Abstract
The impact of gold mining activities on the Lom River in Wakaso (Adamawa Cameroon) and the potential of Moringa Oleifera seeds for the removal of pollutants from wastewater is evaluated on this paper. Water samples were collected for physicochemical (hydrogen potential, electrical conductivity, turbidity and suspended solids) and chemical (major ions and heavy metals) analyses. To evaluate the effect of mining activities on waters and sediments, a combination of multivariate statistical analysis (MSA) and methods to assess the sediment quality was used. The restorative effect of Moringa oleifera seeds was studied with the determination of the maximum removal efficiencies and the maximum adsorption capacities. The results of the physicochemical characterization of waters showed that these waters were slightly acidic to slightly basic (6.12–8.12), weakly conductive (185.8–584.1 μS cm−1), turbid (345–801NTU) and had high content of suspended solids (167–700 mg L−1). The average concentrations of studied heavy metals (Ni, Cd, Fe, Mn, As and Hg) in waters exceeded the limits recommended by the World Health Organization (WHO) standards. Physicochemical characterization of sediments revealed that they were acidic to neutral (5.95–6.80) and organic matter (OM) content ranged from 11.11 to 15.78%. The concentrations of studied trace metals (Ni, Cd, Fe, Hg, Mn and As) in sediments were below the limits recommended by the WHO except for Cd and Hg. The study of the capacity of Moringa oleifera seeds to remove pollutants in waters showed that 54% of the electrical conductivity, 80% of turbidity and 94% of suspended solids were reduced. The maximum removal efficiency of 91.66, 92.30 and 24.48 and the maximum adsorption capacity of 2.4, 2.6 and 16.6 mg g−1were observed for Cd (II), Hg (II) and Fe (II), respectively. Thus, the Moringa oleifera seeds which are locally available natural bio-adsorbent exhibit attractive property to treat wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The exploitation of mineral resources has been developed in sub-Saharan Africa since the 19th century. It is characterized by the coexistence of large-scale exploitation scale practiced by large companies and constitutes a significant source of revenue for the State and small-scale exploitation (Butaré and Keita 2010). Mining activities are important sources of contamination of the environment (Mitchell 2009). Mining operations use water for mineral processing, metal recovery, dust control and supply of water needs of workers on site (Lottermoser 2010). In the locality of Wakaso (Adamawa, Cameroon), an artisanal semi-mechanized gold exploitation is carried out and the extraction of gold can negatively impact water resources.
In the world, physicochemical characterization studies have been carried out to assess the water and sediment quality. Kaizer and Osakwe (2010) have studied the physicochemical characteristics and heavy metal content of the various river systems with a view of accessing the effects of land use on the quality of rivers. In Pakistan, Malik and Nadeem (2011) describe the spatial and temporal variations in surface water in the Rawal Lake Reservoir, identify possible sources of heavy metal contamination and compare with quality guidelines. In India, Jasmin and Mallikarjuna (2014) study the spatial and temporal evaluation of the physicochemical parameters of groundwater quality of the basin to assess its suitability for drinking purpose through the development of drinking water quality index maps. Asare‑Donkor et al. (2018) evaluate the hydrochemical characteristics of surface water from the Birim River basin to assess the ecological risk and the suitability for domestic and irrigation uses. Other studies have specifically shown the impact of artisanal and small-scale gold mining on water resources and sediments. Acheampong et al. (2013) characterize the tailings dam effluent of Central Africa Gold Limited in Bibiani (Ghana), thereby generating reliable data for planning and design of an effluent treatment system. Banunle et al. (2018) determine the physicochemical properties and heavy metal status of the Tano River along the catchment of the Ahafo Mine in the Brong-Ahafo Region of Ghana. On Tropical River, Goix et al. (2019) have shown the impact of some artisanal and small-scale gold mining. The study combined two different models to discriminate the part of the anthropogenic Hg in bottom sediments of tropical rivers affected by ASGM and the part of liquid Hg used by gold-miners in the anthropogenic Hg sources. These studies show that the mining activities have environmental consequences, so urgent action by policymakers is required to combat the negative influence of the mining activities.
In Cameroon, very few studies evaluate the impact of gold mining exploitation on the waters and sediments quality (Rakotondrabe et al. 2017; Mambou et al. 2020a, b). The present study is one of the first with that of Djouzami (Ayiwouo et al. 2020) which assess the effect of gold mining activities on water resources in the Adamawa region. The main differences between the present study and that carried out at Djouzami are the type of adsorbent and the number of metals adsorbed. In this study, Moringa Oleifera seeds are used to eliminate heavy metals in the water contrary to the smectic clay used in Djouzami. The adsorption is carried out on three metals in particular: Cd (II), Hg (II) and Fe (II), while only Pb (II) is adsorbed in Djouzami. In addition, the characterization of the sediments is more complete with the determination of the pH, organic matter (OM), particle size and mineralogical analysis. This waters and sediment characterization study is important because it permits to estimate the real impacts of mining activities on water resources. These impacts are particularly manifested by the presence of heavy metals in nearby rivers with concentrations above national and international standards. It is therefore possible to determine the effects of pollution and anticipate them in order to provide adequate remediation methods.
Research works have been carried out on depollution techniques by adsorption of water contaminated with heavy metals using natural materials less expensive and widely available such as natural zeolites, volcanic ash and clay (Bailey et al. 1999; Etoh et al. 2015). The Moringa oleifera plant belongs to the family Moringaceaeis cultivated across the whole of the tropical belt for different purposes. There has been increased interest in the subject of natural coagulants for treatment of water and wastewater. Moringa oleifera seeds proteins have been found to possess coagulating properties similar to those of alum (Ashrith and Sibi 2019). The aim of the present paper is to evaluate the effect of semi-mechanized gold mining site of Wakaso (Adamawa Cameroon) on the Lom River and the capacity Moringa Oleifera seeds for water treatment.
Materials and methods
Study area and sample collection
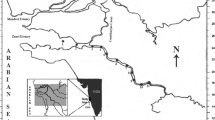
Wakaso is located in the Adamawa region of Cameroon, Mbere division. Its distance from Meiganga, central town of the division, is 31.63 km by air and 49.02 km of land toward the East of the country and its distance from Garoua-Boulay is 48.76 km by air and 93.89 km of land. The gold mining site is located in the Western part of Wakaso. The study area is located at an average altitude of 880 m. The relief is characterized by the presence of a plateau dissected by U-shaped valleys and V-shaped valleys. The hydrography of the study area is not very important. It is marked by the presence of the Lom, Yoyo, Kaya, Foum rivers and intermittent stream Goumba consisting of many meanders, which can justify the presence of the gold mining site and the mining exploitation is carried out on the Lom River (Olivry 1986). The mean annual temperature is 22.6 °C (Suchel 1987).
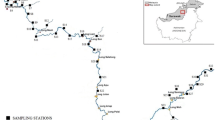
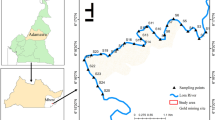
The study was conducted in the Lom River. Surface water and sediment were collected on May 4, June 5 and July 4, 2019 (rainy season) at different points of the study area. The sampling points were selected based on the criteria of accessibility of the site and representativeness. The geographical coordinates of the selected sampling points were obtained using a GARMIN ETREX 30 Global Positioning System (GPS). All the samples were taken from the Lom River. Sampling was done manually, and water samples were taken in 1-L polyethylene bottles and 50 cm below the surface. Before sampling, the bottles were washed three times with double deionized water in the laboratory and with the water at the sampling site during field sampling. Sediment samples were collected at 0–10 cm of the surface water/sediment. The samples were placed in sterilized polyethylene bags and then stored and transported to the laboratory for further analyses. The location map of the study area and the sampling map are presented in Fig. 1.
The samples denoted as WU and SAM0 correspond, respectively, to the water and sediment unaffected reference points located in the upstream of the gold mining site; WM and SMI0 were samples taken in the middle of the gold mining site and WD and SAV0 correspond to water and sediment sampling points downstream the gold mining site.
Laboratory analysis and hydrochemical assessment of water
The water samples were analyzed at laboratory of Hydrobiology and Environment laboratory of the University of Yaounde I (Cameroon). pH and electrical conductivity (EC) were measured using a pH meter HACH HW 11d and conductivity meter HACH HQ14d, respectively. The values of turbidity (Tu) and suspended solids(SS) of water samples were made by a nephelometry method using a HACH DR/2000 spectrophotometer. It was configured to read turbidity at the wavelength of 750 nm specified for measuring turbidity. The study of chemical analyses was done on major ions (Ca2+, Mg2+, K+, Na+, Cl−, NO3−, SO42− and HCO3−) and heavy metals (Ni, Cd, Fe, Mn, As and Hg). Major ions and heavy metals were analyzed by ion by using a Dionex brand chromatograph and atomic absorption spectrometry (AAS) Shimadzu spectrometer, respectively. AAS is a spectroscopy technique used to determine the concentration of trace metals (alkali metals, alkaline earth metals, transition metals), as well as metalloids in a sample.
The sediment’s samples were analyzed at the support framework for promoting artisanal mining (CAPAM) and promotion of local materials (MIPROMALO) laboratories in Yaounde (Cameroon). pH values were measured by a pH meter (Consort). For OM determination, a container was cleaned and dried, it is weighed (M0). The sediments were introduced into the container, dried in an oven at 105 °C for 24 h and weighed M1, after which the container containing the material was inserted in an oven and the temperature was raised to 550 °C for 4 h, allowed to cool and then weighed again (M2). The organic matter (OM) was calculated in percentage using the formula:
Heavy metals analyzed on sediment samples were Ni, Cd, Fe, Mn, As and Hg. Heavy metal’s concentrations were determined by using X-ray fluorescence (XRF) Skyray Instrument EDX Pocket. Uncertainty was high (> 5%) for trace metals displaying a low concentration (< 0.1 ppm).
The hydrochemical facies of water was determined by using Piper, Schoeller and Wilcox diagrams. The Piper trilinear diagram is a graphical representation of hydrochemical data which is helpful in determining connections between different dissolved constituents and for classifying groundwater and surface water based on its chemical characteristics (Tiwari et al. 2017). The semi-logarithmic diagram of Schoeller (Schoeller 1965) allows the major ions of samples to be represent on a single graph, in which samples with similar patterns can be easily distinguished. The Wilcox diagram is used to determine the viability of water for irrigation purposes (Wilcox 1955). The sodium adsorption ratio (SAR), or alkali hazard, is determined by the absolute and relative concentration of cations and is calculated according to the formula:
Percent Na (Na%) is widely utilized for assessing the suitability of groundwater quality for irrigation purpose (Wilcox 1955) and is calculated according to the formula:
Multivariate statistical analysis
To determine the different relationships between the studied parameters, statistical analysis has been made. Firstly, descriptive statistics (minimum, mean, median, maximum and standard deviation) were performed. Secondly, multivariate statistical analysis (MSA) was applied by the determination of correlation matrix, principle component analysis (PCA) and hierarchical cluster analysis (HCA) by using XLSTAT 2019 software. Pearson’s correlation coefficient (R) analysis was used to identify the interrelationship between two quantitative variables. The variables were correlated with significance level of P = 0.01. Principle component analysis (PCA) was applied to determine associations between studied parameters and explain the significance of variation among the groups (Jolliffe 2002). Hierarchical cluster analysis (HCA) which begins with the most similar pair of objects and forming higher clusters stepwise was employed to produce a dendrogram that provide a visual summary of the clustering process using unsupervised pattern recognition technique.
Estimation of the contamination intensity in sediment
The environmental impact of metals and the pollution level in the sediments can be determined with the help of two parameters: The enrichment factor (EF) and the geo-accumulation index (Igeo). The enrichment factor (EF), due to its universal formula, is a relatively simple and easy tool for assessing the enrichment degree and comparing the contamination of different environmental media (Chiffoleau et al. 2001). Some authors suggest that element concentrations measured in a deeper soil horizon (subsoil) could be considered a “local background” for the upper soil horizons (Blazer et al. 2000). Enrichment factor is expressed as follows:
where (M/Fe) sample is the ratio of metal and Fe concentrations in the sample, and (M/Fe) background is the ratio of metal and Fe concentrations of the background. Average upper continetal crust composition given by Talor and McLennan (1995) to determine mineral concentration of the back ground. The EF can be indicated by different classes (see Table 1) ranging from EF < 2 (efficiency to minimal enrichment) to EF > 40 (extremely high enrichment) (Yongming et al. 2006).
Geo-accumulation index (Igeo) was originally defined by Müller (1979) for metal concentrations in the 2-micron fraction and was developed or global standard shale values. His index is expressed as follows:
where Cn is the measured concentration in the sediment for the metal n, Bn is the background value for the metal n and the factor 1.5 is used because of possible variations in the background data due to lithological variations. Müller (1981) has defined seven classes of the geo-accumulation index (Table 2) ranging from Class 0 (Igeo = 0, unpolluted) to Class 6 (Igeo > 5, extremely polluted). The highest class (Class 6) reflects at least a 100-fold enrichment factor above background values.
Water treatment by the Moringa oleifera seeds
The bio-adsorbent (Moringa oleifera seeds) is presented in Fig. 2
Figure 2 presents the Moringa oleifera seeds and Moringa oleifera powder, which were used to remove the pollutants from wastewater. A wastewater of 250 mL was measured and put into five beakers each, one of the beakers served as the control. The coagulant powder was used in two of the beakers. The control was without the coagulant, just the wastewater. 12.5 mg of the powder was used for the treatment. The solutions were mixed rapidly for 2 min. They were followed in 10 min of gentle mixing using a magnetic stirrer to aid in increasing the contact between the particles hence floc formation. The suspensions were left to stand without disturbance for about 2 h. For determining the adsorption efficiency and maximum adsorption capacity, physical parameters and heavy metals of water were measured before and after treatment. The adsorption efficiency (percentage) is calculated according to the expressions:
where \({C}_{0}\) is the initial concentration (mg L−1) and \({C}_{f}\) is the solution concentration at the end of the sorption process (mg L−1) (Agarwal et al. 2006). The maximum adsorption capacity, \({q}_{max}\)(mg g−1), of the metals is calculated using an equation:
where \({C}_{0}\) and \({C}_{e}\) (mg L−1) are the liquid-phase concentrations of heavy metals at time zero and equilibrium, respectively. V is the volume (mL) of the sample solution, and W is the weight (mg) of the dry sorbent.
Results and discussion
Physicochemical parameters of waters
The descriptive statistics of physical parameters of waters and comparison with WHO limits are summarized in Table 3.
The pH values ranged from slightly acidic to slightly basic (6.12–8.12). The pH values were in agreement with the values recommended by the WHO (WHO 2011). The pH basic values could be linked to oil spills or leakages from excavation machinery and transportation vehicles (Damy 2011). It could be also linked to the lithology or substrate of the study area. The EC values ranged from 185.8 to 584.1 μS cm−1 were below the WHO standard (WHO 2011) as seen in Table 3 for all the samples. This water is weakly conductive, and these values are due to the salinity of the water and the low values of organic matter in sediment. The electrical conductivity increases with the addition of organic matter. These values were higher than those observed in Djouzami gold mining site (Ayiwouo et al. 2020). The turbidity values ranged from 345 to 801NTU exceeded the limit recommended by the WHO (WHO 2011). The waters of the site had a cloudy state. Upstream of the site, turbidity values were lower (345, 410 and 382 NTU) compared to that obtained in other sampling points. These lower values explained the fact that there is no exploitation activity. These results reflect those obtained in previous studies (Rakontondrabe et al. 2017; Ayiwouo et al. 2020). The suspended solids ranged from 167 to 700 mg L−1. For the points on the mining activity area (WM1, WM2, WM3) and after the mine (WD1, WD2, WD3), the SS values were higher than that obtained upstream the site. These results reinforce the influence of the mining activity on the physical water quality. The SS values were higher than those found in the mining sites of Ghana (41–96 mg L−1) (Acheampong et al. 2013) and lower than those observed in Betare-Oya gold district (2–8996 mg L−1) (Rakotondrabe et al. 2017) and Batouri (210–3410 mg L−1) (Mambou et al. 2020a, b).
Determination of major ions
Table 4 shows the statistical analysis of major ions in water samples collected.
The major ion concentrations in the Wakaso gold mining site were in the following order: Mg2+ > HCO32− > Ca2+ > K+ > Na+ > SO43− > NO3− > Cl− for the water samples at upstream; Ca2+ > Mg2+ > Na+ > HCO32− > SO43− > K+ > NO3− > Cl− for water samples at the site and HCO32− > Ca2+ > Na+ > Mg2+ > SO43− > Cl− > K+ > NO3− for the water samples after the site. The values of these ions were lower than that observed in Betare-Oya (Rakotondrabe et al. 2017). Table 4 shows that calcium concentrations ranged from 6.03 to 15.87 mg L−1. Calcium is an earthy alkaline metal. Its content varies essentially according to the nature of the lands crossed (Nollet 2006). It is also responsible for the hardness of water (Wedepohl 1995). The magnesium concentrations ranged from 6.24–10.23 mg L−1. It is also an earthy alkaline metal and a major component of water hardness. Magnesium and calcium are found in the waters that have crossed limestone rocks. Upstream the site, the sulfate concentrations were extremely low (1.58, 2.01 and 1.82 mg L−1) compared to that on the site and downstream. They are below the WHO standard (250 mg L−1). It is therefore deduced that the presence of sulfates is related to the exploitation activity. Gold moving with iron sulfides (pyrite), small amounts of sulfides are brought into contact with water and oxygen during excavation works for the recovery of ore (gold) and generate sulfates (Aranguren et al. 2008). The hydrochemical evaluation is analyzed by the Piper, and Schoeller diagrams are illustrated in Fig. 3.
One main water type has been revealed in the study area (see Fig. 3a) using the Piper trilinear diagram (Piper 1944). According to Piper‘s diagram (Fig. 3a), this water was bicarbonate calcium and magnesium with few dominant cations and high proportions of bicarbonate. This indicates that the main processes occurring are the dissolution of silicate minerals such and the dissolution of halite and gypsum. In Schoeller diagram (Fig. 3b), this water (Lom River) was bicarbonate calcium and magnesium. The Piper diagram confirmed the same result. In Fig. 3b, it appears that the broken lines tend to line up. This means that there is a certain compositional similarity between the different waters analyzed. This supports the uniformity of the facies found for the majority of the waters analyzed. The Wilcox diagram is illustrated in Fig. 4.
The Wilcox diagram represents the percentage of Na and EC at the same time and grades the water samples into five classes, namely class I, class II, class III, class IV and class V, representing excellent to good, good to permissible, permissible to doubtful, doubtful to unsuitable and unsuitable water quality, respectively. The sodium level varied from 25 to 40%. Depending on the percentage of sodium and the electrical conductivity (Wilcox diagram), all samples were suitable (excellent) for irrigation.
Heavy metals in water samples
The spatial and temporal variations in nickel and cadmium concentrations in water samples are illustrated in Fig. 5.
The nickel concentrations (5.1–7.1 mg L−1) and cadmium concentrations (0.12–1.61 mg L−1) exceeded the WHO standard WHO (WHO 2011) in all the samples (see Fig. 5a and b). The presence of nickel could be due to Ni–Cd batteries used in gold mine sites (Pahimi et al. 2015). In addition, nickel is present in the air, in suspended particles, after being released by human activities or natural phenomena, such as volcanic eruptions, forest fires and meteorites from the upper atmosphere (Fay et al. 2005). The high concentration was observed during July month. Cadmium could come from the dissolution of the sphalerite and the leaching of soil containing this trace metal. High concentrations of cadmium have been found to lead to chronic kidney dysfunction. Cadmium (Cd) is one of the most toxic elements with reported carcinogenic effects in humans (Goering et al. 1995). At all the sampling points, iron concentrations (2.6–6.1 mg L−1) exceeded the limit of 0.3 mg L−1 recommended by the WHO (WHO 2011). A peak was observed in the downstream samples with a concentration of 5.8, 4.9 and 6.1 mg L−1, respectively. The high iron concentrations present in these waters could be due to the dissolution of pyrite (FeS) and the leaching of lateritic ferralitic soils with strong complexation with humic acids (Ayiwouo et al. 2020). The manganese concentrations ranged from 0.39 to 0.87 mg L−1 were above the limit of 0.04 mg L−1 set by the WHO (WHO 2011) at all the sampling. Dissolved from some rocks and soils, large quantities of manganese are commonly associated with high iron content and acidic water (Bekkoussa et al. 2013). The arsenic concentrations ranged from 0.1 to 0.31 mg L−1 were above the WHO limit (0.01 mg L−1) at all the sampling points indicating arsenic pollution. Its presence could be due to the presence of arsenopyrite mineral. Naturally, arsenic occurs in rocks and gold ore such as arsenopyrite (Asamoah 2012). The agricultural activities with the use of pesticides, which contains the element, could be another source. These results should be taken into account by the authorities of Cameroon, as there is a serious threat to ecological and human health. The mercury concentrations (0.01–1.83 mg L−1) were above the WHO limit (0.001 mg L−1) in all the samples indicating mercury pollution. The presence of mercury in the water samples could be due to the exploitation activity carried out and the gold washing which at times requires mercury for its separation. The use of mercury is prohibited in the mining code of Cameroon (Mining code 2016). Some studies showed that the amount of fluctuations of anthropogenic activity, agricultural and sewage effluents discharged is the main reasons for the temporal difference of heavy metals concentrations (Balan and Shivakumar 2012). On the other hand, because of the high temperature in a tropical context and organic matter decomposition due to fermentation, the heavy metal could be liberated from the sediment to the overlying water (Omar 2013).
Physicochemical characterization of sediment samples
The values of pH and organic matter in sediment samples are presented in Table 5.
The pH ranged from slightly acidic to neutral (5.95–6.80). The pH variation is the factor whose action on the mobility of metals is the most decisive. The speciation of the metal changes with the pH and influences the fixing on the solid phase. Trace metals (TMs) could be mobilized if conditions change, environmental factors, especially pH, when pH decreases, desorption or dissolution will tend to lead to the release of the cations from the sediment to the dissolved phase (Quevauviller 1998). The OM varied from 11.11 to 15.78%. The quantity of OM was less important at the downstream points SAV01, SAV02 and SAV03 than the upstream points (SAM01, SAM02 and SAM03). This could be explained by the importance of the reducing conditions downstream. More so, the strong west winds noticed at the upstream points also play a major role in the contribution to nutrients, hence, the greater presence of organic matter upstream. The percentage in OM remains generally low, this could be explained by the fact that sampling of sediments was done on the surface, the OM undergoes a significant oxidation, causing its degradation and the release of the metallic trace elements associated with it. The results illustrated that OM was important for heavy metal species, transportation and transformation. Hu et al. (2017) has shown that dissolved organic matter (DOM), as the most active organic carbon in the soil, has a coherent affinity with heavy metals from inherent and exogenous sources. The DOM has important roles in the adsorption of heavy metals in soil. The heterogeneity and variability of the chemical constitution of DOM impede the investigation of its effects on heavy metal adsorption onto soil under natural conditions. Therefore, the changes of DOM in chemical structure and composition may affect not only DOM interaction with heavy metals, but also the nature of soil, which will influence the behaviors of heavy metals adsorption onto soil (Hu et al. 2016). The results of the determination of heavy metals in sediments are presented in Fig. 6.
In Fig. 6, nickel concentrations ranged from 26 to 35 ppm (see Fig. 7a). These concentrations were below the limit of 50 ppm recommended by the WHO. Nickel could derive from ultrabasic rocks. The cadmium concentrations (4–12 ppm) exceeded the WHO limit (0.35 ppm). The sediments were polluted by cadmium, and this pollution increases at downstream points. Its presence could be due to the dissolution of sphalerite mineral. Iron concentrations (250–940 ppm) were high in all the sampling points. These concentrations could be due to the presence of pyrite and also the leaching of ferralitic soils. These concentrations are low compared to that observed in Djouzami gold mining site (Ayiwouo et al. 2020). Manganese concentrations varied between 7 and 19 ppm. The peak concentration was observed during July. Meanwhile, the manganese concentration decreases as we move down, meaning that its presence may not be caused by the exploitation. Arsenic concentrations ranged from 5 to 17 ppm (Fig. 6e). These concentrations exceeded the limit of 1.12 set by the WHO. The soils contain arsenic primary minerals derived directly from the weathering of the bedrock of the subsoil where the most frequent species of primary mineralization are arsenopyrite (FeAsS), realgar (AsS), orpiment (As2S3) (Arranguren et. 2008). Mercury concentrations (2–25 ppm) were above the WHO limit (1 ppm) in all the sampling points (see Fig. 6f). Its presence could be due to the fact that mercury is used during the gold washing process for the separation of gold. The mercury could come from the dissolution of the cinnabar (HgS).
Multivariate statistical analysis
The relationship between physical parameters and heavy metals determined in water samples is presented in Table 6.
In Table 6, correlation matrix using Pearson’s coefficient (with a significance level P = 0.01) was applied. Firstly, a high correlation was obtained between pH/Tu (0.923). The EC had high correlations with SS (0.918), Cd (0.780), Fe (0.966) and Hg (0.704). High and positive correlations indicate that the variables follow the same direction. Average correlation was obtained between Tu/SS (0.66). Excavation and extraction works were the main causes of this correlation. Secondly, the correlation matrix obtained indicated high correlations between Cd/Fe (0.823), Cd/Hg (0.704), Fe/Hg (0.769) and Mn/As (0.626). Generally, the tailings from gold extraction are a major source of heavy metals in water. The chemical products are used during the separation of gold and operation of excavating machines (excavators and heavy-duty trucks). The correlation matrix for the parameters determined in the sediment samples is presented in Table 7.
In Table 7, a high correlation was observed between pH/OM (0.770). Cd had average correlations with Fe (0.742) and Mn (0.739). The high correlations observed indicate that these parameters follow the same direction. High correlation was also observed between As/Hg (0.827). The significant correlations observed between these parameters suggest that they follow a common direction. The results of PCA analysis are presented in Fig. 7.
One important aspect of the current study was to conduct information on their evolution in the same or in the opposite direction for water and sediment variables in the study area. PCA, as an extension of the correlation analysis, was also used to distinguish and verify the same or opposite direction of variables of water and sediment samples. In Fig. 7a, two main groups were observed. The first group was composed of pH, Tu, Ca2+ and NO3−. The presence of pH and Tu indicated that they the follow the same direction. High correlation was observed between these two parameters (see Table 6). The pH of this water could be controlled by cloudy state of this water. The second group or component was associated with EC, SS, Na+, Cl− and SO42−. The dominate ions in seawater are Na+ and Cl− both of them are very active. Electrical conductivity and total dissolved solids are normally used as water quality parameters, especially in the seawater. These two parameters are indicators of salinity level, which make these parameters very useful in studying seawater invasion (Rusydi 2018). Matter present in the dissolved form consists of inorganic salts and organic matter which is represented in the form of total dissolved solids (Marandi et al. 2013) and EC is the measure of liquid capacity to conduct an electric charge (Daniels et al. 2016). Figure 7b represents the PCA analysis of heavy metals in waters. Two groups were observed. The first group was associated with Hg, Fe and Cd. A significant correlation was observed between Fe/Cd (0.823) suggesting common direction that could be derived from lithogenic sources. In a river, the transfer of metal from water to sediment or vice versa is a geochemical process involving the process of adsorption of metal compounds by the surface of suspended particles. This transfer as well as the movement of water involving physical factors (depth and width) and hydrology (flow velocity, water discharge, and level of substratum resistance) of the river (Palapa and Maramis 2018). The second group was composed of Mn and As (0.626). In Fig. 7c, the PCA analysis of physicochemical parameters of sediments was presented. Three groups were observed. The first was composed of Fe, Mn and Cd. The significant correlation was observed between Fe/Cd (0.742). This reinforces the fact that these two metals follow the same direction as observed in Table 7. The second group was composed of As and Hg. The third group was associated with pH and OM (0.770). The pH and OM of the samples were mostly low and could be the cause of the release of trace metals. The result of HCA analysis is presented in Fig. 8.
The results of hierarchical cluster analysis are represented as a dendrogram (Fig. 8a and b). Fig. 8a represents the HCA of water samples. This dendrogram was subdivided into two groups (clusters). The first cluster (cluster A) was composed of samples taken after and in the middle of mining site. This cluster was subdivided into many semi-groups. They were observed that samples taken after the mining sites and those taken in mining activity were associated. This could indicate that pollution increases as moving on the site. The cluster B was composed of downstream samples (WD1 and WD3). This cluster was highly polluted compared to cluster A. Highest values of parameters analyzed such as Tu, SS, Ni, Cd, Fe, Mn, As and Hg were observed. All these values were above the limits recommended by the WHO (WHO 2011). Highest values of Tu and SS were due to extraction and excavation works for the samples taken in the middle and downstream of the mining site. The presence of these metals in water samples may be directly linked to the gold processing activities (Aranguren et al. 2008) and the dissolution of some minerals such as olivine, serpentine for Ni; sphalerite for Cd; pyrite or pyrrhotite for Fe and arsenopyrite for As.
In Fig. 8b the HCA of sediment samples was subdivided into two clusters. Cluster A was composed of downstream sample (SAV03). Highest concentrations of trace metals were observed in this sample. Cluster B was subdivided into B1 and B2. Cluster B1 represented some samples taken after and in the middle of site. This cluster represented the samples, which had the lowest concentrations in trace metals. The cluster B2 illustrated in green (see Fig. 8b) showed that some of middle and downstream samples had average concentrations.
Estimation of the intensity of the contamination
The intensity of the contamination was estimated by the determination of the enrichment factor and the geo-accumulation index. This is a contribution compared to the study carried out in Djouzami (Ayiwouo et al. 2020) in the same region, where these two factors were not calculated. The enrichment factor is determined for all the heavy metals studied (Ni, Cd, As and Hg) except for iron because it is used as a reference metal. The analysis of the sediment samples informed the geochemical background of the sampling points. Enrichment factor results are presented in Fig. 9.
An enrichment factor between 0.5 and 2 is considered as a natural enrichment, while above 2 it indicates an anthropic enrichment (Mencio et al. 2016). For all the sampling points, the EF of the different metals was not superior to two. A minimal enrichment was observed for all the sampling points. The geo-accumulation index is determined for Ni, Cd, As, Hg in all the sampling points. The results of the calculated geo-accumulation index are presented in Fig. 10.
The Igeo analysis showed uncontaminated to moderately contaminated (0.10–0.14) for Ni; moderately contaminated to extremely contaminated (2.29–7.45) for Cd with extreme contamination in the middle of the mining area and downstream. A moderately contamination to heavily contamination was observed for As (1.25–3.40) and Hg (1–3.14).
Water treatment by Moringa oleifera seeds
The results of the physical parameters of water before and after treatment are shown in Table 8.
In Table 8, the addition of the coagulant did not have a great influence on the pH changes. 54% of the electrical conductivity, 80% of turbidity and 94% of suspended solids were reduced. The results of the heavy metal concentration of water before and after treatment are shown in Table 9.
In Table 9, in addition to the Moringa oleifera powder, 91.66, 92.30 and 24.48% of Cd (II), Hg (II) and Fe (II) were eliminated. The adsorption capacities were 2.4, 2.6 and 16.6 mg g−1, respectively. The mechanism of interaction between the coagulant dimeric proteins and heavy metals was ion adsorption and charge neutralization. The coagulant has been termed as potential heavy metal removing agent due to its oxygen- and nitrogen-donating carboxylate and amino groups (Okuda et al. 1999). The mechanism that brings about adsorption of heavy metals is through the positive metal ions that forms a bridge among the anionic polyelectrolyte and negatively charged protein functional groups on the colloidal particle surface. There is formation of complexes with the heavy metals and the organic matter of coagulant seeds such as proteins. Due to hydrophilic character, several hydrogen bonds are formed among polyelectrolyte and water molecules. Polyelectrolyte coagulant aid has structures consisting of repeating units of small molecular weight, forming molecules of colloidal size that carry electrical charges or ionizable groups that provide bonding surface for the flocs. Metal ions in coagulation react with proteins and destroy them in water. Metal adsorption occurs due to the high protein content of the seeds.
Conclusion
This paper evaluated the impact of the semi-mechanized gold mining exploitation of Wakaso (Adamawa Cameroon) on waters and sediments of Lom River and the capacity of Moringa oleifera seeds to remove pollutants from wastewater. The evaluation of the quality of the waters and sediments revealed that these waters were slightly acidic to basic, weakly conductive, cloudy, bicarbonate calcium-magnesium and polluted by heavy metals such as Ni, Cd, Fe, Mn, As and Hg. The sediments were acidic to neutral and polluted by Cd and Hg. Finally, the restorative effect of Moringa oleifera seeds was studied. In contact of this coagulant, 54% of the electrical conductivity, 80% of turbidity and 94% of suspended solids were reduced. The maximum percentage removal of 91.66, 92.30 and 24.48 was observed for Cd (II), Hg (II) and Fe (II), respectively. It appeared that Moringa oleifera seeds were sufficient for the removal of cadmium and mercury contained in polluted waters. In the future works, it will interesting be to (i) Study the spatial variations in other metals as Pb and Cr; (ii) Characterize the water and sediment samples in dry season; (iii) Study the mechanism of adsorption and (iv) Use several ranges of coagulant to see the efficient range.
References
Acheampong MA, Adiyiah J, Ansa EDO (2013) Physico-chemical characteristics of a Gold Mining Tailings Dam wastewater. J Environ Sci Eng 2:469–475
Agarwal GS, Bhuptawat HK, Chaudhari S (2006) Biosorption of aqueous chromium (VI) by Tamarindus indica seeds. Biores Technol 97:949–956
Aranguren SMM, Probst A, Roulet M, Isaure MP (2008) Contamination of surface waters by mining wastes in the Milluni Valley (Cordillera Real, Bolivia): mineralogical and hydrological influences. Appl Geochem 23:1299–1324
Asamoah E, (2012) The impact of small scale gold mining activities on the water quality of River Birim in the Kibi traditional area. Unpublished Master’s Thesis. Kwame Nkrumah University of Science and Technology, Kumasi Ghana
Asare-Donkor NK, Ofosu JO, Adimado AA (2018) Hydrochemical characteristics of surface water and ecological risk assessment of sediments from settlements within the Birim River basin in Ghana. Environ Syst Res 7:1–17
Ashrith KA, Sibi G (2019) Sorption of heavy metals from aqueous solutions by Moringa biomass. Austin Environ Sci 4(1):1033
Ayiwouo NM, Mambou LLN, Mache JR, Kingni ST, Ngounouno I (2020) Waters of the Djouzami gold mining site (Adamawa, Cameroon): physicochemical characterization and treatment test by Bana smectite (West, Cameroon). Case Stud Chem Environ Eng. https://doi.org/10.1016/j.cscee.2020.100016
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A Review of potentially low- cost sorbent for heavy metals. Water Resour 33:2469–2479
Balan IN, Shivakumar M (2012) An assessment of groundwater quality using water quality index in Chennai, Tamil Nadu, India. Chron Young Sci 3(2):146
Banunle A, Fei-Baffoe B, Otchere KG (2018) Determination of the physico-chemical properties and heavy metal status of the Tano River along the catchment of the Ahafo mine in the Brong-Ahafo region of Ghana. J Environ Anal Toxicol 8:574
Bekkoussa B, Jourde H, Batiot-Guilhe C, Meddi M, Khaldi A, Azzaz H (2013) Origine de la salinité et des principaux éléments majeurs des eaux de la nappe phréatique de la plaine de Ghriss, Nord-Ouest Algérien. Hydrol Sci J 5:1111–1127
Blaser P, Zimmermann S, Luster J, Shotyk W (2000) Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci Total Environ 249:257–280
Butaré I, Keita S, (2010) Aspects environnementaux liés au développement du secteur minier en Afrique de l’Ouest (Rapport technique). Dakar, Sénégal: Centre de recherches pour le développement international (CRDI)
Chiffoleau JF, Claisse D, Cossa D, Ficht A, Gonzalez JL, Guyot T, Michel P, Miramand P, Oger C, Petit F, (2001). La contamination métallique. Programme scientifique Seine Aval
Damy P-C, (2011) Synthèse des connaissances sur l’origine et la disponibilité du cadmium dans les eaux continentales. Onema/Agences de l’eau et l’OIEau. 39
Daniels WL, Zipper CE, Orndorff ZW, Skousen J, Barton CD, McDonald LM, Beck MA (2016) Predicting total dissolved solids release from central Appalachian coalmine spoils. Environ Pollut 216:371–379
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Etoh MA, Dina DJD, Ngomo HM, Ketcha JM (2015) Adsorption of Pb2+ Ions on two clays: Smectite and kaolin the role of their textural and some physicochemical properties. Int J Appl Res 1:793–803
Fay M, Wilbur S, Abadin H, Ingerman L, Swarts SG, (2005) Toxicological Profile for Nickel, U.S. Department of Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry (ATSDR)
Goering PL, Waalkes MP, Klaassen CD (1995) Toxicology of cadmium. In: Goyer RA, Cherian MG (eds) Toxicology of metals. Springer, Berlin, pp 189–214
Goix S, Maurice L, Laffont L, Rinaldo R, Lagane C, Chmeleff J, Menges J, Heimbürger LE, Maury-Brachet R, Sonke JE (2019) Quantifying the impacts of artisanal gold mining on a tropical river system using mercury isotopes. Chemosphere 219:684–694
Hu S, Cong L, Chengjun Z, Yuanjing Z, Hairui Y, Yaoguo W (2016) Effects of fresh and degraded dissolved organic matter derived from maize straw on copper sorption onto farmland loess. J Soils Sediments 16(2):327–338
Hu S, Yaoguo W, Na Y, Yuanjing Z, Xu X (2017) Chemical properties of dissolved organic matter derived from sugarcane rind and the impacts on copper adsorption onto red soil. Environ Sci Pollut Res 24(27):21750–21760
Jasmin I, Mallikarjuna P (2014) Physicochemical quality evaluation of groundwater and development of drinking water quality index for Araniar River Basin, Tamil Nadu, India. Environ Monit Assess 186:935–948
Jolliffe I (2002) Principal component analysis. Wiley, Amsterdam
Kaizer AN, Osakwe SA (2010) Physicochemical characteristics and heavy metal levels in water samples from five river systems in Delta state, Nigeria. J Appl Sci Environ Manag 14(1):83–87
Lottermoser B (2010) Mine waste: characterization, treatment and impact. Springer, New York, p 40
Malik RN, Nadeem M (2011) Spatial and temporal characterization of trace elements and nutrients in the Rawal Lake Reservoir, Pakistan using multivariate analysis techniques. Environ Geochem Health 33:525–541
Mambou NLL, Mache JR, Ayiwouo NM, Takougang KS, Abende SRY, Roukaiyatou S (2020a) Physicochemical characterization of mining waste from the Betare-Oya gold area (East Cameroon) and an adsorption test by Sabga smectite (North-West Cameroon). Scientifica 2020:12
Mambou NLL, Takougang SK, Ayiwouo NM, Ndi AA (2020b) Impact of gold mining exploitation on the physicochemical quality of water: case of Batouri (Cameroon). Int J Energy Water Resour. https://doi.org/10.1007/s42108-020-00106-0
Marandi A, Polikarpus M, Jõeleht A (2013) A new approach for describing the relationship between electrical conductivity and major anion concentration in natural waters. Appl Geochem 38:103–109
Menció A, Guasch H, Soler D, Canelles A, Zamorano M, Brusi D (2016) Influence of regional hydrogeological systems at a local scale: analyzing the coupled effects of hydrochemistry and biological activity in a Fe and CO2 rich spring. Sci Total Environ 569–570:700–715
Mining code, (2016) Loi n°2016‐17 du 14 décembre (2016) portant Code minier
Mitchell JW (2009) An assessment of lead mine pollution using macro-invertebrates at Greenside Mines, Glenridding. Earth Environ 4:27–57
Müller G (1979) Schwermetalle in den sedimenten des RheinseVeranderungen seitt 1971. Umschau 79:778–783
Müller G (1981) Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflusse: eine Bestandsaufnahme. Chem Ztg 105:157–164
Nollet ML (2007) Hand Book of water analysis, 2nd edn. CRC Press, Taylor & Francis Group, London New York, p 786
Okuda T, Baes AU, Nishijima W, Okada M (1999) Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Resour 33:3373–3378
Olivry JC, (1986) Fleuves et rivières du Cameroun. Monographies Hydrologiques ORSTOM, ORSTOM, Marseille, France
Omar HEDM (2013) Seasonal variation of heavy metals accumulation in muscles of the African Catfish Clarias gariepinus and in River Nile water and sediments at Assiut Governorate. Egypt J Biol Earth Sci 3(2):236
Pahimi H, Panda CR, Ngassoum MB, Tchameni R (2015) environmental impacts of mining in the volcano-sedimentary basins of Cameroon: case study of artisanal gold mine tailings (Betare Oya, EastCameroon). Int J Energy Environ Eng 2:5–15
Palapa MT, Maramis AA, (2018) The relationship between mercury in water and sediment with physicochemicals of water of stream near an amalgamation and cyanidation unit of Talawaan-Tatelu traditional gold mine, North Sulawesi. In: The 3rd International Seminar on Chemistry
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Quevauviller P (1998) Operationally defined extraction procedures for soil and sediment analysis I. Stand Trac Trends Anal Chem 17:289–298
Rakotondrabe F, Ngoupayou JRN, Mfonka Z, Rasolomana EH, Abolo AJN, Asone BL, Ako AA, Rakotondrabe MH (2017) Assessment of surface water quality of Bétaré-Oya gold mining area (East-Cameroon). J Water Resour Prot 9:960–984
Rusydi AF (2018) Correlation between conductivity and total dissolved solid in various type of water: a review. IOP Conf Ser Earth Environ Sci 118(1):012019
Schoeller H (1965). Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigation and development. Water Research, Series-33. UNESCO, Delft, pp. 54–83
Suchel JB, (1987) Les Climats du Cameroun. Tome III [Ph.D. thesis], Université de St Etienne, St. Etienne, France
Taylor SR, McLennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33(2):241–265
Tiwari AK, Singh AK, Singh MP (2017) Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district, Uttar Pradesh, India. Appl Water Sci 7(4):1609–1623
Wedepohl HK (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
WHO (2011) World health organization guidelines for drinking-water quality. Recommandations. 4th Edition, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. U.S Department of Agriculture, Washington, DC
Yongming H, Peixuan D, Junji C, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Cent, China. Sci Total Environ 355:176–186
Acknowledgements
The authors would like to thank the laboratory of Hydrobiology and Environment laboratory of the University of Yaounde I (Cameroon), the promotion of local materials ‘’(MIPROMALO)’’ and the Framework of Support for the Promotion of Mining Handicrafts ‘’(CAPAM)’’ for their support during the analysis works.
Funding
No funding and no organisation supported the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngounouno, M.A., Ngueyep, L.L.M., Kingni, S.T. et al. Evaluation of the impact of gold mining activities on the waters and sediments of Lom River, Wakaso, Cameroon and the restorative effect of Moringa Oleifera seeds. Appl Water Sci 11, 113 (2021). https://doi.org/10.1007/s13201-021-01445-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01445-x