Abstract
Climate change and population growth around Mt. Meru experienced lower availability of water for domestic and agricultural uses. Reduction in quantity of water is compounded by lack of information on water quality which could lead to undesired health risks and agricultural effects when such water is used for irrigation. Thus, major ions from 54 different water types (springs, streams, dug wells, boreholes, and lakes) were used to assess hydrogeochemical characteristics and suitability of water for domestic and agricultural purposes. Results showed dominance of the major cation and anion in the order of Na+ > K+ > Ca2+ > Mg2+ and HCO3− > CO32− > Cl− > SO42− > NO3− > F−, respectively. It is revealed that Mt. Meru is the recharge zone. Geology, water–rock interaction time, and climatic conditions control water chemistry. Major freshwater aquifers were found to be fractured mafic volcanics, breccia, and tuff. Lahars, due to their susceptibility to weathering, were found to host groundwater of low quality. The suitability of water for domestic and irrigation purposes was moderate, in order of lakes < dug wells < boreholes < streams < springs. Fluoride was found to be the major natural contaminant affecting water quality for domestic purposes with mean value of 17.6 mg/L, while elevated Na+ (mean = 118 mg/L), K+ (mean = 59 mg/L) and HCO3− (mean = 390 mg/L) relative to other ions were found to affect water quality for irrigation purposes. In some few cases, anthropogenic pollutions were recognized through NO3− and Cl−.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Access and provision of safe and clean water to people, which is among human rights, has been a continued aim globally. Population growth, poverty, and economic instability among countries have been pointed out to be causative factors toward not achieving goal No. 7 of the Millennium Development Goals (MDGs) particularly (UNICEF 2015; UN 2015). It is estimated that about 11% of the population globally have no access to safe and clean water with a large number of people living in sub-Saharan Africa and Oceania (UNICEF 2015). Majority of population in these areas continue to depend largely on rivers, lakes, ponds, and irrigation canals as their major sources of drinking water (UNICEF 2015). Some of these sources are polluted naturally, and most of them are vulnerable to anthropogenic pollution.
Most of the developing countries are facing economic water scarcity (UNOCHA 2010). Tanzania, one among the developing countries is significantly facing economic water scarcity despite having several freshwater sources such as lakes and rivers (Mjemah et al. 2011; Kashaigili 2012; Mashindano et al. 2013; Mtoni et al. 2013). Water supply from these potential sources to rural and urban areas is still at low scale. In the year 2015, Tanzania was still among the few countries with lowest coverage in accessing an improved drinking water sources and sanitation (Kashaigili 2012; Mashindano et al. 2013; UNICEF 2015). The water demand is currently increasing due to both population growth and climate change (Mjemah et al. 2011, 2012; Kashaigili 2012; Mashindano et al. 2013). Due to these factors, there is a positive trend toward utilization of groundwater as the main water source for domestic purposes (Mjemah et al. 2011; Kashaigili 2012; Mtoni et al. 2012, 2013).
Anthropogenic activities are pronounced in both urban and rural areas. In urban areas, contamination is mainly attributed to industrial activities and onsite sanitation (Mjemah et al. 2011; Napacho and Manyele 2010; Elisante and Muzuka 2015, 2016a, b) where as in sub-urban to rural areas it is mainly caused by agriculture activities (Bowell et al. 1996; Nkotagu 1996a, b; Bowell et al. 1997; Mohammed 2002; Mjemah et al. 2011; Napacho and Manyele 2010; Elisante and Muzuka 2015, 2016a, b). Fluoride is one among the natural contaminants in groundwater systems which are common in volcanic regions of Tanzania (Nanyaro et al. 1983; Ghiglieri et al. 2010; 2012; Malago et al. 2017). According to the fluoride groundwater survey in Tanzania, it was found that 30% of waters used for drinking exceed the recommended standard by WHO, 1.5 mg/L(Thole 2013). The most affected zones are the areas along the rift system, the central to Lake zone parts (Malago et al. 2017). For instance, along the rift system, Nanyaro et al. (1983) reported high concentration of 690 mg/L from Lake Momella (Fig. 2). Since then, high fluoride values have been reported in other water sources along the rift system (Ghiglieri et al. 2010, 2012; Malago et al. 2017).
Several geological and hydrological studies have been conducted within the area (e.g., Nanyaro et al. 1983; Dawson 2008; Ghiglieri et al. 2010, 2012). Most of the researches were limited to fluoride (e.g., Nanyaro et al. 1983), some studies were constrained in specific parts (e.g., Ghiglieri et al. 2010, 2012, northern part of Mt. Meru) and most of them lacked a continuous data set for the whole district that could integrate all water types from the windward to leeward sides for detailed study of hydrogeochemical characteristics, groundwater evolution and water quality for both domestic and irrigation purposes. Furthermore, according the Meru District Council report (MDC 2013, unpublished) and Vye-Brown et al. (2014), there is high population growth in Meru district leading to rapid expansion of agricultural activities and increased water demand for domestic purposes. Agricultural expansion is associated with application of fertilizers and therefore likely to cause contamination in both surface and groundwater systems. Therefore, this study aimed to characterize all water sources in the area and assess their suitability for domestic and agricultural purposes. It also intended to gather information on hydrogeological processes which integrate water types and its evolution. Through this, zones with different water types and quality and zones susceptible to rapid significant hydrogeochemical changes can be identified. Such information is also important in water management plans especially in water allocation for specific uses, protection, and conservation of water resources and in locating future potential boreholes for domestic purposes.
The study area
Location, topography, and climate
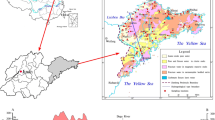
Meru district is among the districts of Tanzania situated along the Eastern branch of the East Africa Rift System, in the northern part of Tanzania (Fig. 1). The topography varies from ~ 860 m in the southern parts to 4565 m (peak of Mt. Meru which is the second highest mountain in Tanzania (Fig. 1). The area is characterized by steep slope toward the peak of the mountain and very gentle slope in the southern parts (Fig. 1). Its climate is significantly controlled by Mt. Meru which divides the district into two climatic zones—windward and leeward sides creating variations in hydrological and hydrogeological processes. The windward side receives high rainfall averaging at about 1000 mm per annual (Oettli and Camberlin 2005) with most of the springs originating on the slopes of the mountain (Fig. 1). The leeward zone receives low rainfall: the mean annual rainfall being less than 500 mm (Oettli and Camberlin 2005; Ghiglieri et al. 2012). There are two groups of lakes surrounding Mt. Meru. The first group is composed of crater lakes of Duluti and Ngurdoto in the windward side of the mountain (Fig. 1). The second group is the Momella series lakes in the leeward side of the mountain (Fig. 1) which are believed to have been formed from the collapse of Mt. Meru (Dawson 2008). Unlike crater lakes, Momella lakes are alkaline and saline (Ghiglieri et al. 2010).
Physiography
The population of Meru District Council (MDC) is 268,144 people (NBS and Office of Chief Stastitian Zanzibar 2013). The MDC has a population growth rate of 3.1% and a population density of 228 inhabitants per square kilometer (Meru District Council 2013) which is four times higher than the national population density of 51 inhabitants per square kilometer (NBS and Office of Chief Stastitian Zanzibar 2013). Such population density is expected to increase pressure on water resources.
Within the study area, there are three agro-ecological zones (high, mid, and low) which have been established based on the topography (Fig. 1) and rainfall distribution. The highland zone (~ 1400–1800 m a.s.l) is characterized by high rainfall (~ 1000 mm/yr) and high population density (Wameru as the dominant ethnical group) (MDC 2013). Agriculture is the major economic activity in this zone. The common practiced crops are coffee, pyrethrum, banana, and potatoes. Most of the crops are grown throughout the year because of water availability for irrigation purposes. In the middle zone (~ 1000–1400 m a.s.l) where rainfall is relatively low (~ 500 mm/yr), both livestock keeping and agriculture activities are practiced. There are small to large-scale farming schemes which include floriculture and horticulture. Some of these schemes are irrigation schemes which lead to water scarcity for the downstream users. The lowland zone (~ 800–1000 m a.s.l), southern part of the study area (Fig. 1), is characterized by low rainfall (~ 300 mm/yr) and low population density. Major economic activities are livestock keeping and agriculture (MDC 2013). Livestock keeping is largely practiced by Maasai, while agricultural activities involving maize, beans, and fruits are practiced by Wameru and Waarusha ethnical groups (MDC 2013).
In all areas with water scarcity, local people have been drilling deep bore holes and/or constructing shallow hand-dug wells. Vulnerability to contamination is high as some wells are drilled close to pit latrines and cow sheds. Furthermore, according to the interview with the local people, most of the drilled hand-dug wells are utilized without testing the suitability of such water for specific uses. This is obviously affecting the human health through direct or indirect consumption of such water, and it is likely to lower crop production.
Geological and hydrogeological setting
The study area is studded by volcanic materials from Mt. Meru. Various groups of volcanic rocks from different volcanic eruption episodes with the latest being in 1910 (Ghiglieri et al. 2010, Vye-Brown et al. 2014) have been documented by many researchers (e.g., Wilkinson et al. 1986, Dawson 2008, Ghiglieri et al. 2010, 2012). The dominant volcanic groups include lahars of various ages, nephelinites and phonolites, pyroclastic and ashes, basaltic lava and scoria, parasitic cones, tuffs, and breccias (Fig. 1). These rocks are generally young, spanning from ~ 300,000 yrs BP to recent (Wilkinson et al. 1986). Earliest formation (~ 300,000 yrs BP) includes phyroclastic with phonolitic-to-nephelinitic lavas surrounding the Meru ash cone (Figs. 2 and 3). The dominant group is lahar materials of various ages between 80,000 and 7000 yrs B.P (Wilkinson et al. 1986). Recent sediments are ashes and pyroclastics, lacustrine, and alluvial sediments, being distributed in some specific areas depending on the land morphology (Figs. 2 and 3). The rock materials control occurrence, distribution, and quality of groundwater (Ghiglieri et al. 2010; Chacha et al. 2018). Because of the limited stratigraphic and groundwater information in the study area, the aquifer types and water quality are discussed with the aid of geology and existing boreholes/dug wells (Fig. 2).
Geological map (after Wilkinson et al. 1986) with key locations of boreholes (BH1: Mandela, BH2: Lodhia), dug wells (D1: Maroroni), springs (S1: Nduruma, S2: Makisolo, S3: Makumira, S4: Arusha National Park), stream (ST1: Ngarenanyuki, ST2: Kyamang’ata), and lake samples used to describe hydrogeology of the area
Cross section from Mt. Meru to low land area as derived from the traverse A–A′ in Fig. 2
Pyroclastics with nephelinitic-to-phonolitic lavas which are surrounding Meru ash cone (Fig. 2) are generally fractured forming the potential aquifers (Ghiglieri et al. 2010). They occupy a main recharge zone with high precipitation (> 1000 mm/yr) (Oettli and Camberlin 2005). High precipitation and fracturing supported by steep slope cause most of the springs to emerge on this formation particularly in the windward side (Fig. 2). Groundwater flows down the slope through the fractures (Ghiglieri et al. 2010). Since the areas is affected by tectonism ((Wilkinson et al. 1986), both shallow and deep groundwater movement through the fractures are possible. Due to high hydraulic gradient, water–rock interaction time is low in this zone as evidenced by relatively low total dissolved solids (TDSs) and low fluoride in spring water sources (Malago et al. 2017). Lahars cover large part in the study areas (Fig. 2). They are characterized by volcanoclastic sediments which are poorly sorted (Ghiglieri et al. 2010; Fig. 4). They form good aquifer because of being fractured and porous (Ghiglieri et al. 2010; Fig. 4). There is variation in water quality in these aquifers, likely to be influenced by chemical composition and thickness of aquifers. For instance, in this study, poor water quality (F = 32 mg/L and TDS = 1820 mg/L) was obtained from the dug well D1 (Fig. 3) which intersected Ngarenanyuki lahars from the surface to a depth of 28 m. Such high fluoride values have been recorded in various water sources in areas which are dominated by Ngarenanyuki lahars (Nanyaro et al. 1983; Malago et al. 2017). Groundwater from the boreholes BH1a, b (Fig. 3), drilled at Nelson Mandela to the depths of 98 and 130 m, respectively, in lahars of various age, was relatively good (TDS ~ 390 mg/L) and F− = 1.0–3.2 mg/L. Lithological logs indicated that lahar is not pronounced, and groundwater is hosted largely in fractured mafic volcanics (Fig. 4). The yields from BH1a were found to be 33 m3/hr suggesting that these formation are permeable enough and sufficient to release water for public uses. Breccia and tuffs cover the eastern part of the study area. They have been reported to be porous and fractured in Ngarenanyuki area and hence potential aquifers (Ghiglieri et al. 2010). This is well supported by the deep borehole (BH2; Fig. 3) drilled by LODHIA gypsum industry to a depth of 150 m. There is no lithological logs; water sample was found to be good (TDS = 452 mg/L, F− = 1.65 mg/L). Yield is likely to be good as it fulfills water need for the industry, communities around the industry, pastoralists, and small irrigation schemes. Tholoids are also common in the study area (Fig. 2); these are generally impermeable and act as a barrier to groundwater movement. Mantling ash are pronounced in the northern part of Mt. Meru (Fig. 1). Physical observation and the work of Ghiglieri et al. 2010 indicated mantling ash and alluvial and lacustrine sediments fall in the same group of materials with low transmissivity due to high clay content.
Lithological logs of two boreholes BH1a, b (~ 100 m apart), represented by BH1 in Fig. 2, drilled on lahars of various age and one dug well (D1) drilled on Ngarenanyuki lahars (Fig. 2). Lithological logs for BH1a is based on the existing borehole completion report while borehole BH1b and D1 were logged directly by the authors during drilling
Methodology
Fieldwork
A total of 54 water samples (16 dug wells, 10 boreholes, 4 lakes, 15 springs, and 9 streams) presented in Fig. 1 were collected within Meru districts between May and August 2015. Sampling criteria were largely based on water types, geology, morphology, and climate variation within the study area. Despite that water sources are not uniformly distributed in the study area (Fig. 1), these criteria led to good distribution of representative samples for assessing variation in hydrogeochemical characteristics. Boreholes and wells have been drilled for various purposes including domestic, agriculture and washings. Therefore, apart from water quality, sampling focused on assessing the types and distribution of Aquifers in the study area.
The sampled windward springs were 10 (south of Mt. Meru) and the leeward springs were 5 (North of Mt. Meru in Oldonyosambu and Ngarenayuki ward (Fig. 1). Two springs (S1 and S2) (Fig. 2) were the only springs in the leeward side which are not on the slope of Mt. Meru. They are emerging in mantling ash and volcanic soils (Fig. 2), about 5–10 km away from the foot of Mt. Meru which is locally defined by an altitude of 1500 m above sea level (Fig. 1). Streams were represented by two samples from the leeward side in Ngarenanyuki ward (Fig. 1) and seven samples from the windward sides (south to southern east of Mt. Meru) (Fig. 1). Important factors such as tributaries joining the streams, the presence of agricultural activities, ephemeral and perennial streams, and stream depths and flows were considered. These are important factors for assessing water–rock interaction processes because they provide insight on levels and source of various ions. Stagnant water bodies were represented by two crater lakes located in the windward side (Ngurdoto and Duluti) and two lakes from the leeward side of Mt. Meru (Mlolozi and Small Momella) (Fig. 2).
During sampling, water sampling procedures were followed. All sampling precautions mainly bottle cleanness, labeling and tightness, duplicates and proper water mixing before sampling were taken in care. In situ measurements of total dissolved solids (TDS), pH, dissolved oxygen (DO), electrical conductivity (EC), and temperature were done using the Hanna—HI 9829 multi-parameter under appropriate daily calibration. Finally, the collected samples were filtered using 0.45-μm membrane filters. The filtered samples were collected in triplicate in 1000, 500 and 500-ml polyethylene bottles. For two bottles of 500 ml each, samples were acidified immediately by concentrated HNO3 and H2SO4 to pH < 2 and kept in cool boxes for preservation purposes before analyses of major cations. The 1000-ml bottles were not acidified and were used for immediate analyses of fluoride and major anions.
Analytical work
Fluoride analyses were done at the Nelson Mandela African Institution of Science and Technology (NM-AIST) laboratory using the fluoride ion-selective electrode (FISE) within 6 days of sampling. The reagent used was the total ionic strength adjustment buffer (TISAB II) with CDTA. The daily calibration of the instrument was done using fluoride standards of 1 mg/L and 10 mg/L with the magnetic steer at 25 °C. The ratio of TISAB to field sample was 1:1 making a total of 10 ml for analyses. Various measures were taken for quality control and assurance. The FISE was first tested using both the solutions that were used in calibration and the certified drinking water bottled samples with the known fluoride concentration. Secondly, three portions of each field sample were prepared, and appropriate average fluoride concentrations were recorded. Thirdly, 10 water samples were taken at Ngurudoto (the near defluoridation water laboratory center) for analysis and the results were compared.
Major ions
The concentrations of major cations (Na+, K+, Ca2+, and Mg2+) were measured using inductive coupled plasma optical emission spectroscopy (ICP-OES) at SEAMIC, Dar es Salaam. Titration technique was employed to determine total alkalinity and hence concentrations of HCO3− and CO32−. Different techniques were used to determine concentrations of other major anions. Cl− was determined by Argentometric titration, SO42− was measured by turbidimetric method, and NO3− was determined by cadmium reduction method (spectrophotometric cadmium reduction). For quality purposes, duplicates were used, and some samples were cross-checked at the nearby Ngurdoto Laboratory Defluoridation Centre (NLDC). Accuracy of the major analysis was checked using cation–anion balance error (CBE). Generally, CBE was found to be < 10% indicating that analysis was fair.
Major parameters for agriculture purposes
The important parameters for irrigation purposes [residue sodium carbonate (RSC), sodium percent (%Na), sodium adsorption ratio (SAR), magnesium adsorption ratio (MAR), and Kelley’s ratio (KR)] are summarized below with the appropriate equations (values in meq/L) which were used in evaluation of Meru groundwater (Eq. 1–5).
Residue sodium carbonate (RSC)
It is an important factor in irrigation when water samples are characterized by high [(HCO3) + CO3)] relative to [Ca + Mg] (Bashir et al. 2013). RSC was calculated using Eq. 1.
Sodium percent (%Na)
Sodium percent is an important factor in irrigation as it relates directly to soil structure. It was calculated using Eq. 2:
Sodium adsorption ratio (SAR)
This is the measure of the amount of Na in water relative to Mg and Ca for irrigation purposes. It was calculated using Eq. 3 (Bashir et al. 2013):
Magnesium adsorption ratio (MAR)
Magnesium content in water is highly related to infiltration process during irrigation. The magnesium adsorption ratio was calculated using Eq. 4:
Kelley’s ratio (KR)
This is basically a ratio of Na + to (Mg2+ + Ca2+), used to evaluate the suitability of water for irrigation purpose. It was calculated using Eq. 5:
Results
Physicochemical parameters and major ions
Statistical summary of physicochemical parameter results is presented in Table 1 and the corresponding summary of the major ion results is presented in Table 2.
The pH values for all water sources ranged from 6.46 to 10.91 and averaged 8.16 ± 0.9. The mean values were nearly uniform between 8.04 ± 0.57 and 8.43 ± 0.91 in borehole, dug well, stream, and lake samples and slightly low in springs (7.7 ± 1.25) as shown in Table 1. The extremely low and high pH values of 6.46 and 10.91 were obtained in springs leading to a wide range of pH values in springs relative to other water types (Fig. 5a). Classification of pH values based on three categories of nearly neutral (6.4–7.5), slightly alkaline (7.5–8.5), and alkaline (8.5–11) indicated that 53% of the springs were nearly neutral, whereas most of the boreholes (70%), dug wells (56%), and lakes (50%) were slightly alkaline, and most of the streams (44%) were alkaline. Intra-classification of pH within spring water type indicated that 80% of the windward springs were under nearly neutral category, whereas 40% and 60% of the leeward springs were slightly alkaline and alkaline, respectively (Fig. 5b).
The mean dissolved oxygen (DO) in all sample types was 4.4 ± 1.5 ppm (Table 1). Generally, it showed an increasing trend from lakes–dug wells–springs–boreholes to streams (Fig. 6a). 50% of the lakes and 62.5% of the dug wells had low DO between 0 and 3.5 ppm, whereas 78% of the streams and 70% of the boreholes had high DO between 5 and 7.5 ppm (Fig. 6a). Despite the wide range of DO for all samples, there were neither stream samples with DO less than 3.5 nor dug wells with DO above 5 ppm (Fig. 6a).
Total dissolved solid (TDS) was classified into three categories as very fresh water (60–300 mg/L), freshwater (300–1000 mg/L), and brine to saline water (1000–4400 mg/L) (Fig. 6b). However, the first two categories are within the recommended WHO freshwater standard of TDS < 1000 (WHO 2011). TDS showed a general increasing trend in the order of spring < stream < boreholes < dug wells < lakes. The low TDS category (60–300 mg/L) was dominated by springs (67%), lakes (50%), and streams (44%) (Fig. 6b). Dug wells water type was the lowest percentage in this category (6.25%), while dominating the high TDS category (100–4400 mg/L) to 56% (Fig. 6b). Streams and boreholes dominated the intermediate category (300–1000 mg/L) with the percentage of 56 and 60, respectively (Fig. 6b). While springs and streams are completely absent in the high TDS category (Fig. 6b), the lakes showed two distinct ranges of TDS); the low TDS (60–300 mg/L) comprising windward lakes (Duluti and Ngurdoto) and the high TDS (1000–4400 mg/L) comprising leeward lakes (Mlolozi and Small Momella).
Temperature in water types varied significantly from 13.76 to 27.84 °C with the mean value of 21.94 ± 3.4 °C. It was low in springs and streams with the mean values of 18.57 ± 2.57 °C and 19.78 ± 2.22 °C respectively and high in boreholes with the mean value of 25.06 ± 1.28 °C. Temperature was intermediate in Lakes (mean = 22.15 ± 3.9 °C) and relatively high in dug wells (mean = 24.32 ± 1.45 °C) (Table 1).
Major ion distribution
Sodium was found to be the dominant cation in all water types comprising about 61% of the total cations with the mean value of 118 mg/L. However, it showed a strong variation in different water types and within each group (Fig. 7a). The high mean value was in lakes (215.52 ± 257.12 mg/L), whereas the low values were in streams (61.62 ± 55.29 mg/L) and springs (39.40 ± 36.92 mg/L). Sodium was classified based on the WHO threshold taste of 200 mg/L (WHO 2011) into low (0–50 mg/L), intermediate (50–200 mg/L) and high sodium (200–600 mg/L). Classification showed that springs (73%), streams (56%), and windward lakes (50%) dominate the low Na+ category, whereas dug wells (50%) and leeward lakes (50%) dominate the high Na+ category. Both springs and streams were absent in the high Na+ category (Fig. 7a).
Potassium was the second dominant cation comprising about 30% of the total cations. Its dominance pattern was found to be similar to that of Na+, being characterized by elevated K+ concentrations in lakes (mean = 139.36 ± 186.69 mg/L) and dug wells (mean = 104.36 ± 64.13 mg/L) and low concentrations in streams (mean = 28.86 ± 29.29 mg/L) and springs (mean = 18.02 ± 13.8 mg/L). Based on the recommended WHO maximum standard of K+ in drinking water (12 mg/L) (WHO 2008), 80% of all samples were above the recommended standard (Fig. 7b). Only 47% of the springs, 33% of the streams, and 25% of the boreholes were within the acceptable standard (Fig. 7b). High K+ category group (Fig. 7b) was dominated by dug wells (37.5%) and leeward lakes (50%), lacking spring and stream sample types (Fig. 7b).
Calcium and magnesium were low in water samples comprising only 9% of the total cations with the mean values of 13.3 ± 14.4 mg/L and 3.6 ± 6.0 mg/L, respectively (Table 2). With exception of two dug well samples, the concentration of Ca2+ and Mg2+ in other samples was within the recommended WHO standard of 50 and 75 mg/L, respectively (WHO 2008). The mean concentrations of Ca2+ were nearly uniform in boreholes, dug well, and lakes between 16 and 17.2 mg/L. Mean uniformity was also observed in spring and surface water types where the mean Ca2+ concentrations were slightly low, restricted between 8.4 and 9.8 mg/L. Classification of Ca2+ values showed the dominance of the springs (53%) in the very low Ca2+ category (0–4 mg/L) and the dominance of lakes (75%) in the high Ca2+ category (20–85 mg/L) (Fig. 7c). The mean values for Mg2+ in different water types ranged from 2.78 mg/L (surface water) to 4.84 mg/L (dug wells) and averaged 3.7 mg/L. Despite that dug well and the spring water types showed a wide range of Mg2+ concentrations, 67% of the springs had the concentration less than 1 mg/L and 62.5% of the dug wells had Mg2+ concentration between 1 and 3 mg/L (Fig. 7d). Unlike dug wells and springs, all lakes fell in one category with Mg2+ concentration between 3 and 10 mg/L (Fig. 7d). The cation results with the dominance pattern of Na+ > K+ > Ca2+ > Mg2+ compare well with the results obtained in the northern part of the same rift system in Ethiopia (Rango et al. 2010).
Bicarbonate was found to be the dominant anion comprising 73% of the total anions in water samples. Overall, it showed a wide range in all sample types with an increasing trend in the order of springs < streams < boreholes < dug well < lakes (Fig. 8d). Based on classification of HCO3− into very low (0–100 mg/L), low (100–300 mg/L), intermediate (300–1000 mg/L), and high (1000–3000 mg/L), dug wells and boreholes were absent in the very low category, while springs and streams were absent in the high category (Fig. 8d). Springs had relatively low HCO3− with 53% and 33% in the categories of very low and low, respectively (Fig. 8d). 40% and 50% of the boreholes were restricted in the low and intermediate categories, respectively. However, the intermediate category was dominated by dug wells to 56.25% (Fig. 8d). Lakes exhibited a wide range of HCO3− concentrations varying from 98.9 mg/L (Ngurudoto crater) to 2889 mg/L (Lake Small Momella) and were uniformly distributed in four categories (Fig. 8d). In alkaline water samples, HCO3− occurred together with carbonates. About 70% of the samples had CO32− concentrations varying between 1.2 and 1009 mg/L (mean = 53 ± 164 mg/L). However, the concentrations were less than 12 mg/L in most of the samples. CO32− showed a wide range of concentration in all water sample types with the dominance pattern similar to that of HCO −.3
In this study, fluoride was found to be among six dominant anions, comprising about 3% of total anions. It varied between 0.8 and 259 mg/L with mean value of 17.6 ± 37.4 mg/L. Classification of fluoride values based largely on the WHO and Tanzania standards showed that 78% of all samples had fluoride above the recommended WHO standard of 1.5 mg/L (WHO 2011) and 52% had fluoride above Tanzanian standard of 4 mg/L. Within the low fluoride category (0–1.5 mg/L), the dominant sample types were springs (40%), streams (33%), and lakes (25%; lake Ngurudoto), while dug wells and boreholes were limited to 6.25% and 10% respectively (Fig. 8a). Unlike the boreholes which dominated the relatively low fluoride category of 1.5–4.0 mg/L to 60%, the dug wells had relatively high fluoride dominating the category of 20–100 mg/L to 50% (Fig. 8a). The Leeward lakes—Small Momella and Mlolozi—exhibited extremely high fluoride of 259 mg/L and 32.5 mg/L, respectively, being reflected in the high fluoride categories shown in Fig. 8a.
Chloride comprised about 7% of the total anions ranging between 2 and 300 mg/L with the mean value of 39.3 ± 55.2 mg/L. Based on the minimum WHO provisional standard of 250 mg/L (WHO 2011), only one dug well sample (Cl− = 300 mg/L) was above the recommended standard. Classification of chloride into very low (0–10 mg/L), low (10–30 mg/L), intermediate (30–200 mg/L), and high (200–300 mg/L) showed the dominance of the springs (67%), boreholes (80%) and dug wells (69%) in the very low, low and intermediate categories, respectively (Fig. 8b). Streams had relatively low chloride with 44.4, 33.3, and 22.2% in the very low, low, and intermediate categories, respectively (Fig. 8b). The high chloride category was represented by lake Small Momella (230 mg/L) and one dug well as discussed previously.
Nitrate comprised about 4% of the total anions ranging between 0.35 and 198 mg/L with the mean value of 22 ± 40 mg/L. Elevated NO3− values were found in dug wells (mean = 42.3 ± 61.7 mg/L) and surface water (mean = 22.9 ± 42.9 mg/L) (Table 2). NO3− distribution presented in Fig. 8c showed nearly uniform distribution of NO3− in dug wells in all categories, whereas in surface water, it dominates the intermediate categories represented by NO3− values between 5 and 20 mg/L (Fig. 8c). With the minimum WHO provisional standard of 50 mg/L for NO3− (WHO 2011), two dug wells with NO3− concentrations of 101.2 mg/L and 198 mg/L and one surface water (NO3− = 136.4 mg/L) were considerably above the recommended minimum standard. These samples represent the high NO3− category (200–300 mg/L) (Fig. 8c). Unlike other ions, the lake samples had the lowest NO3− concentrations (mean = 7.15 ± 4.94 mg/L (Table 2) with 75% in the categories of low concentrations (NO3− < 10 mg/L) (Fig. 8c). Interestingly, based on WHO (2011), all NO3− concentrations in borehole, spring, and lake water types were below the recommended WHO standard of 50 mg/L.
Sulfate comprised about 6% of the total anions ranging between 0 and 300 mg/L with the mean value of 32.4 ± 60 mg/L. Overall, it showed an increasing trend in the order of springs < boreholes < stream < dug wells < lakes (Table 2 and Fig. 9). In all samples, only two water types: one dug well sample (320 mg/L) and one lake sample (Lake small Momella; 300 mg/L), exceeded the minimum WHO provisional standard of 250 mg/L for SO42− (WHO 2011). Based on the SO42− classification into very low (0–10 mg/L), low (10–30 mg/L), intermediate (30–200 mg/L), and high (200–300 mg/L), most of the springs and boreholes fell in the very low category (Fig. 9). Despite that this category included also 50% of the lake and 44.4% of the stream samples, the percentage of dug wells was very low (6.25%) (Fig. 9). The anion results showed a dominance pattern of HCO3− > CO32− > Cl− > SO42− > NO3− > F−.
Physicochemical parameters (TDC and EC) and major ions were further assessed using correlation matrix (Table 3). Strong positive correlations (r > = 0.9) were observed between HCO3− with TDS and EC (r = 0.96), Na+ (r = 0.92), K+, and F− (r = 0.90) (Table 3, Figs. 10, 11). Such strong correlations were also observed between Cl− and SO42− (r = 0.90) and F− with CO32− (r = 0.93) (Table 3). Apart from strong positive correlation between F− with CO32− and HCO3−, F− was also found to correlate positively with TDS (r = 0.84), EC (r = 0.84), K+ (r = 0.72), Na+ (r = 0.86), SO42− (r = 0.69), and Cl− (r = 0.73) (Fig. 11). It had no significant correlation with Mg2+ (r = − 0.06) and Ca2+ (r = − 0.19) (Table 3 and Fig. 12). TDC and EC were found to have significant positive correlation with all major ions with exception of Mg2+, Ca2+, and NO3−. Cations (Mg2+ and Ca2+) correlated positively (r = 0.85) (Fig. 10e), which is the same correlation coefficient for the other two major cations (Na+ and K+) (Fig. 10d).
Major ion characteristics in specific water types
Relative abundance
The general ionic abundance pattern in all water types was Lake > Dug wells > Boreholes > Rivers/Streams > Springs (Fig. 13). Figures 14 and 15 represent water sources that showed ion deviations from others in specific water types.
Major ions variation (a) in the sampled lakes (b) in two streams which showed elevated ions relative to other streams. The lakes and sampling locations for Ngarenanyuki and Kyamang’ata streams are shown in Fig. 2
Major ions characterization in springs. a Leeward and windward springs. b Windward springs showing elevated ions relative to other windward springs. All named springs are shown in Fig. 2
Lake
The leeward lakes (Small Momella and Mlolozi shown in Fig. 2) were characterized by high dissolved ions relative to the windward crater lakes (Duluti and Ngurudoto shown in Figs. 2) (Fig. 14a). However, in lake Small Momella, Ca2+ (1.26 mg/L) and Mg2+ (3.3 mg/L) were less relative to the values obtained in other lakes which varied between 20 and 25 mg/L and 4.4–4.9 mg/L, respectively (Fig. 14a). Also, despite that NO3− values were low in all lakes within the acceptable international standard as discussed previously, Lake Duluti exhibited high NO3− value (12.76 mg/L) relative to other lakes where NO3− averaged 5.28 mg/L.
Streams
Two streams basically in the leeward side of Mt. Meru (Ngarenayuki and Kyamang’ata) (Fig. 2) showed elevated dissolved ions relative to all other sampled streams (Fig. 14b). Generally, within these two streams, Ngarenanyuki river exhibited higher dissolved ions than Kyamang’ata stream with Na+ (175.5 mg/L), K+ (99.7 mg/L), F− (30 mg/L), Cl− (56 mg/L), CO32− (80 mg/L), and SO42− (80 mg/L) as the most discerned ions (Fig. 14b). However, Kyamang’ata stream had extremely high NO3− (136.4 mg/L) relative to both Ngarenanyuki (0.35 mg/L) and mean of other streams (9.9 mg/L) (Fig. 14b). The ions which were low in these two streams relative to the corresponding mean of the other streams were CO32− (0.3 mg/L) in Kyamang’ata stream and Mg2+ (2.02 mg/L) and NO3− (0.35 mg/L) in Ngarenanyuki stream (Fig. 14b).
Springs
Windward springs dominating southern slope of Mt. Meru (Fig. 1), and Leeward springs in Oldonyosambu and Ngarenanyuki wards, north of Mt. Meru (Fig. 1), showed different chemical characteristics. There were high dissolved ions in the leeward springs relative to the windward springs (Fig. 15a). However, unlike other ions, the concentrations of Mg2+ and Ca2+ were high in the windward side relative to leeward side. Furthermore, results showed that a group of six windward springs located on the slope of Mt. Meru (Figs. 1) had low dissolved ions relative to other windward springs which are not on the slope of the mountain (e.g., Nduruma, Makisolo, Kalamu-Makumira and Arusha National Park shown in Fig. 2) (Fig. 15b).
Hydrogeochemical characteristics and classification
Piper diagram, a common method which has been widely used in groundwater characterization and geochemical evolution (e.g., Glover et al. 2012; Narany et al. 2014; Srinivas et al. 2014), was used to characterize water samples from Meru district (Fig. 16). Based on four diamond quadrants of the Piper diagram (Fig. 13), > 92% of Meru waters belong to Na–K–HCO3 type. Only four samples plotted out of this field, with 3 samples (2 springs and 1 lake) in the fields of CaHCO3 and one dug well sample in the field of Na–K–Cl type. Despite the fact that Na–K–HCO3 is the dominant type for all samples, the springs and lakes tend to show a wide range in chemical composition compared to the borehole and dug well samples. The leeward springs showed the dominance of Na+, K+, and HCO3− ions relative to the windward spring being concentrating at the bottom corner of the diamond plot.
Other methods which have been used in water characterization include Langelier and Ludwig (1942), Chadha’s diagram and Gibb’s ratios (1970) (Glover et al. 2012; Narany et al. 2014; Srinivas et al. 2014). Both Langelier and Ludwig (1942) and Chadha’s diagrams (Figs. 17 and 18) showed that over 90% of all water types from Meru district plot in the field of Na–K–HCO3 hydrochemical facies. The figures showed that two springs (Nduruma and Makisolo) and one lake (Ngurdoto) (Fig. 2) belong to Ca2+ (Mg2+)–HCO3− hydrochemical facies. One spring at Oldonyosambu ward (Fig. 1) showed depletion of HCO3− relative to other samples (Fig. 17) and one dug well at Maji ya Chai ward (Fig. 1) showed depletion of HCO3−, belonging to Na–Cl hydrochemical facies (Fig. 18).
Compositions of different water types in Meru district (after Langelier and Ludwig 1942)
Gibb’s plot (Fig. 19) indicated that all water types are interacting with the surrounding geological materials. However, they differ in the degree of interaction as reflected by the positions of various water types in Fig. 19. Surface water types are well characterized by Gibb’s plot. For instance, leeward lakes (Small Momella and Mlolozi, Fig. 2) are typically affected by evaporation (Fig. 19) and about 30% of the windward springs fall in the field of rainfall dominance (Fig. 20).
Modified Gibb’s plot for groundwater from Meru district (After Narany et al. 2014)
Water quality for irrigation purposes
To assess water quality for irrigation purposes, pH, electrical conductivity (EC), residual sodium carbonate (RSC), sodium adsorption ratio (SAR), sodium percent (%Na), magnesium adsorption ratio (MAR), and Kelley’s ratio (KR) were considered. The results are presented in Table 4 together with the acceptable recommended standards.
pH The suitable pH range for agriculture purposes is between 6.5 and 8.4 (Bashir et al. 2013). Based on this range and Tables 3, 4, 20% of the boreholes, 56.25% of dug wells, 25% of the lakes, 33.3% of the springs, and 55.6% of streams had pH beyond the recommended standard.
Residual sodium carbonate (RSC) Results indicated that most of the dug wells and boreholes were above the recommended minimum standard values of 2.5 (Bashir et al. 2013) and that most of the springs were within the permissible limit, while the stream and lakes had intermediate values (Table 4).
Sodium percent (%Na) Results indicated that 57% of all samples belong to unsuitable category with %Na above 80. The dug wells were totally affected with greater than 80% of the samples falling under this category and 12.5% falling in the doubtful category (Table 4). Also, for the remaining water types (lake, stream and spring), only 26% were under the permissible limit of %Na below 60% while 47% fell in the doubtful category.
Sodium adsorption ratio (SAR) Results showed that springs, streams and boreholes had average SAR values of 4.4, 5.1 and 7.8, respectively. These values are within the excellent range of SAR < 8 (Bashir et al. 2013). The lakes and dug wells had average SAR values of 18.8 and 16.5, respectively, classified in the intermediate water quality for irrigation based on SAR (Bashir et al. 2013). Only one borehole sample, one lake sample, and four dug wells exceeded the minimum standard of SAR = 26 (Bashir et al. 2013).
Magnesium adsorption ratio (MAR) Results showed that all sample types with exception of one lake sample (Small Momella, MAR = 81.2%) and one spring (Mburukuu, MAR = 50.3%) had MAR values within the acceptable range of MAR < 50% (Bashir et al. 2013).
Kelley’s ratio (KR) Results indicated that only 18.5% of the samples had KR values less than a unity which is good for irrigation (Bashir et al. 2013). For all sample types, the percentage of samples exceeding the recommended standard of a unity is high compared to all other measured irrigation parameters (Table 4).
For evaluation purposes of all water types, the obtained irrigation parameter results were plotted using standard diagrams which combine different parameters as shown in Figs. 21 and 22. Integrating the two diagrams, results indicated that the suitability of water types for irrigation is moderate in the order of springs > streams > boreholes > lakes > dug wells.
Classification of Meru water types for irrigation purposes (After Wilcox 1955). a Classification based on RCS and SAR with exception of Lake small Momella with RCS = 80.65 and SAR = 60.15. b Classification based on percent sodium and electrical conductivity with exception of Lake small Momella with EC = 8715 and %Na = 99.06
Discussion
Hydrogeochemical characteristics of Meru waters
Characterization of physicochemical parameters
The pH of the water samples indicated that waters were under nearly neutral to alkaline conditions. Nearly neutral pH values for the windward springs located on the southern slope of Mt. Meru (Fig. 1) suggest short water–rock interaction time. This is highly supported by the Gibb’s plots where the windward springs were plotted within and near the rainfall dominance field (Fig. 20) and their low dissolved ions were relative to other sample types (Fig. 6). These springs emerge on the fractured nephelinitic-to-phonolitic formation on the slope of Mt. Meru (Fig. 2; Ghiglieri et al. 2012) which is the main recharge zone. Therefore, short water–rock interaction time is attributed to both fractured formation and high hydraulic gradient. The alkaline condition for most of the springs in the leeward side (north of Mt. Meru, Fig. 1) could be attributed to a significant water–rock interaction time and low dilution (low rainfall) enhanced by evaporation relative to windward side. The low DO in dug wells relative to boreholes, springs, and surface water types is mainly due to poor aeration, high levels of turbidity, and organic matter. Poor aeration is due to the fact that most of the dug wells were not frequently in use because of either poor water quality for domestic purposes or the intended purposes such as seasonal irrigation and washings which do not require continuous supply of water. Most of the dug wells were shallow, constructed locally in residential areas and not protected from surface runoff. Such well’s condition favored accumulation of organic matter (OM) and sediments leading to high turbidity and growth of oxygen consuming organisms. Low DO in Ngurdoto Crater Lake (Fig. 2) was attributed to abundance of aquatic plants as observed during the sampling campaign.
Total dissolved solids (TDSs) and electrical conductivity (EC) reflect mainly the rock solubility, water–rock interaction time, precipitation, and evaporation rates. More ions are dissolved as water interacts with rocks and their concentration increases with increasing evaporation rates while decreasing with increasing precipitation. Thus, as lake Small Momella is in the leeward side of Mt. Meru (Fig. 2), abnormal high TDS (4357 mg/L) in this lake could be attributed to low precipitation, intensive water–rock interaction time, and evaporation. The studies by Dawson (2008) and Delcamp et al. (2013) revealed that Momella lakes were formed as a result of mass movement/collapse of Mt. Meru blocks. Therefore, it is likely that such mechanism enhanced dissolution process by generating huge loose materials leading to high dissolved ions. However, this study indicated high sulfate concentration (300 mg/L) which is not reflected in the surrounding Mt. Meru rocks suggesting the link between lake water and deep aquifers. As the windward side is characterized by high precipitation relative to leeward side (Oettli and Camberlin 2005), the low TDS in crater lakes Duluti and Ngurdoto (Fig. 2) in windward side is largely the result of high precipitation and low evaporation.
Slightly high TDS in streams relative to springs indicate that streams are largely gaining streams, receiving water which has been interacted with the surrounding rocks particularly lahars which are dominant in the study area (Fig. 2). Such lahars which have been documented by many researchers along the rift system (e.g., Nanyaro et al. 1983; Deocampo 2004; Dawson 2008; Ghiglieri et al. 2010, 2012) account for more dissolved ions in groundwater due to their susceptibility to weathering through texture and high ash content. Furthermore, field observation indicated that most of the streams have developed great depths sometimes above 10 m which is likely to favor movement of water from the surroundings to streams.
The significant contrast in TDS for the boreholes and dug wells is mainly attributed to their spatial distribution and evaporation–crystallization circle. Unlike the boreholes, most of the sampled dug wells were in areas dominated by lahars which are susceptible to weathering (e.g., Maji ya Chai-Kikatiti-Maroroni zone) (Figs. 1 and 2). Through Gibb’s plot, it is revealed that evaporation–crystallization is the dominant process for dug well water type. Such processes have been known to contribute to dissolved ions in surface and groundwater (Nielsen 1999; Kaseva 2006) with salt crystallization in the dry season and salt dissolution in the rainy season. Therefore, such circles near the earth surface contribute to high dissolved ions in the dug well.
Hot springs which are connected to deep aquifers were not encountered in this study. Therefore, the temperature difference in water types could be largely attributed to altitude and geographic positions (climatic condition) in relation to Mt. Meru. For instance, the maximum temperature of 27.84 °C was observed at Lake Mlolozi in the leeward side of the mountain. The lowest temperatures generally between 17 and 13 °C were mostly observed in the springs at an altitude above 2000 m a.s.l, whereas elevated temperatures generally between 24 and 28 °C were mostly observed in the borehole and dug wells at an altitude below 1000 m a.s.l.
Water quality for domestic purposes
The general ion dominance pattern in water types was lakes > dug wells > borehole > streams > springs with the ions showing the dominance pattern of Na+ > K+ > Ca2+ > Mg2+ in all water types. Since the rocks along the East Africa continental rift are generally alkaline (Gaciri and Davies 1993; Peccerillo et al. 2007; Ghiglieri et al. 2012), high Na+ as well as K+ in water is basically the results of dissolution of alkaline rocks. The positive correlation of these two cations (R = 0.85) suggest that they are likely to be released from the common source. For instance, the rock such as nephelinite with nepheline (Na3KAl4Si4O16) as the dominant mineral which is among the dominant rocks in the study area (Fig. 2) is likely to release high Na+ and K+ through weathering process. Furthermore, a study by Ghiglieri et al. 2012 in the northern part of Mt. Meru indicated that lahars, which are also dominant in the study area (Fig. 2) are rich in Na-bearing minerals such as augite, anorthoclase, and albite. These minerals can also contribute to high Na+ in groundwater. This is well supported by elevated Na+ and TDS for the dug wells drilled in lahar materials. For instance, for dug well D1 (Fig. 2), Na+ and TDS were as high as 390 and 1823 mg/L, respectively.
Therefore, the natural occurrence of Na+ and K+ explain their abundance in water type. High K+ above the recommended standard of 12 mg/L (WHO 2011) for 80% of the samples and high Na+ above the recommended WHO threshold taste of 200 mg/L (WHO 2011) for 50% of the dug well and two leeward lakes is attributed to water–rock interaction, susceptibility of materials to weathering, evaporation and precipitation rates as discussed previously. The low K+ relative to Na+ could be attributed to the dominance of Na+ relative to K+ bearing minerals in parent materials. The resistance of K-feldspar relative to plagioclase minerals in weathering could be the other factor, but these minerals are generally rare relative to nepheline and leucite minerals in weakly fractionated rocks.
The low Mg2+ and Ca2+ reflect their low abundance in parent rocks relative to Na+ and K+. Their positive correlation (R = 0.85) indicate that common source materials. These two cations were under the recommended drinking water quality limit of 75 and 50 mg/L, respectively (WHO 2008) with exception of one dug wells where elevated Ca2+ up to 82 mg/L could be attributed to the geology at that particular area. Also, as Dawson (2008) reported carbonatitic materials around Lake Ngurdoto (Fig. 2), the elevated Ca2+ in this lake is likely to be attributed to carbonatite. The high concentration of Mg2+ and Ca2+ in the windward side relative to leeward side was mainly attributed to abnormal elevated concentrations of Ca2+ (40.18 mg/L), Mg2+ (23.65 mg/L) at Nduruma spring and Ca2+ (20.12 mg/L), Mg2+ (7.39 mg/L) at Makisolo spring (Fig. 2). These springs are located away from the foot of Mt. Meru relative to other springs (not on the slope of the mountain) suggesting a reasonable water–rock interaction time from the recharge to discharge point particularly through mantling ash as shown in Fig. 2. Thus, as the geology is too erratic because of explosive eruptions along the East Africa Rift System (Rango et al. 2010), elevated Ca2+ and Mg2+ values are likely to result mainly from dissolution of Ca–Mg-bearing minerals such as pyroxene being concentrated in some specific zones.
In the study area, carbonate rocks cannot justify high HCO3− in water systems, and therefore, it is strongly linked to volcanic activities. Two main factors: weathering of silicate minerals and CO2 emission through volcanic activities, explain high HCO3− + CO32− in Meru waters. It has been reported elsewhere that HCO3− being derived from CO2 gas is common in active volcanic areas (Pecoraino et al. 2015). Along the East Africa Rift System, emission of gases such as CO2 and SO2 are common and recently in 2011, there was emission of strong SO2 gas near the border between Ethiopia and Eritrea along the rift (Vye-Brown et al. 2014). Therefore, with the recent eruptions at Mt. Meru, the last eruption being in 1910 (Vye-Brown et al. 2014), weathering of silicate minerals through hydrolysis under presence of the emitted CO2 has been leading to high HCO3− + CO32− in water systems. Since CO2 acts as a catalyst in water–rock interaction process (Pecoraino et al. 2015), its presence in volcanic materials, on the other hand, supports the obtained strong positive correlation between HCO3− and TDS (R = 0.96). This fact together with other factors such as common source explains a strong positive correlation between HCO3− with other ions particularly Na+ (R = 0.92), K+ (R = 0.90), F− (R = 0.90), Cl− (R = 0.73), CO32− (R = 0.79) and SO42− (R = 0.66) in groundwater.
High fluoride content along the East Africa Rift System has been reported by many researchers (e.g., Nanyaro et al. 1983; Gaciri and Davies 1993; Ghiglieri et al. 2010, 2012; Rango et al. 2010). The findings from this study are in agreement with the previous researchers. The mean F− values were found to be above the recommended standard of 1.5 mg/L, (WHO 2008) in all water sources. The most affected areas were the leeward side of Mt. Meru (Ngarenanyuki and Oldonyosambu) and in the windward side at the foot of Mt. Meru (Maji ya Chai-Kikatiti-Makiba–Maroroni) (Fig. 1). Integrated factors leading to high fluoride in such areas include abundance of volcanic materials rich in fluoride, significant water–rock interaction time, high evaporation, and low precipitation. In these areas, the communities are strongly affected by dental and skeletal fluorosis, and in some places, severe effects such as crippling skeletal fluorosis are pronounced. Livestock keeping is one of the major activities in such areas with cattle consuming large amount of water with high fluoride per time. The effects of fluoride in such cattle are likely to be high, but justification is still difficult because of the little attention on effects of fluoride on animals.
The high fluoride in lakes and dug wells relative to other water types follows the same mechanism that led to high TDS and ions such as Na+, K+, and HCO3− in waters. This is supported by the obtained strong positive correlation of F− with TDS, HCO3–, CO32−, Na+, and K+ (Fig. 11). The lack of a significant correlation between F– with Ca2+ and Mg2+ is basically due to the strong affinity between Ca2+ and Mg2+ with F− relative to Na+. That, under favorable condition, in areas with high Ca2+ and Mg2+, CaF2 and MgF2 will precipitate leading to low fluoride in water system.
Chloride abundance and correlation behavior was similar to fluoride suggesting the common source. However, the correlation of Cl− with F− was not so strong (0.6) (Fig. 11) suggesting that Cl− is also likely to result from anthropogenic processes. Anthropogenic activity is further supported by a significant Cl− contrast in boreholes and dug wells with the highest Cl− (300 mg/L) above the recommended standard of 200 mg/L (WHO 2008) in the dug well located about 5 m from the pit latrine.
The wide range of sulfate in dug well (8–320 mg/L, mean = 56.8 ± 77 mg/L) and lakes (2–300 mg/L, mean = 88.5 ± 142.8 mg/L) led to high standard deviation above the mean values. Positive correlation of sulfate with TDS (R = 0.76), K+ (R = 0.79), Cl− (R = 0.90), Na+ (R = 0.66), F− (R = 0.67), HCO3− (R = 0.66) (Table 3) suggest SO42− to result from water–rock interaction. A strong positive correlation between SO42− and Cl− suggest the common source. The low concentration of SO42− in springs relative to other sources could be attributed to short water–rock interaction time. That high SO42− in dug wells and lakes is related to intensive water–rock interaction time. On the contrary, as SO42− can results from anthropogenic activities particularly sewage effluent and agricultural activities (Jiang et al. 2009), and most of the dug wells were located in high density areas, some close to pit latrines, high SO42− in dug wells relative to other sources could indicate anthropogenic source. This is supported by high level of SO42− up to 320 mg/L for one hand-dug well located close to pit latrine. Anthropogenic cannot explain high SO42− in lake Small Momella (300 mg/L) because the lake is free from both human activities and surface inflow. Since there is no documented sulfate deposit surrounding the lake which could account for high SO42− in the lake, it is suggested that there are SO42−-rich springs feeding the lakes which are connected to deep aquifers. Therefore, with the above view, both natural and anthropogenic sources account for the obtained SO42− values in the study area.
The levels of nitrate in groundwater have been used as an indicator of pollution (Wick et al. 2012; Elisante and Muzuka 2015) owing to the fact that there are no reported rock nitrate deposits in nature. The observed high nitrate concentration in dug wells, in which 12.5% of the dug well sampled had NO3− above the recommended limit of 50 mg/L (WHO 2008), suggests anthropogenic activities and not water–rock interaction processes. Most of the dug wells were located in residential areas which had also high number of pit latrines, cowsheds, and other sewage systems that are likely to be releasing nitrate to local constructed dug wells. Thus, springs, lakes, and borehole water types had low nitrate concentration because of either being free from human activities or low surface input. This is well supported by the observation within the lakes; that lake Duluti, the only lake centered close to local communities had high NO3− (12.76 mg/L) relative to all other lakes (mean = 5.3 mg/L, n = 3). Elevated nitrate up to 136.4 mg/L in one surface sample (Kyamang’ata stream) (Fig. 2) could be attributed to animal excreta because it was a cow path with low water discharge. The obtained anion dominance pattern of HCO3− > CO32− > Cl− > SO42− > NO3− > F− compare well with the pattern obtained by Rango et al. 2010 (HCO3− > Cl− > SO42− > F− > NO3−) in the northern part of the same rift system in Ethiopia with exception of F− and NO3− which are interchanged. The interchange of F− and NO3− indicates nitrate contamination by anthropogenic activities in the study area.
Groundwater evolution and geochemical processes
Using Gibb’s plots, it is clear that weathering of rocks is the major process controlling groundwater chemistry in the study area (Figs. 19, 20). There is relatively high dissolution in shallow parts which are dominated by lahars. The distribution of borehole and dug well hydrogeochemical data in Gibb’s plot indicated that evaporation–crystallization process dominated in the shallow part of the earth surface where dug wells are constructed (< 20 m below the surface) and weathering of the rocks dominated at great depth, generally between 20 and 150 m which is a depth range of the sampled boreholes. This is in agreement with the study by Kaseva (2006) in the study area that there is significant concentration of ions such as F− in water resulting from crystallized salts at or near the surface.
Water–rock interaction and evaporation processes are well indicated by hydrogeochemical data of the streams and springs in Gibb’s plot (Figs. 19, 20). Water sample from Ngarenayuki river (Fig. 2), the only perennial river in the leeward side of Mt. Meru, plots in the rock dominance field but close to evaporation–precipitation field indicating that apart from weathering of rocks particularly Ngarenayuki lahars (Fig. 1), evaporation also plays a significant role in controlling water chemistry of the river. This accounts for high dissolved ions particularly Na+, K+, HCO3−, F−, and SO42− in this river relative to other rivers. However, as SO42− can also results from human activities (Jiang et al. 2009), high SO42− could have been also attributed to agricultural activities particularly tomatoes which are commonly practiced in this area as discussed previously. Three stream samples in the windward side of the mountain: one falling in the field of rainy dominance and two falling close to the boundary of rainfall and rock dominance (Fig. 20), indicate dilution effect (high precipitation) enhanced by low evaporation for the windward streams relative to leeward streams.
It is clearly indicated that in the windward side where most of the springs and streams are located and evaporation is not intensive, stream samples showed elevated TDS relative to spring samples. It was also noted that spring samples falling in the field of rainfall dominance were typically located on the high slope of Mt. Meru and most of the springs falling in the field of rock dominance were either on the leeward side or on the lower slope/foot of the mountain. Such observations indicate that the slopes on Mt. Meru are the main recharge zone. Springs have undergone short water–rock interaction time relative to streams and water chemistry change progressively as water interacts with rocks. Such findings agree with the study by Ghiglieri et al. (2012) where short water interaction time was inferred through isotopic studies.
Two different fields for the lake samples in Gibb’s plot are mainly attributed to rainfall and temperature differences in the windward and leeward sides of Mt. Meru as discussed previously. Due to this fact, water chemistry in Lakes Small Momella and Mlolozi (Fig. 2) which are in the leeward side is mainly controlled by evaporation–crystallization processes whereas the water chemistry in the crater lakes in the windward side (Duluti and Ngurudoto) is mainly controlled by weathering process (Fig. 19). However, it is further noted that the intensity of these processes varies depending on the other factors such as geology, geographic position relative to Mt. Meru and vegetation cover. For instance, the effect of evaporation is well reflected at lake Small Momella (plotting in the evaporation field in Figs. 19 and 20). The effect of rock weathering is low for lake Ngurdoto as it plots close to the rainfall dominance and this is attributed to high rainfall supported by intensive vegetation cover which reduces evaporation. For Lake Duluti, despite being in the windward side, the weathering process dominates indicating interaction of lake water with the surrounding lahars and mantling ash (Fig. 2).
The dominance of weathering process is further supported by Piper, Langelier, and Ludwig (1942) and Chadha’s diagrams (Figs. 16, 17, 18), with Na–K–HCO3 as the major water type which is a typical characteristic of groundwater in areas where weathering and ion exchange processes dominate. However, excessive evaporation and low rainfall led to elevated Na+, K+, and HCO3− ions in the leeward relative to windward water types as evidenced by springs. One sample belonging to Na–K–Cl water type is basically the effect of Cl− contamination by anthropogenic processes and few samples belonging to Ca2+ (Mg2+)–HCO3− water type is probably indication of abundance of Mg2+ and Ca2+ in source materials in that areas. Overall, water chemistry changes progressively to more alkaline (enrichment of Na+, K+ and HCO3−) as it interacts with the rocks and atmosphere through weathering and evaporation processes.
Water suitability for irrigation purposes
Groundwater in Meru district was characterized by a wide range of suitability for irrigation purposes from excellent to poor quality. It is clearly shown that geology and water–rock interaction time plays a significant role in controlling water suitability for irrigation. The dug well water types were found to be of low quality due to their high TDS relative to all water types as discussed previously. The dominance of Na+, K+, and HCO3− as reflected in the RCS, %Na and Kelley’s ratio is the key factor contributing to low water quality for irrigation purposes. High RCS and %Na above the recommended standard and Kelley’s ratio above the unity have effects on crop productivity particularly through soil texture. Continuous application of such water leads to soil clogging and thus lowering soil permeability (Bo et al. 2013; Kumar et al. 2013; Srinivas et al. 2014; Nagaraju et al. 2016). Effects such as crop burn in the gardens (flowers and pawpaw) which are likely to be attributed to poor water quality for irrigation were observed in the study areas in some specific areas (Kikatiti–Maroroni, Fig. 1) where crops were irrigated using dug well water with high RCS, %Na, and Kelley’s ratio above unity.
Unlike Lakes Duluti and Ngurdoto in the windward side, lakes Small Momella and Mlolozi in the leeward side were found to be characterized by low water quality for irrigation. The low quality was mainly attributed to high dissolved ions as explained previously. Generally, the overall order of water quality for irrigation follows the trend of water quality for domestic purposes in the order of springs > streams > boreholes > lakes > dug wells. However, this is a general trend in which the irrigation parameter diagrams (Fig. 21, 22) indicate that water suitability is not a function of water type only; dug wells are generally of poor quality, but there are some few dug wells with suitable water for irrigation. Other factors related to geology and water rock interaction processes might have significant contribution in controlling the water quality. Furthermore, it should be noted that apart from water chemistry, the factors like soil texture, structure and composition, local irrigation practices and types of crops are important factors in assessing water quality for irrigation purposes (Srinivas et al. 2014).
Conclusions and recommendations
Groundwater in Meru district showed variation in hydrogeochemical characteristics. Despite the internal variation within the water types, significant variation was according to water types. Geology, water–rock interaction time and climatic conditions were found to be the main factors which control hydrogeochemical characteristics of groundwater. Overall, the dominant patterns of major cation and anion in water types were Na+ > K+ > Ca2+ > Mg2+ and HCO3− > CO3− > Cl− > SO42− > NO3− > F−, respectively, with the dominant pattern of dissolved ion being in the order of lakes > dug wells > borehole > streams > springs. Springs particularly from the windward side on the slope of Mt. Meru were characterized by short water interaction time relative to all other water type. This mainly due to proximity to the recharge zone (Mt. Meru), fractured nephelinitic-to-phonolitic lavas where most of the springs emerge and high hydraulic gradient on the slope of Mt. Meru. This was evidenced by low dissolved ions and their position in water–rock interaction plots where the samples fell close to the rainfall dominance. Water chemistry changes progressively to more alkaline as water interacts with the rocks from recharge zone (Mt. Meru) to discharge zones. Springs in the leeward sides showed slightly high dissolved ions relative to windward springs indicating that low precipitation and excessive evaporation have great influence in controlling water chemistry. Climate effect is well observed in water–rock interaction plots where the typical leeward water types showed that weathering of rocks together with evaporation–crystallization are the dominant process whereas rainfall and weathering of rocks dominate in the leeward sides. Hydrochemical facies indicated that Na–K–HCO3 water type dominates to about 90%. High Na+ and K+ is the reflection of their dominance in parent materials which are alkaline in nature, likely to be released from minerals such as nepheline, augite, anorthoclase which are dominant in the lahars and nehelinitic lavas. Correlation analysis indicated that HCO3− correlated positively with TDC, EC, Na+, K+, F−, and Cl− implying that they originate from the rocks through weathering and CO2 triggered the dissolution process.
This study revealed that more ions are dissolved close to the earth surface (0–20 m, dug well zone) particularly in areas dominated by lahars, ash and pyroclastic materials. This indicates that these materials are weak to weathering and therefore responsible for high dissolution of ions including fluoride. Unlike areas which are dominated by fractured mafic volcanics, nephelinitic-to-phonolitic lava, breccia and tuffs which host shallow and deep aquifers, shallow aquifers in lahar materials are characterized by groundwater of low quality in terms of fluoride and TDS. In such areas, salts crystallization and dissolution in dry and wet seasons, respectively, could be the other factor contributing to high dissolved ions in shallow groundwater as well as streams in the leeward side such as Ngarenanyuki and Kyamang’ata. Similarly, apart from intensive evaporation, high dissolved ions in the leeward lakes relative to windward lakes could also be attributed to the presence of these materials. However, high sulfate concentrations in Momella lakes which are free from surface input suggest the lakes to be fed by underground springs.
The suitability of water for domestic and agricultural purposes was found to be moderate, following the same order pattern of springs > streams > boreholes > lakes > dug wells. Fluoride was found to be the major natural pollutant lowering the water quality for domestic purposes (mean = 17.6 mg/L). 78% and 52% of the samples had fluoride above the recommended WHO and Tanzanian standard, respectively. Anthropogenic pollution was recognized through nitrate particularly in the dug wells located in residential areas where latrines are locally constructed. Chloride and sulfate were found to result from both natural and anthropogenic sources. High TDS, particularly in dug wells, along with abundance of Na+ (mean = 118 mg/L) and HCO3− (mean = 390 mg/L) in Meru waters were found to lower significantly the water quality for irrigation purposes.
To great extent, the effects of high fluoride in drinking water are known to the communities. However, the use of such water along with Na+ and HCO3− beyond the recommended standard in irrigation and livestock purposes has been the common practice. Thus, lowering of crop productivity and consumption of agriculture products with high fluoride unknowingly is inevitable. Therefore, this study recommends (1) intensive water quality analyses pre-utilization and effective utilization of spring water for domestic purposes; (2) more researches on fluoride mobility from soil to plants, its levels and distribution in both plants and livestock; (3) researches on crop productivity effects resulting from of high Na+ and HCO3− in water system; (4) further geological works for intensive assessment of rocks and their spatial distribution including fluoride bearing materials; (5) leaching process so as to assess the materials responsible for high dissolved ions including fluoride; and (6) further geological, geophysical, and geochemical works for delineation of low fluoride groundwater zone for domestic and agriculture purposes.
References
Bashir E, Naseem S, Hanif H, Pirzada T (2013) Geochemical study of groundwater of Uthal and Bela areas, Balochistan and its appraisal for drinking and irrigation water quality. Int J Agric Environ 2:1–13
Bo Y, Liu C, Jiao P, Chen Y, Cao Y (2013) Hydrochemical characteristics and controlling factors for waters’ chemical composition in the Tarim Basin, Western China. Chemie der Erde-Geochemistry 73:343–356
Bowell RJ, McEldowney S, Warren A, Mathew B, Bwankuzo M (1996) Biogeochemical factors affecting groundwater quality in central Tanzania. Geol Soc London Spec Publ 113(1):107–130
Bowell RJ, Robertson S, Walles K, Bwankuzo M, Cowan J, Kimaro N, Mjengera HA, Terkhell K, Warren A, Wood A (1997) Improving water quality assessment and supply. In: Pickford J (ed) Water and sanitation for all: partnerships and innovations. Proceedings of the 23rd WEDC Conference, Durban, South Africa, pp 146–149
Chacha N, Njau KN, Lugomela GV, Muzuka ANN (2018) Groundwater age dating and recharge mechanism of Arusha aquifer, northern Tanzania: application of radioisotope and stable isotope techniques. Hydrogeol J 26(8):2693–2706
Dawson JB (2008) The Gregory rift valley and Neogene-recent volcanoes of northern Tanzania. Geological Society of London, London
Delcamp A, Kwelwa S, Macheyeki A, Kervyn De Meerendre M (2013) Multiple collapses at Mt Meru volcano, Tanzania: remote sensing and field evidences from debris avalanche deposits, EGU General Assembly Conference Abstracts, p 7775
Deocampo DM (2004) Hydrogeochemistry in the Ngorongoro Crater, Tanzania, and implications for land use in a World Heritage Site. Appl Geochem 19:755–767
Elisante E, Muzuka AN (2015) Occurrence of nitrate in Tanzanian groundwater aquifers: a review. Appl Water Sci 7:1–17
Elisante E, Muzuka AN (2016a) Assessment of sources and transformation of nitrate in groundwater on the slopes of Mount Meru, Tanzania. Environ Earth Sci 75:277. https://doi.org/10.1007/s12665-015-5015-1
Elisante E, Muzuka AN (2016b) Sources and seasonal variation of coliform bacteria abundance in groundwater around the slopes of Mount Meru, Arusha, Tanzania. Environ Monit Assess 188:395. https://doi.org/10.1007/s10661-016-5384-2
Gaciri S, Davies T (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Ghiglieri G, Balia R, Oggiano G, Pittalis D (2010) Prospecting for safe (low fluoride) groundwater in the Eastern African Rift: the Meru District (Northern Tanzania). Hydrol Earth Syst Sci Dis 14:1081–1091
Ghiglieri G, Pittalis D, Cerri G, Oggiano G (2012) Hydrogeology and hydrogeochemistry of an alkaline volcanic area: the NE Mt. Meru slope (East African Rift-Northern Tanzania). Hydrol Earth Syst Sci 16:529–541
Glover E, Akiti T, Osae S (2012) Major ion chemistry and identification of hydrogeochemical processes of ground water in the Accra Plains. Geoscience 50:10279–10288
Jiang Y, Wu Y, Groves C, Yuan D, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J Contam Hydrol 109(1–4):49–61
Kaseva M (2006) Contribution of trona (magadi) into excessive fluorosis—a case study in Maji ya Chai ward, northern Tanzania. Sci Total Environ 366(1):92–100. https://doi.org/10.1016/j.scitotenv.2005.08.049
Kashaigili J (2012) Tanzania. In: Pravelic P, Giordano M, Keraita B, Ramesh V, Rao T (eds) Groundwater availability and use in Sub-Saharan Africa: a review of 15 countries. International Water Management Institute, Battaramulla, pp 195–216
Kumar XRA, Giridharan L, Shyamala J, Velmurugan P, Jayaprakash M (2013) Urbanisation impact of groundwater quality in Cuddalore District, East Coast of India. J Environ Chem Ecotoxicol 5:63–73
Langelier W, Ludwig H (1942) Graphical methods for indicating the mineral character of natural waters. J Am Water Works Assoc 34(3):335–352
Malago J, Makoba E, Muzuka ANN (2017) Fluoride levels in surface and groundwater in Africa: a review. Am J Water Sci Eng 3(1):1–17. https://doi.org/10.11648/j.ajwse.20170301.11
Mashindano O, Hassan AK, Kida T, Nzuki M, Kazi V, Mbilinyi AV (2013) Post MDGs development agenda: what future do we want and how can we achieve it? The Economic and Social Research Foundation. TAKNET Policy Brief Series No. 018–2013. http://www.taknet.or.tz/topics/PB_MDGS.PDF. Accessed on 24 Sept 2015
Mjemah IC, Van Camp M, Martens K, Walraevens K (2011) Groundwater exploitation and recharge rate estimation of a quaternary sand aquifer in Dar-es-Salaam area, Tanzania. Environmental Earth Sciences. 63(3):559–569
Mjemah IC, Mtoni Y, Elisante E, Tungaraza CT, Mtakwa PW, Walraevens K (2012) Sources of salinity in the Quaternary sand aquifer of Dar es Salaam, Tanzania. In: da Silva Jr GC, Montenegro SMGL (eds) Proceedings of the 22nd Saltwater Intrusion Meeting (SWIM), 17–21 June, 2012. META. Buzios, Brazil (pp 264–267)
Mohammed SM (2002) A review of water quality and pollution studies in Tanzania. AMBIO J Hum Environ 31:617–620
Mtoni YE, Mjemah I, Msindai K, Van Camp M, Walraevens K (2012) Saltwater intrusion in the Quaternary aquifer of the Dar es salaam Region, Tanzania. Geol Belg 15(1–2):16–25
Mtoni Y, Mjemah IC, Bakundukize C, Van Camp M, Martens K, Walraevens K (2013) Saltwater intrusion and nitrate pollution in the coastal aquifer of Dar es Salaam, Tanzania. Environ Earth Sci 70(3):1091–1111
Nagaraju A, Muralidhar P, Sreedhar Y (2016) Hydrogeochemistry and groundwater quality assessment of Rapur Area, Andhra Pradesh, South India. J Geosci Environ Protect 4:88
Nanyaro J, Aswathanarayana U, Mungure J, Lahermo P (1983) A geochemical model for the abnormal fluoride concentrations in waters in parts of northern Tanzania. J Afr Earth Sc 2:129–140
Napacho ZA, Manyele SV (2010) Quality assessment of drinking water in Temeke District (part II): Characterization of chemical parameters. Afr J Environ Sci Tech 4(11):775–789
Narany ST, Ramli MF, Aris AZ, Sulaiman WNA, Juahir H, Fakharian K (2014) Identification of the hydrogeochemical processes in groundwater using classic integrated geochemical methods and geostatistical techniques. Sci World J. https://doi.org/10.1155/2014/419058
Nielsen JM (1999) East African magadi (trona): fluoride concentration and mineralogical composition. J Afr Earth Sci 29:423–428. https://doi.org/10.1016/s0899-5362(99)00107-4
Nkotagu H (1996a) Origins of high nitrate in groundwater in Tanzania. J Afr Earth Sci 22 (4):471–478
Nkotagu H (1996b) Application of environmental isotopes to groundwater recharge studies in a semi-arid fractured crystalline basement area of Dodoma, Tanzania. J Afr Earth Sci 22(4):443–457
Oettli P, Camberlin P (2005) Influence of topography on monthly rainfall distribution over East Africa. Climate Res 28:199–212. https://doi.org/10.3354/cr028199
Peccerillo A, Donati C, Santo AP, Orlando A, Yirgu G, Ayalew D (2007) Petrogenesis of silicic peralkaline rocks in the Ethiopian rift: geochemical evidence and volcanological implications. J Afr Earth Sc 48:161–173
Pecoraino G, D’Alessandro W, Inguaggiato S (2015) The other side of the coin: geochemistry of alkaline lakes in volcanic areas. In: Volcanic lakes chapter, Part of the series Advances in Volcanology, pp 219–237
Rango T, Bianchini G, Beccaluva L, Tassinari R (2010) Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J Afr Earth Sc 57:479–491
Srinivas Y, Hudson OD, Stanley RA, Chandrasekar N (2014) Quality assessment and hydrogeochemical characteristics of groundwater in Agastheeswaram taluk, Kanyakumari district, Tamil Nadu, India. Chin J Geochem 33:221–235
Thole B (2013) Ground water contamination with fluoride and potential fluoride removal technologies for east and Southern Africa. INTECH Open Access Publisher
UN (2015) The millennium development goals Report. United Nations, New York. http://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20%28July%201%29.pdf. Accessed 24 Sept 2015
UNICEF (2015) Progress on sanitation and drinking water-2015 update and MDG assessment. United State of America
United Nation Office for the Coordination of Humanitarian Affairs (UNOCHA) (2010) Water scarcity and humanitarian action: key emerging trends and challenges. Policy Development and Studies Branch, OCHA Occasional Policy Briefing Series—No. 4
Vye-Brown C, Crummy J, Smith K, Mruma A, Kabelwa, H (2014) Volcanic hazards in Tanzania. British Geological Survey, p 38
Wick K, Heumesser C, Schmid E (2012) Groundwater nitrate contamination: factors and indicators. J Environ Manage 111(3):178–186
Wilcox LV (1955) Classification and use of irrigation water. Washington: USDA, Circular 969, Wash., US. Dept. Agric., DC
Wilkinson P, Mitchell J, Cattermole P, Downie C (1986) Volcanic chronology of the Meru-Kilimanjaro region, northern Tanzania. J Geol Soc Lond 143:601–605
World Health Organization (WHO) (2008) Guidelines for drinking water quality. Third Edition, incorporating the first and second addenda, Volume 1, Recommendations. WHO, Geneva
World Health Organization (WHO) (2011) Guidelines for drinking-water quality, 4th edn
Acknowledgements
We thank the Tanzania Commission for Science and Technology (COSTECH), and VLIR-UOS Project through Nelson Mandela African Institution of Science and Technology (NM-AIST) for financial support. Appreciations are extended to unanimous reviewers for their constructive comments in reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Makoba, E., Muzuka, A.N.N. Water quality and hydrogeochemical characteristics of groundwater around Mt. Meru, Northern Tanzania. Appl Water Sci 9, 120 (2019). https://doi.org/10.1007/s13201-019-0955-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0955-3