Abstract
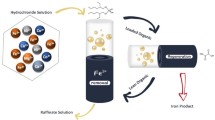
The potential impact of the liquid–liquid extraction technique for the removal and extraction of iron (III) metal ions has been investigated utilizing different basic extractants. In this respect, Octylamine and N,N-dimethylaniline as primary and tertiary amines were employed for the elimination of Fe(III) ions using benzine as a diluent and with the examination of various supportive parameters as solution pH, contact period, extractant concentration, metal ion concentration, diluent type and loading capacity. The solvent extraction results demonstrated that the maximum removal % of Fe(III) was found to be 96 and 92% for Octylamine and N, N-dimethylaniline, respectively, and it was fast, reached equilibrium after 30 min., and optimized at pH 2 with 0.05 M of the utilized extractants. According to the distribution coefficient calculations, two moles of Octylamine extractant are required for the extraction of a mole of Fe(III) ion, while for N,N-dimethylaniline one mole of it is for extraction of a mole of Fe(III) ion. Moreover, the maximum loading capacity of Fe(III) ions in the organic phase after 5 subsequent stages was 49.8 and 45.4 g/L for Octylamine and N,N-dimethylaniline, respectively. Therefore, the proposed system emphasizes and highlights the promising capability for future progress in the field of extraction techniques and wastewater management.







Similar content being viewed by others
References
Agrawal A, Kumari S, Sahu K (2011) Studies on solvent extraction of iron (III) as a step for conversion of a waste effluent to a value added product. J Environ Manag 92(12):3105–3111
Alguacil FJ, Amer S (1986a) Extraction equilibrium between primary amine primene 81R and iron (III) sulphate. Polyhedron 5(11):1747–1753
Alguacil FJ, Amer S (1986b) The extraction of iron (III) from aqueous sulphate solutions by primene sulphate. Hydrometallurgy 15(3):337–350
Alguacil FJ, Amer S, Luis A (1987) The application of Primene 81R for the purification of concentrated aluminium sulphate solutions from leaching of clay minerals. Hydrometallurgy 18(1):75–92
Ali M, Biswas R, Salam S, Akhter A, Karmakar A, Ullah M (2011) Cyanex 302: an extractant for Fe3+ from chloride medium. Bangladesh J Sci Ind Res 46(4):407–414
Asano H, Itabashi H, Kawamoto H (2001) Separation of iron (III) by di (2-ethylhexyl) phosphate/4-methyl-2-pentanone extraction. Tetsu-to-Hagane 87(9):623–625
Biswas R, Singha H (2006) Purified Cyanex 272: Its interfacial adsorption and extraction characteristics towards iron (III). Hydrometallurgy 82(1–2):63–74
de San Miguel ER, Aguilar JC, Rodrı́guez MT, De Gyves J (2000) Solvent extraction of Ga (III), Cd (II), Fe (III), Zn (II), Cu (II), and Pb (II) with ADOGEN 364 dissolved in kerosene from 1–4 mol dm−3 HCl media. Hydrometallurgy 57(2):151–165
Deep A, Correia PF, Carvalho JM (2006) Selective recoveries of Fe (III) and Cr (III) from a tannery filtrate using Cyanex 923. Anal Chim Acta 558(1–2):254–260
Desouky O, Daher A, Abdel-Monem Y, Galhoum A (2009) Liquid–liquid extraction of yttrium using primene-JMT from acidic sulfate solutions. Hydrometallurgy 96(4):313–317
El-Nadi Y, El-Hefny N (2010) Removal of iron from Cr-electroplating solution by extraction with di (2-ethylhexyl) phosphoric acid in kerosene. Chem Eng Process 49(2):159–164
Ismael M, Carvalho J (2003) Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Miner Eng 16(1):31–39
Jayachandran J, Dhadke P (1997) Liquid-liquid extraction separation of iron (III) with 2-ethyl hexyl phosphonic acid mono 2-ethyl hexyl ester. Talanta 44(7):1285–1290
Khatri N, Tyagi S, Rawtani D (2017) Recent strategies for the removal of iron from water: a review. J Water Process Eng 19:291–304
Li M, He Z, Zhou L (2011) Removal of iron from industrial grade aluminum sulfate by primary amine extraction system. Hydrometallurgy 106(3–4):170–174
Lommelen R, Binnemans K (2021) Hard–Soft Interactions in Solvent Extraction with Basic Extractants: Comparing Zinc and Cadmium Halides. Acs Omega 6(42):27924–27935
Luo F, Li D, Wei P (2004a) Synergistic extraction of zinc (II) and cadmium (II) with mixtures of primary amine N1923 and neutral organophosphorous derivatives. Hydrometallurgy 73(1–2):31–40
Luo F, Li D, Wu Y (2004b) Extraction and separation of cadmium (II), iron (III), zinc (II), and europium (III) by Cyanex302 solutions using hollow fiber membrane modules. Solvent Extr Ion Exch 22(1):105–120
Maes S, Zhuang W-Q, Rabaey K, Alvarez-Cohen L, Hennebel T (2017) Concomitant leaching and electrochemical extraction of rare earth elements from monazite. Environ Sci Technol 51(3):1654–1661
Mao X (2015) Solvent extraction of iron (III) from chloride acid solutions by decanol. In: 3rd international conference on material, mechanical and manufacturing engineering (IC3ME 2015)
Marczenko Z (1975) Spectrophotometric determination of elements. E. Horwood
Mingyu L, Zhimei H, Li Z (2011) Extraction of iron by primary amine N1923 from industrial aluminium sulfate. In: 2011 international conference on computer distributed control and intelligent environmental monitoring
Mishra R, Rout P, Sarangi K, Nathsarma K (2010) A comparative study on extraction of Fe (III) from chloride leach liquor using TBP, Cyanex 921 and Cyanex 923. Hydrometallurgy 104(2):298–303
Mishra R, Rout P, Sarangi K, Nathsarma K (2011) Solvent extraction of Fe (III) from the chloride leach liquor of low grade iron ore tailings using Aliquat 336. Hydrometallurgy 108(1–2):93–99
Nieto LM, Alami SBD, Hodaifa G, Faur C, Rodríguez S, Giménez JA, Ochando J (2010) Adsorption of iron on crude olive stones. Ind Crops Prod 32(3):467–471
Paiva A, Costa M (2005) Application of N, N′-tetrasubstituted malonamides to the recovery of iron (III) from chloride solutions. Hydrometallurgy 77(1–2):103–108
Pośpiech B, Walkowiak W (2010) Studies on iron (III) removal from chloride aqueous solutions by solvent extraction and transport through polymer inclusion membranes with D2EHPA. Physicochem Probl Miner Process 44:195–204
Pouillon D, Doyle F (1988) Solvent extraction of metals with carboxylic acids—Theoretical analysis of extraction behaviour. Hydrometallurgy 19(3):269–288
Remya P, Pavithran R, Reddy M (2004) 3-Phenyl-4-acyl-5-isoxazolones as reagents for the solvent extraction separation of titanium (IV) and iron (III) from multivalent metal chloride solutions. Solvent Extr Ion Exch 22(3):473–490
Ritcey GM, Ashbrook A (1984) Solvent extraction. principles and applications to process metallurgy. Part I
Saji J, Reddy M (2001) Liquid–liquid extraction separation of iron (III) from titania wastes using TBP–MIBK mixed solvent system. Hydrometallurgy 61(2):81–87
Sokolov A, Valeev D, Kasikov A (2021) Solvent extraction of iron (III) from Al chloride solution of bauxite HCl leaching by mixture of aliphatic alcohol and Ketone. Metals 11(2):321
Stefanakis M, Monhemius A (1987) Computer modelling of the solvent extraction of iron by versatic acid from aluminium nitrate solutions. Hydrometallurgy 19(2):187–198
Su W, Chen J, Jing Y (2016) Aqueous partition mechanism of organophosphorus extractants in rare earths extraction. Ind Eng Chem Res 55(30):8424–8431
Sun J, O’Keefe T (2002) An evaluation of steel scrap as a reducing agent in the galvanic stripping of iron from D2EHPA. Miner Eng 15(3):177–185
Zhao X, Cui K, Huang K (2022) Enhanced interfacial salt effect on extraction and separation of Er (III) from Mg (II), Al (III), Fe (III) sulfate aqueous solutions using bubble-supported organic liquid membrane. Sep Purif Technol 285:120344
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest with any ethical problems.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Bagawi, A.H., Alanazi, T.Y.A. Prospective investigation for iron (III) removal from aqueous solutions with solvent extraction technique using Octylamine and N,N-dimethylaniline extractant. Chem. Pap. 77, 6739–6745 (2023). https://doi.org/10.1007/s11696-023-02973-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02973-3