Abstract
In wastewater treatment field, physicochemical techniques containing conservative substance such as metal or substance that are non-biodegradable are typically employed. However, these techniques have their own drawbacks and none of them is universal in treating all kinds of metal present in the wastewater. Separation procedure is known to be very suitable for the separation and recovery of metal ions. The solvent extraction or liquid–liquid extraction, LLE, was opted in this study to identify the influencing factors on the extraction of cadmium(II) and copper(II) ions from aqueous solution by using soybean-based oil as organic solvent. The experiment was approached using fractional factorial design to determine the significance of six factors which are concentration of di-2-ethylhexylphosphoric acid (D2EHPA), concentration of tributyl phosphate (TBP), shaking time, the concentration of Na2SO4, O/A phase ratio and aqueous pH. The results have shown that %E shows that Cu(II) was obtained in the range from 35.77 to 91.81%, while the result obtained from the factorial plot of screening experiment shows that the interaction effect AB (shaking time × [D2EHPA]), CE ([TBP] × O/A) and DF ([Na2SO4] × pH) influences significantly the Cu(II) %E. Meanwhile, %E for a sample containing Cd(II) ion was obtained in a range from 54.77 to 87.35%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is one of the most critical issues since early 1960’s due to the rapid industrialization activity worldwide. Direct emission of industrial wastewater containing toxic chemical and metal can lead to negative impact toward the environment (Kamaruddin et al. 2018). Besides, untreated discharge of industrial wastewater containing toxic heavy metals into the environment will give dangerous effects toward human health, aquatic lives and other living organism. The most common detected heavy metals in polluted industrial wastewaters are mercury, copper, arsenic, nickel, cadmium and lead.
Water stream may be polluted by two types of sources which are point source and non-point source. The point source or man-made pollution is regarded as the direct discharge from outlets such as effluents outlets or any pipes from industrial factories (Kamaruddin et al. 2017). Non-point source pollutions occur when there is no identifiable point such as surface runoff from urbanization and agricultures area, and it is very difficult to control. Besides, many reports established have found that direct discharge of untreated industrial wastewater to the nearby stream could create water pollution risks. Minamata is one of the disasters that occurred in the year of 1952 resulting from the continuous discharge of wastewater containing mercury into the Minamata Bay, Japan. All the populations nearby Minamata Bay suffered from mercury poisoning caused by consumption of fish polluted with mercury and its impact of bringing over mercury fatality until now (Maruyama et al. 2012). Therefore, the treatment of wastewater containing toxic chemical including heavy metal need in order to prevent adverse impact towards the environmental receptors are needed to be addressed in holistic approach.
In general, wastewater treatment techniques are classified into two categories, namely biological treatment and physiochemical treatment (Kamaruddin et al. 2015). The treatment techniques are selected according to specific wastewater characteristic. The physiochemical techniques are preferable to treat wastewater containing a conservative substance such as metal or substance that is non-biodegradable. The most widely used physicochemical techniques in the industry include ion-exchange, coagulation–flocculation, adsorption and much more. However, these techniques have their own limitation, and none of them is universal in treating broad range of metals present in the wastewater (Kamaruddin et al. 2013).
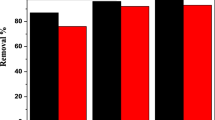
Figure 1 shows typical concentration of heavy metal ion detected in untreated wastewater illustrated in percentage form. The data were at three different manufacturing industries in Malaysia. As mentioned earlier, the copper and cadmium ions are mostly found in wastewater. High concentrations of metal ions are detected in copper plating and electroplating industry. The wastewater characteristic for electroplating industry found from Bernard et al. (2013) research studies while copper plating industry found by Chang et al. (2011) and the textile manufacturing obtained from Halimoon and Yin (2010) study. From the reports, it indicated that copper and cadmium are the most presence in the industrial wastewater.
The liquid–liquid extraction (LLE) or solvent extraction techniques have been recognized as the most promising separation methods between two different immiscible solvents in terms of extraction of the organic and inorganic compound from the aqueous solution at certain pH. LLE gives more advantage in the aspect of toxicological analysis such as the simplest technique, low cost and very useful with any analytical systems. Indeed, this technique consists and involves several main materials such as solvent extraction reagent and an organic solvent. These two components play an importance role in the solvent extraction process (Kamaruddin et al. 2015).
Organic solvent and the solvent extraction reagent play influencing role in the separation of the metal ion. The solvent extraction reagent also known as the carrier will decide the success of the extraction process (Guezzen and Didi 2012). Di-2-ethylhexylphosphoric acid (D2EHPA) is excellent carrier compound and has been used in numerous works. The previous studies conducted by Chang et al. (2010) found that the D2EHPA is effective in the extraction of copper(II) ions. Moreover, Pośpiech and Walkowiak (2010) research study found that the D2EHPA is suitable carrier used for various metal ions extractions. Besides, tributyl phosphate (TBP) is a major extractant and modifier with many applications in hydrometallurgy. From the research obtained by Fatmehsari et al. (2009) observed that by increasing the concentration of tributyl phosphate (TBP) in D2EHPA could improve the separation efficiency. Therefore, the TBP could be used to achieve the high required metal recovery and its separation from solutions containing metals.
In this work, soybean-based oil was used as organic diluent to replace the petroleum derivation. The vegetable oil is non-toxic material and produced from the renewable source. Besides, vegetable oils are readily available commercially. Soybean oil is chosen as diluent compared to others for several reasons. The vegetable oil is sold commercially and can be obtained easily as illustrated in Fig. 2. Besides, Ahmad et al. (2015) claimed and proved that the commercial vegetable oil could be used as diluent.
Major vegetable oil production worldwide in 2015–2016, million metric tonne (Portal 2016)
In the present study, the solvent extraction techniques have been proposed to extract two different toxic heavy metals from the synthetic wastewater. The solvent extraction technique is recognized as simplest and does not require the sophisticated equipment to run the experiments. Besides that, moreover, in real industries, this technique has minimum energy consumption and is suitable for large-scale operations.
Materials and methods
Organic solvent preparation
The organic solvent or diluent was prepared by loading soybean oil with 50 mM and 100 mM of di-2-ethylhexylphosphoric acid(D2EHPA) together with 30 mM and 60 mM of tributyl phosphate (TBP).
Standard solution preparation
An aqueous solution was prepared in 1000 mL of 100 mg/L of Cu with deionized water and loaded in 150 mM and 200 mM. The metal ions were prepared in 100 mg/L because by choosing high concentration stock, it would be much easier to extract rather than used at low concentration.
The aqueous solution containing Cu2+ ions was prepared with 0.4 g of CuSO4 in 21.3 g/L of NaSO4 in deionised water in 1000 mL of volumetric flask, while the aqueous solution containing Cd2+ was prepared with 0.4 g of CdSO4 in 28.41 g/L of Na2SO4 in deionized water. The weight was measured using the analytical balance. A few drop of nitric acid (HNO3) was added as the sample preservative, and the solution is shaken for a few minutes to make it mix uniformly. Figure 3 shows the preparation of the aqueous solution.
The copper standard solution was prepared at 1.0, 3.0, 5.0, 8.0 and 10.0 ppm, respectively. Meanwhile, for cadmium, standard solution was prepared at 0.05, 0.5, 1.0, 1.5 and 2.0 ppm, respectively. These standard solutions were used for flame atomic absorption spectroscopy calibration.
Analytical procedure
The pH value of the samples was measured using Hach Sension 3 meter. The pH variation test was set up with the difference in concentration of di-2-ethylhexylphosphoric acid (D2EHPA) in the ranges of 10, 50,100,150 and 200 mM loaded in soybean oil. A few fixed variables for the pH variation were assigned such as initial aqueous phase of pH 4.70, initial metal concentration of 100 mg/L in 150 mM sodium sulfate, shaking time of 20 min and shaking speed of 150 rpm using the orbital shaker. The pH was measured every 1 min until no change of ΔpH.
For LLE procedure, two stages of procedures involved. The first part involved mixing part, where Erlenmeyer flask (250 mL) was filled with the aqueous phase and the organic phase and shaken using orbital shaker (Certomat, Sartorius, Germany) at 150 rpm and the pH of the aqueous phase was measured before and after mixing. The second part of experiment dealt with settling in which the separating funnel was used for the settling or separation process of the organic and aqueous phase. Figure 3 illustrates the experimental setup for the LLE protocol.
Extraction procedure
The experiment was conducted at room temperature, and the shaking speed was fixed at 150 rpm. The sample condition was developed based on design matrix that has been prepared using Minitab software. The aqueous solution containing Cu(II) was prepared with the pH of 4.0 and 4.5. Sample in the ratio of 1:1 was prepared with 20 mL of organic solution mixed with 20 mL of prepared Cu(II) aqueous solution in the conical flask. Then, the sample in the conical flask was shaken by the orbital shaker at 150 rpm about 5 min.
Statistical approach
The screening procedure was followed once the extraction process has completed. A two-level 26–1 factorial design of experiment was carried out in this study to investigate the effects of six different factors. Minitab software version 16 was used to develop the design matrix and for analysis of results. The model adequacy checking of six factors used in this experiment is shown in Table 1. The values are represented in (− 1 and + 1) which meant for coded values at low (− 1) and high (+ 1) levels of the factors studied, respectively. The design matrix is run random to avoid residual error. A total of 32 samples for each metal were experimentally run to complete the experiment. In addition to this, total sample for both metals was 64.
After mixing process, the sample was left idle for about 3 min until the two layers were formed. Then, 3 mL of sample aqueous solution at bottom layer was taken using the small tube syringe, and pH was measured using the pH meter. There are pH differences to be compared with the initial pH (4.0 and 4.5). The aqueous solution was returned to the conical flask. 1 M of sodium hydroxide, NaOH, and 1 M of sulfuric acid, H2SO4, are used to adjust the pH to get the initial value. Then, the sample was mixed again for 5 min and allowed to settle down.
Similar steps were repeated until initial pH was obtained. After obtaining the pH equivalent to its original value, again the sample was mixed using the orbital shaker for 5 min. The mixture was transferred into the separating funnel for the separation process. Finally, the sample was left until two different layers were completely formed. About 15 mL of the aqueous solution was sampled for the analysis by using flame atomic absorption spectroscopy (FAAS, PinAAcle 500, USA). The filtration of the sample was performed by using nylon syringe filter (Target2™, USA) with 13 mm diameter and filter paper of 0.45 μ pore size. The achievements of extraction process were reported in terms of percentage in extraction. The percentage extraction is calculated as per Eq. 1:
where Mi,aq is the initial metal ion in the aqueous phase and Mf,aq is final concentration of metal ion in the aqueous phase after the extraction process. The analysis of variation (ANOVA) was used in analyzing the statistical data. The design of experiment for LLE includes shaking time, the concentration of D2EHPA, concentration of TBP, pH of feed phase and concentration of the stripping agent.
Results and discussion
pH variation
Tables 2 and 3 show the result of pH obtained after the extraction of aqueous solution containing both copper and cadmium ions. The tables indicated that marginal amount of D2EHPA consumed would result in shorter time for it to reach its initial pH value. Principally, this test was carried out to determine the effect of different concentrations of D2EHPA on the extraction time. In this work, it was found that the pH difference occurs due to the reaction of metal–carrier complex between the D2EHPA carrier and the H+ (Talebi et al. 2015).
Extraction result analysis
Tables 4 and 5 summarize the average percentage extraction, %E, for both metals. A total of 32 samples for each metal ion was conducted in one response under the homogeneous condition in one block measurement. According to Chang et al. (2011), the experiment sequence (Std order) was fixed to randomize in order to reduce effects of independent factors. The normal probability plot of standardized and Pareto chart at 5% significance level with 95% confident level is used to show the significant effect variable. In addition, factorial design was applied by following the same methodology as Khattak et al. (2011).
From Table 4, the %E for copper ions was in the range from 35.77% to 91.81%. The highest value of 91.81% extraction was at run order 20, and the lowest value of %E is 35.77% at run order 18. It was observed that, for example, samples with the run order of 20 with factor [A = 10; B = 100; C = 60; D = 150; E = 1; F = 4.5]. Meanwhile, for run order of 18 with factor [A = 10; B = 50; C = 30; D = 150; E = 1; F = 4.5], were tabulated by the software. From both factors hierarchy, there is a different level of effect for B and C, where factor B represents the concentration of Na2SO4 and factor C represents the concentration of TBP. It implies that high level for both factors results in high %E for copper ions.
Meanwhile, from Table 5, it was observed that %E for sample containing cadmium(II) ion results in highest %E of 87.35% at run order 24 with factors (A = 10; B = 100; C = 30; D = 150; E = 1.5; F = 4.5), while the lowest %E was observed to be 54.77% at run order 2 with factor (A = 10; B = 50; C = 60; D = 150; E = 1; F = 4). From both factors hierarchy, there was different level for effect B, C, D and F. For high %E, it needs to have high level for factors B, E and F together with the low level of factor C and vice versa. It can be concluded that different levels of factors that have been obtained from the extraction of different metal ions were not comparable to each other. In order to investigate the interaction between the factors, factorial plots were plotted and discussed in the next section.
Statistical plots
Normal probability plot for Cu(II)
Figure 4 shows the resulting plot for normal probability against standardized effects labeled for copper(II) ion extraction. Briefly, the normal probability plot of the effects was plotted to determine the magnitude, direction and the importance of the effects between the variables. Accordingly, for normal probability plot of the effects, effects that are further from 0 are statistically significant. The least marginal effects, CE and D, which have an absolute value of 0.05, were plotted at 3.3 and 8% probability. Next is the effects of factor F at 12.6% and so on. Many factors lie near to the straight line and categorized as insignificant effects. Besides, interaction effects CE, DF and AB were deviate obviously away from the straight line indicating significant interactions between variables. The interaction effects AB were represented as shaking time and concentration of D2EHPA, DF is a concentration of sodium sulfate and pH, and CE is a concentration of TBP and organic to aqueous ratio. In conclusion for the copper(II) ion extraction, the interaction between those factors will give the best %E.
Normal probability plot for Cd(II)
Figure 5 shows the plot for normal probability against standardized effects labeled for cadmium ion(II) extraction. The smallest effects, BC and CE, which each have an absolute value of 0.05 are plotted at 3.3 and 7.9% probability. Next, is the AF interaction accounted at 12.6% and the other interactions were as followed: he effects of B, F, and AB-plot at 87.4, 92 and 96.7% were very relatively significant because it distributes away from the straight line. In conclusion, the main effect concentration of D2EHPA, the aqueous pH, and interaction effects of the shaking time and concentration of D2EHPA played an importance role in the best %E achievement.
Pareto chart plot
The Pareto chart was used to display the determination magnitude and important effect on the percentage extraction, %E. The chart also shows the absolute values of the standardized effects from the largest effect to the smallest effect. Principally, on the Pareto chart, bars that cross the reference line are statistically significant. Figure 6 shows the Pareto chart plotted for Cu(II) %E. The effects or interactions beyond reference line with value 2.23 were considered potentially significant. The Pareto chart displayed the sequence of interaction effects for copper ion extraction with AB > CE > DF with respect to decreasing %E. Meanwhile, Fig. 7 shows Pareto chart plotted for Cd(II) %E. It was observed that factors AB, F and B were extant beyond the 2.228 reference line in which these factors were significant, while the Pareto chart of standardized effects also displays decreasing sequence of main and interaction effects AB > F > B on the %E. To prove the affecting effects obtained using the factorial plot, the analysis of variance (ANOVA) was used to measure the coefficient of determination, R2, and p value via the regression analysis.
Statistical analysis
Regression model for Cu(II)
Table 6 presents the estimated effects and coefficients for the regression model for % extraction in coded units for the copper ions. The term (Coef) corresponds to the regression coefficient, (SDcoef) is corresponding standard derivation. T is t-statistic and p is the probability value. Among the six factors studied, the interaction factors AB, CE and DF are significant with p value less than 0.05. Equation 2 shows the second-order polynomial model after adjustment by considering insignificant factors in the coded unit which correlated %E as shown in Table 6. Meanwhile, Table 7 shows the ANOVA for the Cu(II) after adjustment by neglecting insignificant factors.
Bendebane et al. (2010) used the correlation between theoretical and experimental %E to check the performance of the polynomial regression. Figure 8 shows correlation between theoretical and experimental %E for copper ion extraction together with it coefficient of determination, R2. The R2 for this model was considered good due to R2 value near to 1 with (R2 = 0.811). Besides, the good correlation was also proven by data which were distributed along the regression line. In conclusion, among six variable studied, the interaction factors AB, CE and DF were found to influence more the %E due to their p value less than 0.05 and its R2 was closer to 1 value.
Meanwhile, Cd(II) ions after adjustment by neglecting the insignificant term at 5% significant level mean the p value is greater than 0.05 as shown in Table 8. After the adjustment, the p value of main and interaction effects was found less than 0.05. It indicated that the main variables and their interactions gave significance interaction towards the model the reduction model produce regression as in the following equation:
Table 9 shows the ANOVA for Cd(II) after neglecting insignificant terms. Figure 8 illustrates correlation between theoretical and experimental %E for copper ion extraction together with it coefficient of determination, R2, for Cd(II). The R2 values of 0.872 indicated that this model has good correlation.
Conclusion
In conclusion, the average percentage extraction, %E, of copper(II) ions was obtained in the range from 35.77 to 91.81%. While the highest %E was with factor (A = 10; B = 100; C = 60; D = 150; E = 1; F = 4.5), the lowest %E was with factor (A = 10; B = 50; C = 30; D = 150; E = 1; F = 4.5). Using the factorial plot approach of screening experiment on effecting copper(II) ions obtained that the interaction effect of factors AB (shaking time × [D2EHPA]), CE ([TBP] × O/A) and DF([NaSO4] × pH) is significant. Meanwhile, the Pareto chart shows the order of significance with respect to decreasing of influence on %E. It was observed that AB (shaking time \( \times \) [D2EHPA]) > CE gives that the interactions effects AB, CE, and DF come out with p-value are less than 0.05. This means the null hypothesis is accepted for those interaction effects. In additions, the model shows good correlations due to it correlation coefficient, R2 = 0.811.
The average percentage extraction, %E, for a sample containing cadmium(II) ion was obtained in the range of 54.77–87.35% extraction. The highest %E was with factor (A = 10; B = 100; C = 30; D = 150; E = 1.5; F = 4.5) and for lowest %E was with factor (A = 10; B = 50; C = 60; D = 150; E = 1; F = 4). The factorial plot of screening experiment on effecting cadmium(II) ions obtained that the main and interaction effect of factors AB (shaking time × [D2EHPA]), F (pH) and B ([D2EHPA]) is significant. The Pareto chart shows order of significance with respect to decreasing influence on %E in which effects AB (shaking time × [D2EHPA]) > F (pH) and B ([D2EHPA]). Meanwhile, the result of ANOVA gives that the interactions effects AB, F and B come out with p value less than 0.05. This means the null hypothesis is accepted for those interaction effects. In additions, the model shows good correlations due to it correlation coefficient, R2 = 0.872. The high R2 value indicated that the model obtained were sufficient and further strengthen the estimated of response were in the range of studied.
References
Ahmad A, Buddin MS, Ooi B (2015) Extraction of Cd (II) Ions by Emulsion Liquid Membrane (ELM) Using Aliquat 336 as Carrier. Am J Chem 5(3A):1–6
Bendebane F, Bouziane L, Ismail F (2010) Extraction of naphthalene. Optimization and application to an industrial rejected fuel oil. J Ind Eng Chem 16(2):314–320
Bernard E, Jimoh A, Odigure J (2013) Heavy metals removal from industrial wastewater by activated carbon prepared from coconut shell. Res J Chem Sci
Chang SH, Teng TT, Ismail N (2010) Extraction of Cu(II) from aqueous solutions by vegetable oil-based organic solvents. J Hazard Mater 181(1):868–872
Chang SH, Teng TT, Ismail N (2011) Soybean oil-based bulk liquid membrane for simultaneous extraction and stripping of Cu(II) from aqueous solutions. Int J Environ Sci Dev 2(5):389
Fatmehsari DH, Darvishi D, Etemadi S, Hollagh AE, Alamdari EK, Salardini A (2009) Interaction between TBP and D2EHPA during Zn, Cd, Mn, Cu, Co and Ni solvent extraction: A thermodynamic and empirical approach. Hydrometallurgy 98(1):143–147
Guezzen B, Didi MA (2012) Removal of Zn(II) from aqueous acetate solution using di (2-ethylhexyl) phosphoric acid & tributylphosphate. Int J Chem 4(3):32
Halimoon N, Yin RGS (2010) Removal of heavy metals from textile wastewater using zeolite. Environ Asia 3(special issue):124–130
Kamaruddin MA, Yusoff MS, Aziz HA, Basri NK (2013) Removal of COD, ammoniacal nitrogen and colour from stabilized landfill leachate by anaerobic organism. Appl Water Sci 3(2):359–366
Kamaruddin MA, Yusoff MS, Aziz HA, Hung YT (2015) Sustainable treatment of landfill leachate. Appl Water Sci 5(2):113–126
Kamaruddin MA, Yusoff MS, Rui LM, Isa AM, Zawawi MH, Alrozi R (2017) An overview of municipal solid waste management and landfill leachate treatment: Malaysia and Asian perspectives. Environ Sci Pollut Res 24:1–33
Kamaruddin MA, Yusoff MS, Adam NH, Maz MRR, Abdullah MMAB, Alrozi R, Zawawi MH (2018) Degradation of organic matter from stabilized leachate by using zinc sulphate as coagulant agent. In: IOP conference series: materials science and engineering, vol 374(1), p 012047. IOP Publishing
Khattak A, Khan B, Irfan M, Ahmed S (2011) Factorial design approach to investigate the effect of different factors on the resilient modulus of bituminous paving mixes
Maruyama K, Yorifuji T, Tsuda T, Sekikawa T, Nakadaira H, Saito H (2012) Methyl mercury exposure at Niigata, Japan: results of neurological examinations of 103 adults. BioMed Res Int 2012:1–7
Portal S (2016) Production of major vegetable oils worldwide from 2012/13 to 2016/17, by type (in million metric tons). https://www.statista.com/statistics/263933/production-of-vegetable-oils-worldwidesince-2000/. Accessed 13 May 2018
Pośpiech B, Walkowiak W (2010) Studies on iron (III) removal from chloride aqueous solutions by solvent extraction and transport through polymer inclusion membranes with D2EHPA. Physicochem Probl Min Process 44:195–204
Talebi A, Teng TT, Alkarkhi AF, Ismail N (2015) Nickel ion coupled counter complexation and decomplexation through a modified supported liquid membrane system. RSC Adv 5(48):38424–38434
Acknowledgements
The authors would like to acknowledge financial assistance received from Universiti Sains Malaysia under Research University Grant Scheme (304/PTEKIND/6315062) that enabled this work to be conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kamaruddin, M.A., Ismail, N., Osman, U.N. et al. Sustainable separation of Cu(II) and Cd(II) from aqueous solution by using solvent extraction technique with di-2-ethylhexylphosphoric acid (D2EHPA) as carrier: optimization study. Appl Water Sci 9, 141 (2019). https://doi.org/10.1007/s13201-019-1008-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1008-7