Abstract
A new cellulosic material “corn silk” was modified with titanium dioxide nanoparticles as a novel photocatalyst support. In this study, the prepared support was tested for the removal of Reactive Black 5 (RB5) as an azo dye pollutant candidate from synthetic samples. High capability of decolorization (> 99%) was achieved after 30 s using the corn silk/TiO2 photo-biocatalyst. The effect of important parameters such as pH of the medium, the amount of photocatalyst, mixing rate and dye concentration was investigated and modified. UV–Vis spectroscopy, scanning electron microscopy (SEM), X-ray powder diffraction and Fourier-transform IR spectrometry were applied to characterize the effect of functionalization, structure, surface morphology and photocatalyst properties of the support and mineralization of pollutants. It was observed that the maximum decolorization of RB5 occurred at pH 3.0, 25 °C, 300 rpm, 30 s using the corn silk/TiO2 composite material for this study. The results reveal that corn silk/TiO2 composite has high and significant photocatalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive production of pollutants with increasing rate threatens the global environment seriously. On the other hand, by increasing public awareness, revolutionary approach to overcome to the problems of the environment is taking place. Finding appropriate solutions and valuable ideas that utilize economic policies, healthy and environmentally friendly methods, without destructive side effects with maximum response in removing pollutants of ecosystem is on the agenda of scientists. Among the wide spectrum of environmental pollutants, especially water contaminants, textile dyes have a larger stake. Remediation of dye-contaminated wastewater released from the textile and other dye industries is necessary to prevent pollution of soil, surface and ground water (Erturk 2010).
More than 0.7 million tons of organic dyes are produced each year globally. It is noted that there are over 10,000 commercially accessible dyes that are classified by their utilization fields, namely acid, reactive, disperse, vat, metal complex, mordant, direct, basic and sulfur dyes. Reactive dyes have been identified as the most environmentally problematic compounds in textile dye effluents for several reasons. First, reactive dyes are intensively used due to their preferable performance and their increasing market share. Second, they are very soluble and approximately 10–15% of the weight of applied reactive dyes is discharged from the dye houses. Third, conventional wastewater treatment units, which reliance on sorption and aerobic biodegradation, have a low removal output for reactive and other anionic soluble dyes. Consequently, they cause to be colored water channels and public complaints, and the pollutants are being transferred to another phase and not being eliminated (Dojcinovic et al. 2012).
Currently, various methods such as physical, chemical, physicochemical and biological treatments (Erturk 2010; Dojcinovic et al. 2012; Laohaprapanon et al. 2015), advanced oxidation (Wu et al. 2008) and electrochemical oxidation such as anodic oxidation (Dhaouadi et al. 2009) and electro-Fenton processes (Ramirez et al. 2013) are used to decolorization of this wastewater. Biological techniques are the most common used methods in textile industries as they have low processes cost. Nevertheless, due to complex molecular structure of dyes, aerobic degradation can only remove lesser degree of color. In addition, poor anaerobic degradation of azo dye releases toxic and potentially carcinogenic aromatic amine compounds in the treated waste (Laohaprapanon et al. 2015).
Enzymatic process is another method used for the removal of dyes. However, this technique is not effective as enzymes are inhibited at different pHs, temperatures and by inhibitors (Yanmis et al. 2013). Advanced oxidation processes (AOPs) are widely recognized as highly effective methods for obstinate wastewater treatment. AOPs destroy organic pollutants by forming hydroxyl radicals (Shoabargh et al. 2014). Titanium dioxide nanoparticles have attracted particular interest in AOPs due to their wide applications in various fields and extra benefits such as low cost, high photocatalytic activity and high stableness in comparing with other photocatalysts such as SnO2 and ZnO (Shoabargh et al. 2014). Immobilization of nanoparticles on a proper support can avoid the requirement for the separation of the surplus amount of photocatalyst from sewage. Supports may be organized into macro-particles, such as sand and glass beads (Khataee et al. 2009), glass tubes surrounding the light source in photoreactor (Ling et al. 2004) and some polymers (Mahmoodi et al. 2006; Kasanen et al. 2011).
Cellulosic materials can be a good support for immobilization of photosensitive nanoparticle, such as TiO2, ZnO and SnO2 due to their porosity, abundance, low cost, ease of usage, nontoxic, reusability and replacement. Despite these advantages, application of cellulosic material as support has been understudied. Luffa sponge as a cellulosic source was introduced and modified by ZnO nanoparticles (Nadaroglu et al. 2017). In here, we report the immobilization of TiO2 NPs on corn silk as a novel cellulosic photosensitive support. Corn silk is a fibrous, biodegradable and nontoxic material. We investigated the effect of operational parameters, including dye concentration, mixing speed, amount of CS/TiO2 nanophotocatalyst and pH on dye decolorization. Moreover, to evaluate the performance of prepared photosensitive support, decolorization of Reactive Black 5 under UV-C irradiation was studied in batch reactor and compared with previously reported processes.
Materials and methods
Chemicals
Reactive Black 5, hexadecyltrimethylammonium bromide (CTAB), hydrochloride acid (HCl), sodium hydroxide (NaOH), sodium hypochlorite (NaClO), titanium dioxide nanoparticles (TiO2) (< 20 nm) were purchased from Sigma-Aldrich Chemical. All the chemicals were used without further purification. Distilled water was used for all the tests (GFL 2004). Characteristics and structures of RB5 are summarized in Table 1.
Apparatus
The UV–Vis spectra of dye solutions were recorded from 300 to 900 nm using an Epoch Microplate Spectrophotometer. Decolorization of dye was determined by flowing the dye concentration using their maximum absorbance (597 nm) in a UV–Vis spectrophotometer (Epoch Microplate Spectrophotometer). pH of samples was adjusted by Crison model pH meter. Immobilization of TiO2 nanoparticles on the texture of corn silk was performed in the Ultrasonic bath (Kudos SK 10GT Model). Bachman Coulter model centrifuge was used to separate the solid photocatalyst from solution. The photocatalytic degradation of RB5 on the CS immobilized nano-TiO2 was performed under a 15 W/50 HZ UV-C lamp. The distance between the lamp and the phocatalyst reactor was 15 cm. The surface morphology of CS, TiO2, CS/TiO2 NPs and CS/TiO2/RB5 was monitored by scanning electron microscope (Zeiss Sigma 300 field emission SEM). X-ray diffractometer (XRD) of CS and TiO2 IML-CS, before and after dye treatment, was undertaken using a PANalytical Empyrean model XRD at Cu-Kα radiation (λ = 1.54 Å). The analysis of dried CS, TiO2 NPs-IML-CS and TiO2 IML-CS/RB5 was carried out by continuous scans from 10° to 100° at 2° scan rate at 2θ min−1 in ambient pressure.
FTIR analysis of RB5 dye, TiO2 and IML-CS with TiO2 NPs, before and after tests, was recorded using Vertex 80 Model FTIR Frontier spectrophotometer with attenuated total reflection (ATR) technique in the 4000–400 cm−1 region.
Supply and preparation of corn silk
Corn silk was supplied from local vendors. 25 g corn silk sample was weighed and added to 250 mL, 0.5 M NaClO solution. Then, this mixture was treated in a water bath (80 °C) for 1 h. Corn silk was washed thoroughly with 500 mL of pure water and then incubated in the same conditions with 1 M NaOH solution. Then, corn silk was completely washed with double distilled water and dried in oven at 60 °C for 8 h. The dried corn silk sample was fractionated with pure water in a steel blender. 0.02 g TiO2 NPs was then added to the obtained mixture and treated for 2 h in an ultrasonic bath at 60 °C for immobilizing TiO2 nanoparticles onto the cellulosic structure of corn silk. TiO2 immobilized corn silk was separated from the supernatant and washed 5 times with distilled water to remove unbound TiO2 NPs. The TiO2 immobilized corn silk support was dried in oven at 60 °C for 8 h and used in all the experiments.
Preparation of dye solutions
The stock solutions of RB5 were prepared in 50 mg L−1 concentration and diluted with deionized water. The pH of the solution was adjusted with diluted HCl or NaOH solutions.
Experiments procedure
Reactive Black 5 azo dye degradation was carried out in a closed system consisting of a magnetic stirrer and UV-C lamp (Fig. 1). For this purpose, the reaction medium was prepared by adding 250 mL (50 mg L−1) RB5 azo dye and 0.4 g of TiO2 NPs–CS catalyst in a 250 mL beaker. The same procedures were performed in CS samples. Distilled water was used as a blank sample. The dye removal efficiency was calculated using the following equation:
Removal (%) is dye removal efficiency, C0 (mg L−1) is the initial dye concentration, and Ct (mg L−1) is concentration of dyes at t time.
Effect of various parameters on dye removal
Degradation of RB5 dye was monitored by measuring absorbance at 597 nm. In order to determine the contact time, photodegradation reaction in UV system was followed for 10 min. Samples were taken at regular intervals from the reaction medium, measured against distilled water and degradation % was calculated using Eq. (1).
Also, the effects of stirring speed and medium pH on the photodegradation of RB5 were investigated. For this purpose, pH of RB5 azo dye was adjusted using 0.01 N HCl/NaOH solutions in the range of 3–10. At each pH, the change in absorbance was monitored at 597 nm by establishing the same experiments.
Effect of stirring rate
To study the effect of stirring speed on degradation of dye, 0.4 g corn silk was added to 25 mL RB5 azo dye at 50 mg L−1 concentration solution and reactions were occurred at 25 °C pH 3.0 at 100, 200 and 300 rpm. Absorbance was recorded for each speed at 597 nm against distilled water.
Effect of amount of support material
The different reactions were performed to investigate the effect of support material onto decolorization of RB5 using 0.1 and 0.8 g TiO2 NPs-IML-CS.
Effect of dye concentration
The effect of dye concentration on the photocatalytic degradation of RB5 azo dye was investigated by setting up the reactions using the same conditions (pH 3.0, 25 °C, 300 rpm) and at the broad spectrum of RB5 dye concentrations: 25, 50, 75, 100, 150 and 200 mg L−1.
Reusability tests for TiO2 NPs immobilized corn silk
The elimination of RB5 was performed in the presence of 0.4 g CS/TiO2 NPs photocatalyst in 10 cycles reactions to assess the potential reusability of TiO2 IML-CS-NPs at an initial dye concentration of 50 mg L−1. Before each cycle, TiO2 IML-CS-NPs pieces were washed three times with distilled water. All experiments were carried out in triplicates, and the data are presented as mean value.
Results and discussion
Characterization of support material
The surface morphology of TiO2 nanoparticles immobilized corn silk before and after removal of RB5 azo dye samples are given in Fig. 2. It is appeared to be immobilized TiO2 NPs on the corn silk fibrous structure very well in Fig. 2a. It was also observed from Fig. 2b that the photocatalytic effect of TiO2 NPs immobilized on CS is very obvious. It is quite clear from Fig. 2b that the degradation of Reactive Black 5 by bio-nanophotocatalyst was carried out successfully.
Figure 3 shows the FTIR spectra of CS, TiO2 NPs, TiO2 NPs-IML-CS and TiO2 NPs-IML-CS/RB5. In the FTIR spectrum of CS, the IR spectra had peaks at 1550–1770 cm−1 which was attributed to the C=O stretching and 2800–3000 cm−1 which correspond to the ester C=O stretching vibration and carboxylic acid O–H (Miao and Shanks 2011). The peaks at 3330 and 1612.5 cm−1 in the spectra are due to the stretching and bending vibration of the –OH group. In the spectrum of pure TiO2, the peaks at 560.0 cm−1 show stretching vibration of Ti–O and peaks at 1039.6 cm−1 show stretching vibrations of Ti–O–Ti (Vetrivel et al. 2014–2015). The FTIR spectrum of TiO2 NPs-IML-CS after treatment on RB5 shows the peak at 3331, 2922 and 2879.7 cm−1.
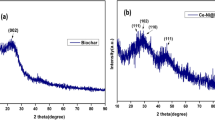
The XRD patterns of TiO2 NPs-IML-CS and TiO2 NPs-IML-CS + RB5 are depicted in Fig. 4. According to the CSPDS card No. 21-1272, the obtained peak at 2θ = 25° (101) belongs to TiO2 NPs (anatase) (Theivasanthi and Alagar 2013). Also, at the 2θ = 32°, 48°, 55° and 62° peaks are approved that TiO2 NPs are bound and immobilized to corn silk fibers (Thamaphat et al. 2008; Antic et al. 2012; Ba-Abbad et al. 2012). Based on this evidence by looking at the XRD diagrams of TiO2 NPs, TiO2 NPs-IML-CS and TiO2 NPs-IML-CS/RB5, it was observed that all the immobilized TiO2 NPs interacted with RB5 azo dye during the photodegradation under UV light. Therefore, all the peaks related to TiO2 in TiO2 NPs-IML-CS/RB5 were not observed and this event confirmed that the photodegradation is performed.
Effect of reaction time
To confirm the photodegradation of RB5 dye, the absorbance of the samples was monitored at several time points during the photoreaction. As seen from Fig. 5, there was a 99.95% decolorization in the presence of TiO2 NPs-CS-IML in first 30 s. It can be concluded that the immobilized TiO2 NPs onto CS fibers provide the possibility of the degradation of RB-5 dye (50 mg L−1) with high efficiency. When only corn silk was used as the degradation agent, 2 and 5% removal of RB5 azo dye was achieved for 30 and 60 s, respectively. For effective removal of RB5 azo dye at 30 s with TiO2 NPs-IML-CS, there are great benefits such as low cost, energy and time save in industrial scale.
Effect of pH
pH is an important factor for photodegradation reactions taking place on the surface of TiO2 nanoparticles. pH variation can in fact influence the adsorption of dye molecules onto the TiO2 surfaces (Saggioro et al. 2011). The effect of solution pH was studied in a range of 3–10 for the RB5 azo dyes using both CS and TiO2 NPs-IML-CS. Figure 6 shows the variation on the efficiency of the photocatalytic degradation of RB5 at different pHs. Photodegradation was found to be higher in acidic media (pH 3) using TiO2 NPs-IML-CS, with degradation rates of 99%. Up to a pH value of 7, the dye degradation efficiency decreased to 87.66% using TiO2 NPs-IML-CS. Above pH 7, the degradation continued to decrease to about 91.32% at pH 10. But, it was observed that TiO2 NPs-IML-CS was not effected by different pHs too much and it was stable during all remediation of RB5 in all pHs values.
TiO2 NPs-IML-CS surface is positively charged in acidic reaction medium (pH < 7.0), whereas under alkaline conditions (pH > 7.0) it is negatively charged (Zielinska et al. 2001). Seeing the structure of RB5 azo dye (Fig. 7), a positive charge excess in the TiO2 surface supports a strong interaction with four SO3− groups of the dye (Fig. 7a). A negative charge excess promotes the repulsion of the RB5 azo dye by the TiO2 NPs-IML-CS surface, reducing the catalytic activity of this photocatalyst (Fig. 7b). These results suggest that the influence of the initial pH of the solution on photocatalysis reaction kinetics is due to the amount of the dye adsorbed on TiO2 NPs-IML-CS (Zielinska et al. 2001; Saggioro et al. 2011). This hypothesis supports with a reaction occurring at TiO2 NPs-IML-CS surface and not in the solution, close to the surface.
Effect of stirring rate
The effect of stirring rate on the photoelimination reaction of RB5 azo dye was surveyed by changing the speed of mixing from 100 to 300 rpm in dye concentration of 50 mg L−1, contact time 30 s, and temperature 25 °C. As it is shown in Fig. 8, it was found that by increasing the stirring speed, photodegradation of RB5 is increased two units in aqueous solution in the presence of TiO2 NPs-CS. This increase in degradation of RB5 azo dye reached its maximum at 300 rpm as 99.96% removal rate using TiO2 NPs-IML-CS. The increase in photodegradation of RB5 might increase the contact between the surface of TiO2 NPs-IML-CS and RB5 by increasing the stirring speed (Gouvea et al. 2000).
Effect of dye concentration
The effect of the initial concentration of RB5 on the photoelimination of the dye under UV photon was investigated (Fig. 9). As seen in Fig. 9, there is no significant change in the color removal process. When the initial dye concentration decreases from 200 to 50 mg L−1, the removal efficiency of RB5 increases from 98.0 to 100%. These results indicate that the photodegradation rate of RB5 dye with this photocatalyst support is very effective and intriguing. The gradient of the photodegradation performance of RB5 dye by increasing the initial concentration of the dye from 50 to 200 mg L−1 is very feeble.
Effect of photocatalyst amount on degradation efficiency
The effect of the amount of TiO2 NPs-IML-CS was also investigated (Fig. 10). The results indicated that by increasing amount of TiO2 NPs-IML-CS from 0.05 to 0.75 g/50 mL, the removal efficiency of RB5 increases from 89.25 to 99.76%. Using CS and TiO2 NPs-IML-CS, RB5 with 0.4 g/50 mL of photocatalyst showed a degradation rate of 7 and 99.76%, respectively. It was observed that the efficiency of photodegradation of RB5 azo dye decreased by increasing the initial amount of photocatalyst (Daneshvar et al. 2003). Most likely, photocatalyst prevented the UV light reaching to the RB5 azo dye.
Reusability of bio-photocatalyst
In order to evaluate the reusability and the decolorization capacity of the prepared Photocatalyst (TiO2 NPs–CS), a series of experiments were designed and carried out in 10 cycles (Fig. 11). All parameters including irradiation time (30 s), pH 3.0, RB5 concentration (50 mg L−1) and amount of photocatalyst (0.4 g) were kept constant. The photocatalyst was separated from the solution mixture at the end of each cycle through filtration. The recovered photocatalyst was washed with distilled water and reused in the next degradation cycle. It was found that after 6 cycles, 93.42% removal of RB5 azo dye was obtained. Results showed no significant reduction in photocatalytic performance in photodegrading of RB5 at the first six times; thus, this indicated the stability of TiO2 NPs-IML-CS as a photocatalyst. At the end of ten degradation cycle, 82.21% of RB5 azo dye was removed. Therefore, based on our results from reusability tests, we can conclude that the dye removal capacity of this novel cellulosic photocatalyst is extremely high.
Conclusion
A novel cellulosic photocatalyst material corn silk–TiO2 was introduced and successfully applied for degradation of Reactive Black 5 (RB5) as a model azo dye under UV irradiation in batch reactors. High decolorization percentage (99.96%) was achieved in a very short time (30 s). XRD patterns showed the high loaded amount of TiO2 nanoparticle on cellulosic texture of corn silk and good treated with dye during photoreaction. Based on the UV–Vis and FTIR spectroscopy results, decolorization of RB5 confirmed the cleavage of azo bonds (N=N) of dye molecule. According to these results, the best reaction condition for RB5 degradation (%) was determined as 0.4 g/50 mL TiO2 NPs-IML-CS, 50.0 mg L−1 initial dye concentration, pH 3.0, mix rate 300 rpm and 25.0 °C temperature. The recycling of TiO2 NPs-IML-CS was performed and found to be adequately used up to six times. This new photocatalyst, in comparison with others, offers higher quality and efficiency.
References
Antic Z, Krsmanovic RM, Nikolic MG, Marinovic-Cincovic M, Mitric M, Polizzi S, Dramicanin MD (2012) Multisite luminescence of rare earth doped TiO2 anatase nanoparticles. Mater Chem Phys 135(2–3):1064–1069
Ba-Abbad MM, Kadhum AAH, Mohamad AB, Takriff MS, Sopian K (2012) Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int J Electrochem Sci 7:4871–4888
Daneshvar N, Salari D, Khataee AR (2003) Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol A Chem 157:111–116
Dhaouadi A, Monser L, Adhoum N (2009) Anodic oxidation and electro-Fenton treatment of rotenone. Electrochim Acta 54:4473–4480
Dojcinovic BP, Roglic GM, Obradovic BM, Kuraica MM, Tosti TB, Markovic MD, Manojlovic DD (2012) Decolorization of Reactive Black 5 using a dielectric barrier discharge in the presence of inorganic salts. J Serb Chem Soc 77:535–548
Erturk HA (2010) The handbook of environmental chemistry, biodegradation of azo dyes, vol 9. Springer, Berlin Heidelberg, p 1
Gouvea CAK, Wypych F, Moraes SG, Duran N, Nagata N, Peralta-Zamora P (2000) Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 40:433–440
Kasanen J, Salstela J, Suvanto M, Pakkanen TT (2011) Photocatalytic degradation of methylene blue in water solution by multilayer TiO2 coating on HDPE. Appl Surf Sci 258:1738–1743
Khataee AR, Pons MN, Zahraa O (2009) Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: influence of dye molecular structure. J Hazard Mater 168:451–457
Laohaprapanon S, Matahum J, Tayo L, You SJ (2015) Photodegradation of Reactive Black 5 in a ZnO/UV slurry membrane reactor. J Taiwan Inst Chem Eng 49:136–141
Ling CM, Mohamed AR, Bhatia S (2004) Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere 57:547–554
Mahmoodi NM, Arami M, Limaee NY, Tabrizi NS (2006) Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J Colloid Interface Sci 295:159–164
Miao SJ, Shanks BH (2011) Mechanism of acetic acid esterification over sulfonic acid-functionalized mesoporous silica. J Catal 279:136–143
Nadaroglu H, Cicek S, Gungor AA (2017) Removing Trypan blue dye using nano-Zn modified Luffa sponge. Spectrochim Acta Part A Mol Biomol Spectrosc 172:2–8
Ramirez C, Saldana A, Hernandez B, Acero R, Guerra R, Garcia-Segura S, Peralta-Hernandez JM (2013) Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J Ind Eng Chem 19:571–579
Saggioro EM, Oliveira AS, Pavesi T, Maia CG, Ferreira LFV, Moreira JC (2011) Use of Titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 16:10370–10386
Shoabargh S, Karimi A, Dehghan G, Khataee A (2014) A hybrid photocatalytic and enzymatic process using glucose oxidase immobilized on TiO2/polyurethane for removal of a dye. J Ind Eng Chem 20:3150–3156
Thamaphat K, Limsuwan P, Ngotawornchai B (2008) Phase Characterization of TiO2 Powder by XRD and TEM. Kasetsart J Nat Sci 42:357–361
Theivasanthi T, Alagar M (2013) Titanium dioxide (TiO2) nanoparticles-XRD analyses-an insight. Centre for Research and Post Graduate Department of Physics, Ayya Nadar Janaki Ammal College, Sivakasi
Vetrivel V, Rajendran K, Kalaiselvi V (2014–2015) Synthesis and characterization of pure Titanium dioxide nanoparticles by sol–gel method. Int J ChemTech Res 7:1090–1097
Wu CH, Chang CL, Kuo CY (2008) Decolorization of Procion Red MX-5B in electrocoagulation (EC), UV/TiO2 and ozone-related systems. Dyes Pigm 76:187–194
Yanmis D, Bozoglu C, Nadaroglu H, Adiguzel A, Gulluce M (2013) Removal of some textile dyes with laccase from Anoxybacillus gonensis (P39). Curr Opin Biotechnol 24:S33
Zielinska B, Grzechulska J, Grzmil B, Morawski AW (2001) Photocatalytic degradation of Reactive Black 5—a comparison between TiO2-Tytanpol A11 and TiO2-Degussa P25 photocatalysts. Appl Catal B Environ 35:L1–L7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nadaroglu, H., Lesani, A., Soleimani, S.S. et al. A newly green photocatalyst support for azo dye remediation under UV light irradiation. Appl Water Sci 8, 107 (2018). https://doi.org/10.1007/s13201-018-0752-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0752-4