Abstract
The canteen and laboratory of every academic organization need a lot of clean water, and it generates equivalent amount of wastewater every hour which is neither purified nor reused. Due to water scarcity, the recycling and reusing of wastewater become very essential. The present study describes the simple and cost-effective method for the design of a small-scale wastewater treatment plant for the purification of wastewater generated by household, canteen and laboratory of an academic institute. The current study explored the process of phytoremediation by Typha latifolia L. and Canna indica L. for removal of metal ions and phosphate ions from the wastewater. The partially treated water after phytoremediation was further purified by sand filtration. The various water quality parameters (pH, hardness, dissolved oxygen, chemical oxygen demand, turbidity, total dissolved solids and metal ions) of the treated and untreated water were analyzed. It was observed that there are significant reduction in hardness, turbidity and chemical oxygen demand and increase in dissolved oxygen value. The treated water can be reused for various household works and agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a transparent liquid, covers approximately 75% of earth surface and is vital for all living forms of life. For survival every living being should have access to sufficient amount of clean water. The word “water scarcity” describes the relationship between demand for water and its availability. Water scarcity is rapidly becoming a major problem for many developing countries which means shortage of enough water (quantity) and lack of access of safe water (quality) (Alcamo et al. 2003; Alcamo et al. 2007). With growing demand for water and depletion of the available water, assured supply of good quality water is becoming a major concern (Alonso-Castro et al. 2009; Anning et al. 2013). The population growth coupled with industrialization and urbanization has resulted in an ever-increasing demand for water (Belinda et al. 2007). There exist numerous high-technology systems to purify wastewater. But for a huge proportion of population in the developing world that lives in the rural areas, such systems would be inappropriate or too expensive (Biswas 2004; Bose et al. 2008; Carranza-Álvarez et al. 2008). The current study describes the process of phytoremediation coupled with sand filter for the purification of wastewater generated from kitchen, canteen and chemistry laboratory of an academic institution. Phytoremediation is the process of purification of wastewater by the use of green plants which include grasses, herbs and woody species (Clark et al. 2012; Demirezen and Aksoy 2004). In the past few decades, phytoremediation has become an important and eco-friendly technique for the removal of various environmental contaminants from soil and water. Plants are known to play very important role in removal of toxic heavy metal ions from wastewater (Dimaano 2015; Dushenkov et al. 1995). They accumulate heavy metal ions in roots, rhizomes and old leaves (Fediuc and Erdei 2002). Various processes (phytoextraction, rhizofiltration, phytodegradation and phytovolatilization) have been described in literature by which plants remove the toxic substances and metal ions from wastewater (Gleick 2000; Gottinger et al. 2011). Among these, phytoextraction and rhizofiltration are the most accepted mechanisms for the removal of heavy metal ions form wastewater. In phytoextraction, impurities are accumulated in shoots and leaves (Gratão et al. 2005; Gupta and Sinha 2007), while in rhizofiltration impurities are accumulated in the massive root system of the plant (Hale and Melia 1913).
The purification of wastewater by sand filtration is very well-known and cost-effective method. Sand filter can be easily fabricated by using sand which is supported by gravels of various sizes. The height of the filter, water flow rate, size of gravels and thickness of sand are very important parameters for designing the sand filtration unit (Hollender et al. 2009). The purification of water by sand filter is largely based on biological process. Microorganisms accumulate and multiply on the filter media and degrade the waste material present in the water. As the water percolated downwards, impurities are strained out by the filter media resulted in the purification of wastewater (Kamal et al. 2004). The aim of the present work is to purify the wastewater generated by the from kitchen, canteen and chemistry laboratory of an academic institution by combining the process of phytoremediation and sand filtration.
Experimental methods
Standard methods have been used for the determination of various water quality parameters; a brief outline of the methods has been given here. Turbidity, pH and total dissolved solids (TDS) were measured by turbidity meter, pH meter and TDS meter, respectively. Total hardness of water was estimated by titrating it with EDTA salt (disodium salt of ethylene diamine tetraacetic acid) solution using Eriochrome Black T as an indicator. Dissolved oxygen and chemical oxygen demand were determined by modified Winkler’s method and open refluxed method, respectively (Kumar et al. 2003). The detailed procedure for estimation of total hardness, dissolved oxygen and chemical oxygen demand has been given in supporting information.
Collection of experimental plant
Two plants, namely Typha latifolia L. (cattail) and Canna indica L. (keli), were collected from the main drainage water channel at Yamuna river bank, India.
Fabrication and working of filtration plant
The tank used for phytoremediation is made by a plastic container with a diameter of 40 cm and height of 46 cm. The plant was grown to the depth of 35 cm using garden soil. The sand filtration unit is very simple in design and operation (Lasat 2002). It consists of a plastic bucket of height 35 cm and diameter 32 cm. The water-carrying capacity of the bucket is 25 L. The retention time in sand filter varies from 5 to 48 h. The flow rate of the water is 15 L/h. It is filled with various layers of gravel (different diameter) and thick sand. The topmost layer of the sand filter consists of thick sand, and the second layer consists of small-sized gravel having diameter 2–4 mm. The third and fourth layer is composed of medium- and large-sized gravel having diameter 6–8 mm and 12–14 mm, respectively. Figure 1 depicts the (a) experimental setup for phytoremediation, (b) design of water purification plant, (c) various gravel layers used for fabrication of sand filter and (d) sand filtration unit. For the removal of metal ions and phosphate ion from the wastewater, the process of phytoremediation using T. latifolia and C. indica has been demonstrated. The partially purified water is further purified by passing it through sand filter prepared by arranging various layers of gravels and sand one over the other (Li Fangyue and Wichmann 2009; Logsdon et al. 2002; Manios et al. 2003). The water is allowed to pass through the filter at a very slow rate, and as water slowly percolates through a bed of carefully arranged sand medium, almost all the suspended and colloidal material is trapped by the top layers of sand. (Marchiol et al. 2004) Clear, filtered water is collected at the bottom of the filter medium. The water quality parameters were checked before and after purification of water. The water quality parameters (color, pH, hardness, total dissolved solid (TDS), dissolved oxygen (DO) and chemical oxygen demand (COD) and turbidity) of the treated and untreated water have been estimated by literature known procedures (Maurer et al. 2005; Muthusaravanan et al. 2018).
Results and discussion
The wastewater generated from academic laboratory is generally composed of organic matter, wide range of chemicals and heavy metals and is most difficult to purify. On the other hand, wastewater produced from canteen or house is highly turbid and contains phosphate ion from dish washing soap and detergent as the major impurity. T. latifolia and C. indica plant has been used for the removal of heavy metal contaminant from the wastewater generated from laboratory and canteen/house respectively (Peter et al. 2012; Prasad et al. 2006). T. latifolia can grow in water contaminated by heavy metals, and it gathers metal ions in its tissue (Sasmaz et al. 2008; Seckler et al. 1999). The wastewater was kept in well-grown plants species, and effluent water was checked for removal of zinc (Zn), cadmium (Cd) and lead (Pb) metal. The reduction in phosphate ions is due to process of mineralization in which soil microorganisms convert organic phosphorous into orthophosphates which can be easily taken up by plants for their growth (Silva et al. 2015; Smakhtin et al. 2004). The procedure for the qualitative detection of metal ions, phosphate ion and various water quality parameters has been given in supporting information. After phytoremediation, the treated water was admitted to sand filter. Slow sand filters are known to substantially remove suspended solids, turbidity and microorganism from wastewater (Suresh and Ravishankar 2004; Taghizadeh et al. 2007).
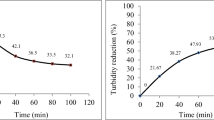
The partially treated water was allowed to enter into sand filter from where it flowed downwards under the action of gravity. As water drains downward, purification occurs by sedimentation where particles settled down by gravitational forces (Vardanyan and Ingole 2006). Sand bed (composed of sand and gravel of different diameter) acts as effective strainer that retained suspended particles and other impurities; those are larger than the interstices between the sand filter (Vogel 1987). Contaminants are physically and chemically adsorbed on sand bed by various electrostatic, van der Waals and chemical interactions (Vogel 1989; Wang et al., 2002). Table 1 shows the value of various water quality parameters for untreated and treated water. Qualitative test for detection of Zn, Cd, Pb and phosphate ion has been done, and it was found that treated water does not have any of these metals. The reduction in metal ions is due to uptake by plants to meet growth requirement (Smakhtin et al. 2004). The percentage reduction in TDS and turbidity of canteen water and laboratory water is 77, 91 and 47, 99.8, respectively. The decrease in TDS and turbidity is due to a decrease in suspended and dissolved solids (Weber-Shirk and Dick 1997; Wotton 2002). The percentage reduction in COD for canteen and laboratory water is 75 and 88, respectively. The appreciable decrease in COD value is due to consumption of organic matter by microorganisms. Microorganisms get food from organic matter present in wastewater and in turn release oxygen which increases the dissolved oxygen content of the water. The significant improvement in various water quality parameters is graphically shown in Fig. 2. The treated water can be reused for various purposes like cleaning, gardening, agriculture, etc.
The purification of wastewater has been done by using sand filtration alone. It was observed that without phytoremediation, there is no appreciable removal of metal ions. On the other hand, there is sufficient improvement in TDS, turbidity and COD values. These results show that plants play important role in removal of metal ions from wastewater. Quantitative estimation of metal ions and various disinfection methods are currently underway so that the treated water can be used for drinking purposes as well.
Conclusions
A simple and cost-effective water treatment plant was built whose construction, operation and maintenance are easy. It can be easily implemented in rural areas because it does not need electricity for its operation. In the nut shell, this paper provides an overview of purification of wastewater by combining the technique of phytoremediation and sand filtration. The initial values of the various water quality parameters are quite high which indicate the high impurity level in untreated water generated from chemistry laboratory and canteen. There is substantial improvement in all the water quality parameters after treatment. The present method provides an eco-friendly and cost-effective small-scale water treatment plant.
References
Alcamo J, Döll P, Henrichs T, Kaspar F, Lehner B, Rösch T, Siebert S (2003) Development and testing of the WaterGAP 2 global model of water use and availability. Hydrol Sci J 48:317–337. https://doi.org/10.1623/hysj.48.3.317.45290
Alcamo J, Flörke M, Märker M (2007) Future long-term changes in global water resources driven by socio-economic and climatic changes. Hydrol Sci J 52:247–275. https://doi.org/10.1623/hysj.52.2.247
Alonso-Castro A, Carranza Alvarez C, Alfaro C, Chávez-Guerrero L, Fernando García-De la Cruz la Cruz R (2009) Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch Environ Contam Toxicol 57:688–696. https://doi.org/10.1007/s00244-009-9351-6
Anning AK, Korsah PE, Addo-Fordjour P (2013) Phytoremediation of wastewater with Limnocharis flava, Thalia geniculata and Typha latifolia in constructed wetlands. Int J Phytoremed 15:452–464. https://doi.org/10.1080/15226514.2012.716098
Belinda EH, Tim DF, Ana D (2007) Treatment performance of gravel filter media: implications for design and application of stormwater infiltration systems. Water Res 41:2513–2524. https://doi.org/10.1016/j.watres.2007.03.014
Biswas AK (2004) Integrated water resources management: a reassessment. Water Int 29:248–256. https://doi.org/10.1080/02508060408691775
Bose S, Jain A, Rai V, Ramanathan AL (2008) Chemical fractionation and translocation of heavy metals in Canna indica L. grown on industrial waste amended soil. J Hazard Mater 160:187–193. https://doi.org/10.1016/j.jhazmat.2008.02.119
Carranza-Álvarez C, Alonso-Castro AJ, Alfaro-De La Torre MC, García-De La Cruz RF (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial Lagoon in San Luis Potosí, Mexico. Water Air Soil Pollut 188:297–309. https://doi.org/10.1007/s11270-007-9545-3
Clark PA, Pinedo CA, Fadus M, Capuzzi S (2012) Slow-sand water filter: design, implementation, accessibility and sustainability in developing countries. Med Sci Monit 18:105–117
Demirezen D, Aksoy A (2004) Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) Living in Sultan Marsh (Kayseri, Turkey). Chemosphere 56:685–696. https://doi.org/10.1016/j.chemosphere.2004.04.011
Dimaano I (2015) Effort in reducing unaccountable water and economic consideration. Water Pract Technol 10:50–58. https://doi.org/10.2166/wpt.2015.007
Dushenkov V, Kumar PBAN, Motto H, Raskin I (1995) Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol 29:1239–1245. https://doi.org/10.1021/es00005a015
Fediuc E, Erdei L (2002) Physiological and biochemical aspects of cadmium toxicity and protective mechanisms induced in Phragmites australis and Typha latifolia. J Plant Physiol 159:265–271. https://doi.org/10.1016/j.ecoleng.2008.05.006
Gleick PH (2000) A look at twenty-first century water resources development. Water Int 25:127–138. https://doi.org/10.1080/02508060008686804
Gottinger AM, McMartin DW, Price D, Hanson B (2011) The effectiveness of slow sand filters to treat Canadian rural prairie water. Can J Civ Eng 38:455–463. https://doi.org/10.1139/l11-018
Gratão PL, Prasad MNV, Cardoso PF, Lea PJ, Azevedo RA (2005) Phytoremediation: green technology for the cleanup of toxic metals in the environment. Braz J Plant Physiol 17:53–64. https://doi.org/10.1590/S1677-04202005000100005
Gupta AK, Sinha S (2007) Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour Technol 98:1788–1794. https://doi.org/10.1016/j.biortech.2006.06.028
Hale FE, Melia TW (1913) Winkler’s method for the determination of oxygen in water; the effect of nitrite and its prevention. J Ind Eng Chem 5:976–980. https://doi.org/10.1021/ie50060a006
Hollender JSG, Zimmermann S, Koepke M, Krauss C, McArdell S, Ort C, Singer H, von Gunten URS, Siegrist H (2009) Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ Sci Technol 43:7862–7869. https://doi.org/10.1021/es9014629
Kamal M, Ghaly AE, Mahmoud N, Côté R (2004) Phytoaccumulation of heavy metals by aquatic plants. Environ Int 29:1029–1039. https://doi.org/10.1016/S0160-4120(03)00091-6
Kumar A, Singhal V, Joshi BD, Rai JPN (2003) Lysimetric approach for ground water pollution control from pulp and paper mill effluent using different soil textures. Ind J Sci Ind Res 63:429–438
Lasat MM (2002) Phytoextraction of toxic metals. J Environ Qual 31:109–120. https://doi.org/10.2134/jeq2002.1090
Li Fangyue K, Wichmann RO (2009) Review of the technological approaches for grey water treatment and reuses. Sci Total Environ 407:3439–3449. https://doi.org/10.1016/j.scitotenv.2009.02.004
Logsdon GS, Kohne R, Abel S, LaBonde S (2002) Slow sand filtration for small water systems. J Environ Eng Sci 1:339–348. https://doi.org/10.1139/s02-025
Manios T, Stentiford EI, Millner PA (2003) The effect of heavy metals accumulation on the chlorophyll concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metaliferus water. Ecol Eng 20:65–74. https://doi.org/10.1016/S0925-8574(03)00004-1
Marchiol L, Assolari S, Sacco P, Zerbi G (2004) Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut 132:21–27. https://doi.org/10.1016/j.envpol.2004.04.001
Maurer M, Rothenberger D, Larsen TA (2005) Decentralised wastewater treatment technologies from a national perspective: at what cost are they competitive? Water Sci Technol Water Supply 5:145–154. https://doi.org/10.2166/ws.2005.0059
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Gayathri V, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359. https://doi.org/10.1007/s10311-018-0762-3
Peter AC, Catalina AP, Matthew F, Stephen C (2012) Slow-sand water filter: design, implementation, accessibility and sustainability in developing countries. Med Sci Monit 18:105–117
Prasad G, Rajput R, Chopra AK (2006) Sand intermittent filtration technology for safer domestic sewage treatment. J Appl Sci Environ Manag 10:73–77. https://doi.org/10.4314/jasem.v10i1.17308
Sasmaz A, Obek E, Hasar H (2008) The accumulation of heavy metals in Typha latifolia L. grown in a stream carrying secondary effluent. Ecol Eng 33:278–284. https://doi.org/10.1016/j.ecoleng.2008.05.006
Seckler D, Barker R, Amarasinghe U (1999) Water scarcity in the twenty-first century. Int J Water Resour Dev 15:29–42. https://doi.org/10.1080/07900629948916
Silva A, Yogafanny E, Fuchs S (2015) Intermittent slow sand filtration for drinking water treatment in developing countries
Smakhtin V, Revenga C, Döll P (2004) A pilot global assessment of environmental water requirements and scarcity. Water Int 29:307–317. https://doi.org/10.1080/02508060408691785
Suresh B, Ravishankar GA (2004) Phytoremediation—a novel and promising approach for environmental clean-up. Crit Rev Biotechnol 24:97–124. https://doi.org/10.1080/07388550490493627
Taghizadeh MM, Torabian A, Borghei M, Hassani AH (2007) Feasibility study of water purification using vertical porous concrete filter. Int J Environ Sci Technol 4:505–512. https://doi.org/10.1007/BF03325987
Vardanyan LG, Ingole BS (2006) Studies on heavy metal accumulation in aquatic macrophytes from Sevan (Armenia) and Carambolim (India) lake systems. Mar Pollut Ecotoxicol 32:208–218. https://doi.org/10.1016/j.envint.2005.08.013
Vogel AI (1987) Vogel’s qualitative inorganic analysis, 6th edn. Longman Scientific and Technical, Wiley, New York
Vogel AI (1989) Vogel’s textbook of quantitative chemical analysis, 5th edn. Longman Scientific and Technical, Wiley, New York
Wang Q, Y Cui, Y Dong (2002) Phytoremediation of polluted waters potentials and prospects of wetland plants. Acta Biotechnol22:199–208. https://doi.org/10.1002/1521-3846(200205)22:1/2<199::AID-ABIO199>3.0.CO;2-T
Weber-Shirk ML, Dick RI (1997) Physical–chemical mechanisms in slow sand filters. J Am Water Works Assoc 89:87–100. https://doi.org/10.1002/j.1551-8833.1997.tb08164.x
Wotton R (2002) Water purification using sand. Hydrobiologia 469:193–201. https://doi.org/10.1023/A:1015503005899
Funding
Partial financial support was received from Gargi College, University of Delhi, India, under DBT-Star College Scheme, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saini, G., Kalra, S. & Kaur, U. The purification of wastewater on a small scale by using plants and sand filter. Appl Water Sci 11, 68 (2021). https://doi.org/10.1007/s13201-021-01406-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01406-4