and artificial neural network. J Mol Liq 299:112154. https://doi.org/10.1016/j.molliq.2019.112154
Dewangan R, Hashmi A, Asthana A et al (2020) Degradation of methylene blue and methyl violet using graphene oxide/NiO/β-cyclodextrin nanocomposites as photocatalyst. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1802443
Ding X, Yu M, Wang Z et al (2019) A promising clean way to textile colouration: Cotton fabric covalently-bonded with carbon black, cobalt blue, cobalt green, and iron oxide red nanoparticles. Green Chem 21:6611–6621. https://doi.org/10.1039/c9gc02084e
Elmorsy AH, Ghurzan S, El-Toony M, Al-Johani E (2019) A comparative study on Co(II) removal capacity from water samples by sorption using limestone and nanolimestone. J Water Reuse Desalin 9:339–349. https://doi.org/10.2166/wrd.2019.060
Esmaeili H, Mahini R, Foroutan R (2018) Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis. Turk J Biochem 43:623–631. https://doi.org/10.1515/tjb-2017-0333
Garcia MD, Hur M, Chen JJ, Bhatti MT (2020) Cobalt toxic optic neuropathy and retinopathy: case report and review of the literature. Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2020.100606
Ghorai S, Sarkar A, Raoufi M et al (2014) Enhanced removal of methylene blue and methyl violet dyes from aqueous solution using a nanocomposite of hydrolyzed polyacrylamide grafted xanthan gum and incorporated nanosilica. ACS Appl Mater Interfaces 6:4766–4777. https://doi.org/10.1021/am4055657
Hamitouche A, Benammar S, Haffas M et al (2016) Biosorption of methyl violet from aqueous solution using algerian biomass. Desalin Water Treat 57:15862–15872. https://doi.org/10.1080/19443994.2015.1077476
Hong YJ, Hong YJ, Liao W et al (2020) Progress in the research of the toxicity effect mechanisms of heavy metals on freshwater organisms and their water quality criteria in China. J Chem. https://doi.org/10.1155/2020/9010348
Imiete IE, Viacheslovovna Alekseeva N (2018) Reverse osmosis purification: a case study of the Niger delta region. Water Sci 32:129–137. https://doi.org/10.1016/j.wsj.2018.04.001
Ivas’kevych LM (2020) Influence of alloying with cobalt and hafnium on the corrosion and hydrogen resistances of refractory nickel alloy. Mater Sci 55:1–7. https://doi.org/10.1007/s11003-020-00365-6
Khan H, Khalil AK, Khan A et al (2016) Photocatalytic degradation of bromophenol blue in aqueous medium using chitosan conjugated magnetic nanoparticles. Korean J Chem Eng 3310(33):2802–2807. https://doi.org/10.1007/S11814-016-0238-8
Khan H, Khalil AK (2019) Photocatalytic degradation of alizarin yellow in aqueous medium and real samples using chitosan conjugated tin magnetic nanocomposites. J Mater Sci Mater Electron 3024(30):21332–21342. https://doi.org/10.1007/S10854-019-02510-7
Khan I, Khan I, Usman M et al (2020a) Nanoclay-mediated photocatalytic activity enhancement of copper oxide nanoparticles for enhanced methyl orange photodegradation. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-020-03431-6
Khan I, Saeed K, Ali N et al (2020b) Heterogeneous photodegradation of industrial dyes: an insight to different mechanisms and rate affecting parameters. J Environ Chem Eng 8:104364. https://doi.org/10.1016/j.jece.2020.104364
Khan M, Khan A, Khan H et al (2021) Development and characterization of regenerable chitosan-coated nickel selenide nano-photocatalytic system for decontamination of toxic azo dyes. Int J Biol Macromol 182:866–878. https://doi.org/10.1016/J.IJBIOMAC.2021.03.192
Khosravirad MM, Bakhtiari F, Ghader S, Abkhoshk E (2020) An improved process methodology for extracting cobalt from zinc plant residues. Hydrometallurgy 191:105163. https://doi.org/10.1016/j.hydromet.2019.105163
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290. https://doi.org/10.1016/j.biori.2019.09.001
Lwalaba JLW, Louis LT, Zvobgo G et al (2020) Physiological and molecular mechanisms of cobalt and copper interaction in causing phyto-toxicity to two barley genotypes differing in Co tolerance. Ecotoxicol Environ Saf 187:109866. https://doi.org/10.1016/j.ecoenv.2019.109866
Mahey S, Kumar R, Sharma M et al (2020) A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl Sci 2:1–12. https://doi.org/10.1007/s42452-020-3020-9
Marand ZR, Kermanpur A, Karimzadeh F et al (2020) Structural and electrical investigation of cobalt-doped niox/perovskite interface for efficient inverted solar cells. Nanomaterials. https://doi.org/10.3390/nano10050872
Ojeda-Hernández DD, Canales-Aguirre AA, Matias-Guiu J et al (2020) Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.00389
Parshetti G, Saratale G, Telke A, Govindwar S (2009) Biodegradation of hazardous triphenylmethane dye methyl violet by Rhizobium radiobacter (MTCC 8161). J Basic Microbiol. https://doi.org/10.1002/jobm.200800200
Peeters N, Binnemans K, Riaño S (2020) Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem 22:4210–4221. https://doi.org/10.1039/d0gc00940g
Pietrelli L, Francolini I, Piozzi A et al (2020) Chromium(III) removal from wastewater by chitosan flakes. Appl Sci 10:1925. https://doi.org/10.3390/app10061925
Rodrigues JAV, Martins LR, Furtado LM et al (2020) Oxidized renewable materials for the removal of cobalt(II) and copper(II) from aqueous solution using in batch and fixed-bed column adsorption. Adv Polym Technol. https://doi.org/10.1155/2020/8620431
Routoula E, Patwardhan SV (2020) Degradation of anthraquinone dyes from effluents: a review focusing on enzymatic dye degradation with industrial potential. Environ Sci Technol 54:647–664
Saeed K, Khan I, Gul T, Sadiq M (2017) Efficient photodegradation of methyl violet dye using TiO2/Pt and TiO2/Pd photocatalysts. Appl Water Sci 7:3841–3848. https://doi.org/10.1007/s13201-017-0535-3
Senthamarai T, Chandrashekhar VG, Gawande MB et al (2020) Ultra-small cobalt nanoparticles from molecularly-defined co-salen complexes for catalytic synthesis of amines. Chem Sci 11:2973–2981. https://doi.org/10.1039/c9sc04963k
Shi J, Cai Y (2020) Environmental chemistry and toxicology of heavy metals. Ecotoxicol Environ Saf 202:110926
Swelam AA, Awad MB, Salem AMA, El-Feky AS (2018) An economically viable method for the removal of cobalt ions from aqueous solution using raw and modified rice straw. HBRC J 14:255–262. https://doi.org/10.1016/j.hbrcj.2016.10.001
Szeto W, Kan CW, Yuen CWM et al (2014) Effective Photodegradation of methyl orange using fluidized bed reactor loaded with cross-linked chitosan embedded nano-CDS photocatalyst. Int J Chem Eng. https://doi.org/10.1155/2014/270946
Upadhyay U, Sreedhar I, Singh SA et al (2020) Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2020.117000
Velasco-Garduño O, Martínez ME, Gimeno M et al (2020) Copper removal from wastewater by a chitosan-based biodegradable composite. Environ Sci Pollut Res 27:28527–28535. https://doi.org/10.1007/s11356-019-07560-2
Wu C, Dong D, Yu X et al (2020) Mesoporous carbon/cobalt ferrite nanocomposite: a charge and pH independent magnetic adsorbent for dye pollutant treatment. Diam Relat Mater 105:107796. https://doi.org/10.1016/j.diamond.2020.107796
Wu JS, Liu CH, Chu KH, Suen SY (2008) Removal of cationic dye methyl violet 2B from water by cation exchange membranes. J Memb Sci 309:239–245. https://doi.org/10.1016/j.memsci.2007.10.035
Wu W, Luo ZD, Wang J, Liu J (2017) Photocatalytic degradation of methyl violet and rhodamine B based on an extremely stable metal-organic framework decorated with carboxylate groups. Inorg Chem Commun 85:2–4. https://doi.org/10.1016/j.inoche.2017.03.025
Yang L, Lu X, Wang S et al (2020) Designed synthesis of nickel-cobalt-based electrode materials for high-performance solid-state hybrid supercapacitors. Nanoscale 12:1921–1938. https://doi.org/10.1039/c9nr08156a
Yang S, Xu Y, Huang Y et al (2013) Photocatalytic degradation of methyl violet with TiSiW12O40/TiO2. Int J Photoenergy. https://doi.org/10.1155/2013/191340
Zhang X, Wang X, Chen Z (2017) Radioactive cobalt(II) removal from aqueous solutions using a reusable nanocomposite: kinetic, isotherms, and mechanistic study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph14121453
Zhuang S, Yin Y, Wang J (2018) Removal of cobalt ions from aqueous solution using chitosan grafted with maleic acid by gamma radiation. Nucl Eng Technol 50:211–215. https://doi.org/10.1016/j.net.2017.11.007
Funding
This work was carried out with the financial support of Bacha Khan University and University of Malakand Chakdara Dir(Lower).
Author information
Authors and Affiliations
Contributions
All the authors contributed equally in the work.
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, N., Khan, I., Zada, N. et al. Utilization of cross-linked chitosan for cobalt adsorption and its reutilization as a photocatalyst for the photodegradation of methyl violet dye in aqueous medium. Appl Water Sci 12, 107 (2022). https://doi.org/10.1007/s13201-022-01633-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01633-3