Abstract
Cadmium is one of the most hazardous heavy metal concerning human health and aquatic pollution. The removal of cadmium through biosorption is a feasible option for restoration of the ecosystem health of the contaminated freshwater ecosystems. In compliance with this proposition and considering the efficiency of calcium carbonate as biosorbent, the shell dust of the economically important snail Bellamya bengalensis was tested for the removal of cadmium from aqueous medium. Following use of the flesh as a cheap source of protein, the shells of B. bengalensis made up of CaCO3 are discarded as aquaculture waste. The biosorption was assessed through batch sorption studies along with studies to characterize the morphology and surface structures of waste shell dust. The data on the biosorption were subjected to the artificial neural network (ANN) model for optimization of the process. The biosorption process changed as functions of pH of the solution, concentration of heavy metal, biomass of the adsorbent and time of exposure. The kinetic process was well represented by pseudo second order (R2 = 0.998), and Langmuir equilibrium (R2 = 0.995) had better fits in the equilibrium process with 30.33 mg g−1 of maximum sorption capacity. The regression equation (R2 = 0.948) in the ANN model supports predicted values of Cd removal satisfactorily. The normalized importance analysis in ANN predicts Cd2+ concentration, and pH has the most influence in removal than biomass dose and time. The SEM and EDX studies show clear peaks for Cd confirming the biosorption process while the FTIR study depicts the main functional groups (–OH, C–H, C=O, C=C) responsible for the biosorption process. The study indicated that the waste shell dust can be used as an efficient, low cost, environment friendly, sustainable adsorbent for the removal of cadmium from aqueous solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heavy metals in aquatic ecosystem can be detrimental to different organisms depending on the tolerance levels and the complexity of the food web. In course of the different industrial and agricultural processes, the heavy metals are released and eventually contaminate the aquatic ecosystems. For instance, cadmium, considered as one of the important heavy metal pollutant, is added to the aquatic ecosystems through activities in mining, electroplating, battery, paint and ceramic industries, in addition to the natural deposits. The disposal of heavy metals in aquatic systems concentrates as a cascading effect in the trophic levels, frequently recognized in the form of bioaccumulation and biomagnifications (Brooks et al. 2004; Tao et al. 2012). Subsequent entry of heavy metals in the living system can create a chain of physiological, biochemical and genetic changes that are concern from public health viewpoint. In case of cadmium, entry in the human body can damage the kidney, substitute calcium in the bones, can cause liver damage, cancer and hypertension (Godt et al. 2006; Bernard 2008). Accumulation of cadmium in the food web causes damage to the wild life and their diversity (Johri et al. 2010; Bernard 2008). As a consequence, the removal of cadmium and similar heavy metals are given priority for the sustenance of the ecological functions of the aquatic ecosystem.
The heavy metal pollutants are non-biodegradable and thus adsorption is considered as one of the convenient way of removal from the aquatic ecosystems. In recent past, several methods based on the principle of ion exchange, chemical precipitation, coagulation, activated charcoal, electrochemical processes and membrane technology alone or in combination have been promoted for the purpose of the heavy metal removal (Du et al. 2011; Liu et al. 2009). However, many of these processes increase the risk of generating secondary pollutants, and therefore pose a concern from ecosystem health and public health viewpoints. As a consequence, the use of biological materials for the removal of these heavy metals is being promoted to minimize the secondary pollutant level in the system as well as cost-effect benefit (Gifford et al. 2006). Application of the biological materials that are component of the ecosystem reduces the possibility of yielding unwanted secondary pollutants. These are substantiated in the observations on the metal adsorption ability of different microorganisms (Hetzer et al. 2006; Sari and Tuzen 2009) and hydrophytes (Sinha et al. 2007) many of which are hyperaccumulator of heavy metals. Application of the aquatic animals in the removal of the heavy metals from the aquatic system has been tested as evident from the studies on living freshwater bivalve (Jana and Das 1997), bivalve shell (Du et al. 2011; Liu et al. 2009; Pena-Rodriguez et al. 2010) crab and arca shell biomass (Dahiya et al. 2008). However, the unregulated use of the living forms of the biological resources may facilitate species invasion, such as many of the plant hyperaccumulators (Sinha et al. 2007). In other instances, such as the case of the freshwater bivalve, despite the metal removal being high (Jana and Das 1997), their potential as aquaculture resources limits their use in bioremediation. Recent studies have demonstrated that calcium carbonate and its derivatives may act as biosorbents of different heavy metals (Du et al. 2011; Pena-Rodriguez et al. 2010). The shells of different molluscs are composed of 95–99% calcite and/or aragonite (principally made up of calcium carbonate) oriented in a matrix of proteins (0.1–5%), and act as natural ceramic with excellent strength and toughness (Kaplan 1998; Boro et al. 2012). The shells of aquatic gastropods can be considered as cheap and available source of calcium carbonate (Hossain and Aditya 2013) and thus can be considered as low-cost biosorbent of heavy metals. In compliance with this proposition, the present study was aimed at evaluation of cadmium removal capacity of shell dust of freshwater gastropod Bellamya bengalensis (Mollusca: Gastropoda: Viviparidae). A common inhabitant of ponds, ditches, rivers and varied types of freshwater wetlands, the operculate snail B. bengalensis a prolific breeder and can easily be cultured (Khan and Choudhury 1984). The flesh of the snail is used as a cheap source of protein and the shells are discarded. The discarded shells that are aquacultural waste may be used as cheap source of calcium carbonate and the Bellamya shell dust (BSD) can be used in the removal of cadmium from aquatic system.
To promote the biosorption as an effective process, the solution for the optimization is required since the process is influenced by several factors (Witek-Krowiak et al. 2014). Improving the performance of the cadmium biosorption in terms of the process efficiency can be achieved through the optimization, which provides an idea about the best operational condition to yield the best possible response (adsorption) (Witek-Krowiak et al. 2011). Apart from the adsorption kinetics which forms the basis for the selection of the conditions, the modeling of the adsorption process, including those of heavy metals such as cadmium, is carried out to portray the changes in the efficiency depending on the various physico-chemical parameters (Çelekli and Geyik 2011; Çelekli et al. 2013). Since the biosorption process is linked with different variables in a non-linear manner and the mechanism of the process is multifaceted, it is difficult to model using conventional mathematical simulation. In recent past, artificial neural network (ANN) model has been used in describing problems in different fields of chemical and environmental engineering (Çelekli et al. 2012; Hossain et al. 2015; Maghsoudi et al. 2015). In a generalized ANN, the outputs provide an overview of the relative importance of the input parameters (factors influencing the biosorption process) that drive the sorption process (Çelekli and Geyik 2011; Çelekli et al. 2013; Ahmad et al. 2014; Witek-Krowiak et al. 2014). In the present instance, the application of the ANN was meant for deducing the efficacy of the B. bengalensis shell dust (BSD) as biosorbent of cadmium, under varied physico-chemical conditions that influence the adsorption process. Thus, the cadmium absorption efficiency of BSD was judged through multilayer perceptron in ANN model, apart from description of the equilibrium and kinetic models. The results will be useful in supplementing the required information in utilizing the BSD, a waste generated from aquaculture as a biosorbent and thus in bioremediation of heavy metal contaminated aquatic ecosystem.
Materials and methods
Preparation of the metal solution
The experiments were initiated through preparation of a cadmium chloride stock solution of 1000 mg L−1 using double distilled water and further working solutions were prepared by appropriate dilution. The pH of the solution was adjusted by adding HNO3 (0.1 N) and NaOH (0.1 N) as required in course of the experiments. All the inorganic chemicals that have been used in these experiments were purchased from Merck India Ltd., India.
Preparation of the Bellamya shell dust
The waste shells of the snail B. bengalensis were collected from local fish markets in Burdwan, India. Following procurement, the remnant tissue portion and dusts in the shells were cleared in warm water and dried in sunlight for 2 days. Next, the shells were placed in hot air oven for 2 days at 60 °C and kept in plastic zipper bags. For preparation of the shell dust, the shells were pulverized in mortar and pestle to fine granules and dried in oven for 2 days at 60 °C. The granules were then sieved through 500 µm and consequently through 200 µm net, to yield two different sized granules, respectively, 500–200 µm and ≥ 200 µm. Initially, the larger-sized granules adsorbed less amount of cadmium and thus were not studied further. The ash content of the dust was 72.34% and the water content capacity was 1859.0 mg g−1. The specific surface area, pore volume and pore diameter were determined by the Brunauer–Emmett–Teller (BET) method using Quantachrome Autosorb automate with nitrogen gas (version 4.0). The surface area of the biosorbent was 10.143 m2 g−1, while the pore volume was 0.079 cc g−1 and the pore diameter was 3.336 nm.
Batch sorption procedure
The batch sorption experiments were performed in a 250-mL Erlenmeyer’s flask that contained 100 mL solution of the particular cadmium ion concentration at required pH and relevant amount of snail shell dust (BSD). The flasks were sealed with wax paper and shaken in a shaking incubator (Lab Companion, SI-300R, India) at a required temperature at 150 rpm for required time. After shaking for particular time, the solution of the flasks was centrifuged at 2000 rpm for 15 min and the supernatant was taken for estimation of metal concentration by atomic absorption spectroscopy (GBC Avanta 1.3, India). The influence of different levels of pH on biosorption equilibrium was studied through changing the pH of the solution in range of 2–7. The effect of contact times between solution and the BSD were monitored by varying it from 10 to 80 min. For equilibrium studies, the metal ion concentrations were used in a varied concentration of 25–1000 mg L−1, and for optimum biosorption study, the BSD biomass was varied between 200 and 1000 mg. The amount of Cadmium ion adsorbed on the BSD was estimated following the equation (Sharma et al. 2011):
where qe is the amount of metal adsorbed, v is the volume of solution, m is the mass of adsorbent, co is the initial concentration of the Cd solution and ce is the equilibrium concentration of the Cd solution.
Estimation of the metal content in tissues and shells by AAS
For estimation of metal, required amount of Bellamya bengalensis tissue/shell of a particular dose was taken into Teflon container for microwave digestion. 4 mL of aqua regia was added and container was placed in microwave oven at 450 W and the sample was digested for 7 min. 4 mL of hydrogen peroxide was then added to the mixture and was again digested for another 7 min. It was diluted with distilled water, and filtered in a 25-mL volumetric flask. Final volume was made up with distilled water. Reagent blank was prepared in the same way. The final samples were then estimated for cadmium by atomic absorption spectroscopy (GBC Avanta 1.3, India) at 228.8 nm wavelength.
Analyses of FT-IR absorbance spectra of BSD
IR spectra of protonated or Cd2+ loaded BSDs were recorded in a (Perkin-Elmer FTIR, Model RX1) Fourier transform infrared (FT-IR) spectroscopy. Samples of 100 mg of KBr disks contain 1% of the finally ground powder of each sample were prepared less than 24 h before recording.
The scanning electron micrograph (SEM) study of BSD
Raw and metal-adsorbed BSDs were dried and prepared for scanning electron microscopic studies. The samples were attached with the stubs by both side cello tapes and gold plated in a sputter coater before use in the SEM. Electron acceleration potential of 20 kV was used for the microscopic observations. Photographs were taken in a HITACHI S530 scanning electron microscope at 800× magnification.
Energy dispersive X-ray (EDX) analysis
For energy dispersive X-ray analysis, the samples were studied by field emission gun-based scanning electron microscope with energy dispersive X-ray analysis (SEM/EDX) (Quanta model by FEI Co., the Netherlands) for the morphological as well as for the presence of elemental information on the samples. The SEM studies were performed at 30 kV in the low vacuum mode. EDX spectra were taken from the area corresponding to the SEM image shown in the in-set. The EDX spectra were taken in the ‘region mode’ with the bombardment of energetic electrons for duration of electron of 100 s.
Equilibrium modeling
The adsorption equilibriums were studied for the estimation of maximum cadmium biosorption by the BSD. For the equilibrium study, the experiments were performed at different initial cadmium ion concentration (25–1000 mg g−1).
Langmuir and Freundlich isotherms were used in describing the equilibrium between adsorbed cadmium ions on the BSD (qe) and in solution (ce) at a particular temperature. The parameters of the Langmuir equation (Langmuir 1918) were determined from a linear form of the following equation:
where a is the maximum amount of metal ions/unit mass of adsorbent to form a monolayer and b is the equilibrium constant. The correlation coefficient of the linear plot of ce/qe against ce indicates the applicability of the Langmuir modeling in the experiment (Tan and Hameed 2017).
Freundlich equation (Freundlich 1906) is described as below:
where kf and 1/n are the Freundlich constants indicating the adsorption capacity and intensity, respectively. The linear model of the isotherm can be expressed logarithmically as in the following:
where the value of KF and n can be determined from the y-intercept and slope of the plot of log ce against log qe. The linear plot of log qe against log ce indicates the applicability of the Freundlich modeling in the experiment.
Temkin’s isotherm model is an empirical equation comprises a factor that clearly indicates the adsorbent–adsorbate interactions. Through overlooking the lower and higher value of concentrations, it assumes that heat of adsorption of the interacting molecules in the layer would decrease linearly rather than logarithmically. The model is given by the following equation (Foo and Hameed 2010; Tanzifi et al. 2017; 2018; Ghaedi and Vafaei 2017):
or,
where AT is the Temkin isotherm equilibrium binding constant (L g−1), bT is the Temkin isotherm constant, R is the universal gas constant (8.314 J−1 mol−1 K), and T is the temperature at 298 K. AT and BT values are obtained from qe versus ln ke plot for the adsorbent.
Dubinin–Radushkevich (D–R) isotherm was used in investigation of Cd adsorption nature based on the equilibrium data. The isotherm is expressed in linear form as:
where β and xm (maximum Cd adsorption capacity) are the constants obtained from the plot of ln qe vs. ɛ2 (Polanyi potential). ‘ɛ’ is calculated from the following equation:
where R is the universal gas constant (J mol−1) and T is the absolute temperature in Kelvin and ‘β’ is associated to the adsorption free energy (E). Adsorption energy is calculated with the following equation (Tanzifi et al. 2017):
Kinetic modeling
The pseudo second order model for prediction of biosorption is not suitable for a long period of adsorption process (Ho and McKay 1999) and the pseudo second order equation:
or
where \(q_{\text{e}}\) is the amount of adsorbed metal ion on biosorbent at equilibrium, q t is the amount of adsorbed metal ion on biosorbent at time ‘t’, and k2 is the second order rate constant (g mg−1 min−1). A linear plot of t/q vs. t indicates whether this model of biosorption is applicable for this case.
Artificial neural network (ANN) model
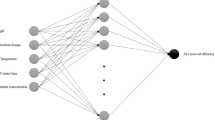
ANN is a non-linear mathematical model that is inspired by the structural and functional aspects of neuron in exploration of a group of input data (through training and testing) as function of output data (including relative error) and to envisage the performance of the given system (Cavas et al. 2011). A three-layered ANN model is used in the analyses of the complex interactions in finding the pattern of experimental data. Multilayer perceptron uses supervised learning technique in relating output responses to the expected responses by regulating the loads of the input signals and remind it. The memory is used in the next time of data feed when the output response will be much closer to the wanted one after the same input signals are delivered (Shanmugaprakash and Sivakumar 2013). The weight of any input signal is calculated by the following equation:
where w ij is the corresponding load of connection between each neuron ‘j’ in input layer and each neuron ‘i’ at hidden layer and x j is the value of input signal ‘j’ at input layer (Çelekli et al. 2013). For a particular function, the output is predicted from the following equation:
where w i is the sum weight of each neuron and b j is the bias, i.e., constant weight of a neuron representing the relative error (Çelekli and Geyik 2011). In predicting the biosorption of cadmium (output), the combined effect of the variables—(1) pH, (2) Cd2+ concentration, (3) biosorbent dose and (4) contact time were used in the analysis. Sixty-nine experimental sets were used in training, validation and testing the ANN model by SPSS® 20, trial version.
Results and discussion
Bioaccumulation of cadmium on shell dust
Effect of pH of the solution on batch sorption
The biosorption procedure was maintained over the experimental range of pH 2–7. The sorption procedure was affected by the pH of the medium in two ways—metal solubility and total charge of the functional groups of the biosorbent. The optimum pH, at which the procedure shows the highest adsorption for the biosorbent was estimated to be 6 which is nearer to the environmental pH of most of the freshwater aquatic system. At high pH, that is, at alkaline condition, precipitation of the metal takes place, and at low pH due to high protonation, metal sorption capacity decreases (Guo et al. 2008). The experiment was carried out in 100 mL of solution having 100 mg L−1 Cd2+ and 100 mg of the BSD at 30 °C in reference to varying pH of the solution. Figure 1a indicates the pH-dependent adsorption of the metal ion by BSD as the metal sorption was negligible at pH 2 and it increases dramatically as increase in pH and at pH 6 it shows the highest adsorption. The metal sorption declined as the pH increase further. In a particular pH range, most metal sorption is enhanced with pH, increasing to a certain value followed by a reduction on further pH increase (Guo et al. 2008; Pavasant et al. 2006; Semerciöz et al. 2017).
a Effect of pH of the solution on biosorption of cadmium on BSD at 30 °C, initial cadmium ion concentration of 100 mg L−1, contact time of 60 min and biosorbent dose of 1 g per 100 mL solution. b Effect of initial cadmium ion concentration of solution on biosorption on BSD at 30 °C, pH 6, contact time of 60 min and biosorbent dose of 1 g per 100 mL solution. c Effect of time on biosorption of cadmium on BSD at 30 °C, pH 6, initial cadmium ion concentration of 100 mg L−1, and biosorbent dose of 1 g per 100 mL solution. d Effect of biosorbent dose on biosorption of cadmium on BSD at 30 °C, pH 6, initial cadmium ion concentration of 100 mg L−1, contact time of 60 min
Effect of initial metal ion concentration on batch sorption
Increase in metal ion concentration added on metal adsorption as concentration of the ion increases. 25–1000 mg L−1 ion concentrations were used for the study taking seven different doses in series. The 25 mg L−1 initial metal ion concentration showed the lowest adsorption while the 400 mg L−1 concentration showed the highest adsorption and the adsorption remained same in further increase in Cd2+ ion increase in the solution (Fig. 1b). The initial metal ion concentration can modify the metal removal efficiency over a combination of factors, the availability of specific surface functional groups and ability of surface functional groups to bind metal ions (Taty-Costodes et al. 2003; Pino et al. 2006)
Effect of contact time on batch sorption
The sorption potentials of the BSD over time were monitored from 10 min to 20, 40, 60, 80 min using 100 mL of 100 mg L−1 Cd2+ at pH 6 (Fig. 1c). At the beginning, metal adsorption was less due to more binding sites remained free when treated for the short period of time and increased rapidly as the treatment time increases. It showed lowest adsorption when treated for 10 min and increased over time to saturate at 60 min and after that the uptake remained almost same. The variation in uptake of the cadmium ions with time was used in fitting the kinetic models.
Influence of the biosorbent dose on batch sorption
The cadmium biosorption potential of the BSD augmented over its amount increased in treating the metal solution. The more the amount of biosorbent the more the free binding sites or exchanging group to adsorb the metal ion from the solution. For a 100 mg L−1 metal ion concentration, the increase in biosorbent resulted increase in metal ion adsorption and above a certain dose it remained same or slightly higher due to comparatively higher number of free sites and lesser number of metal ions (Fig. 1d) (Al-Anber and Matouq 2008; Ghodbane et al. 2008).
FT-IR study of the shell dust
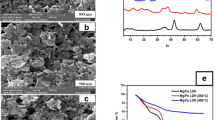
To study the mechanism of cadmium removal and the main functional groups responsible for Cd2+ binding, the FTIR spectrum of the BSD was performed. IR spectra of protonated and cadmium loaded BSD are shown in Fig. 2a, b, respectively. A peak at 3424 cm−1 is indicating the presence of hydroxyl (–OH) groups. Strong band at 2921 cm−1 is due to C–H stretching frequency (Semerciöz et al. 2017) and peak at 1634 cm−1 is due to C=O stretching mode of the primary and secondary amides (Reddy et al. 2010). Weak band at 1462 cm−1 is attributed to aromatic C=C and the strong band at 1041 cm−1 is due to C–O stretching of alcoholic groups (Blazquez et al. 2011). FTIR study reveals that OH, CH, CO, and C=C may be the responsible groups in the biosorption process. The FTIR of metal loaded BSD shows that distinct shift of the above mentioned bands as well as change in intensity informs some ion exchange behavior of the BSD.
The scanning electron micrograph (SEM) analysis of the shell dust
The surface structure of the free and cadmium-loaded BSD was analyzed under scanning electron microscope. The scanning electron micrographs of the dried BSD before and after the Cd2+ treatment at 800× magnification are shown in Fig. 3a, b, respectively. It indicates the irregular morphological structure of the particles and lamellar stratified surface of the BSD. The SEM image of the biosorbent after exposure to the Cd2+ shows a spongy layer indicating surface precipitation occurred during the sorption (Du et al. 2011). In case of cadmium sorption by CaCO3 compound generally follows surface precipitation due to similar ionic radii (Prieto et al. 2003; Perez-Garrido et al. 2007) of divalent calcium and cadmium.
Energy dispersive X-ray (EDX) analysis
The surface structure of the shell dust makes accessible a large unadsorbed surface area for the cadmium ion of the solution. The elemental profile of the BSD before and after the treatment of the cadmium solution was estimated using the energy dispersive X-ray analysis. The projecting peaks in the EDX spectra correspond to CKα, OKα, AlKα, SiKα, PKα, CaKα, CaKβ, FeKα, etc., in the untreated BSD and furthermore to it a CdLα peak in treated BSD (Fig. 4a, b). Both the treated and untreated BSD display strong peaks corresponding to calcium. In addition, the calcium of BSD may facilitate adsorption of cadmium because of the similarities in ionic radii that enhance ion exchange (Purkayastha et al. 2014).
Equilibrium modeling
The biosorption isotherm is important in waste water treatment as it implies estimation of biosorption capacity of the adsorbent. The linear representations of Langmuir and Freundlich isotherm of cadmium adsorption at 30 °C are given in Fig. 5a, b, respectively. The correlation coefficient and constants obtained from the equations are presented in Table 1. The correlation of determination is high in Langmuir equation (R2 = 0.995) contrast to Freundlich equation (R2 = 0.760) (Fig. 5). It indicates that Langmuir model is more suitable for describing the biosorption equilibrium of Cd2+ on the snail shell dust. High qmax value (30.33 mg g−1) from Langmuir equation indicates the biosorption potential of the material. The value of b (0.04) (Table 1) indicates b the affinity of the binding sites and the energy of adsorption (L/mg) (Blazquez et al. 2011). The equilibrium models of cadmium biosorption support that under optimum conditions (pH 6, biosorbent dose of 1 g, Cd2+ concentration of 100 mg L−1 and 60 min time period) 30.33 mg g−1 is the maximum biosorption capacity of BSD. The Temkin isotherm shows BT = 533, TA = 1.15 and the R2 = 0.896 showing moderate fit. The R–D equation (R2 = 0.766) for the biosorption shows the value of the adsorption energy (E) is 13.89 J/mol that corresponds to chemisorption type of uptake based on ion exchange (Wu et al. 2012; Markou et al. 2016). Maximum cadmium biosorption capacities of similar low cost biosorbents are shown in Table 2. BSD is mainly composed of calcium carbonate, degradation of which, if any, will not yield unwanted compound to the ecosystem.
Kinetic modeling
The kinetic model is necessary for determination of optimal condition of the biosorption process. For the evaluation of differences in sorption process, the kinetics of metal uptake were described by pseudo second order model (Ho and McKay 1999). The linear plots obtained from pseudo second order model at 100 mg L−1 initial Cd2+ concentration, pH 6 and at studied temperature are shown in Fig. 6a, b, respectively. The rate constants, expected metal uptake and correlation coefficients have been described in Table 3. The pseudo second order reaction in biosorption is based on the sorption capacity on the solid phase. From Fig. 6b and Table 3, the correlation coefficient in the pseudo second order reaction (R2 = 0.998) is high and the calculated metal adsorption (9.26 mg g−1) is much nearer to the expected value (9.302 mg g−1). These suggest that the biosorption process is based on the pseudo second order model.
Optimization results through ANN model
The ANN model established in this study comprised three layers as shown in Fig. 7a consisting of a hyperbolic tangent transfer function at hidden layer and a linear transfer function at output layer. The input layers have four predictors, viz. biomass, pH, Cd concentration and time, the hidden layer and the output layer have one response variable, the amount of Cd absorbed(Table 4) (Çelekli et al. 2012, 2016). The weight of the neurons was used in the study of the relative influence of each of the input variables on the biosorption process, i.e., on output layer. In the present instance, the sum of squares error (SSE error, a measure of the predicted and the observed values; Çelekli and Geyik 2011) is 0.467; and the relative error being 0.056, justifying the suitability of the multilayer perceptron ANN model. The regression equation of the output (predicted adsorption through ANN model, y) and the corresponding target (observed through the experiments, x) complied with the linear form as y = 0.37 + 0.95x (Fig. 7b). The high (R2 = 0.943) coefficient of determination fits well with the experimental dataset with that of the ANN model of the system (Yang et al. 2011; Khataee et al. 2011). Upon comparison, the data complied well with both the pseudo second order (Fig. 6) as well as the ANN model (Fig. 7b), with high values of coefficient of determination. Among the predictors, the pH of the solution (0.347) appeared to be comparable to the cadmium concentration (0.339) in terms of the relative importance in shaping the adsorption process, similar to those observed for the dye removal by Chara contraria (Çelekli and Geyik 2011). The contact time and the biomass bear comparative low influence in the adsorption, contrast to those observed for the dye removal by the walnut husk (Çelekli et al. 2016).
Conclusion
The biosorption process works as function of pH of the solution, biomass, Cd ion concentration and time of contact. The biosorption study indicates that at pH 6, the maximum cadmium adsorption capacity of BSD is 30.33 mg g−1. The isotherm model follows Langmuir model (R2 = 0.995) better than Temkin isotherm (R2 = 0.896), R–D (R2 = 0.766) and Freundlich model (R2 = 0.760). The main functional group responsible for chelation is OH, C=O, C=C and C–C, as supported by FTIR analysis. EDX study shows clear peaks for cadmium in the treated biomaterial, conveying further support of clear adsorption. The biosorption process followed pseudo second order (R2 = 0.998) kinetics. A multilayer perceptron model in artificial neural network (ANN) model successfully portrayed biosorption of cadmium ion on the LSD with high correlation coefficient (R2 = 0.943) between predicted and observed removal. BSD can be considered as efficient, low cost and environment friendly biosorbent for cadmium bioremediation, opening a new aspect of the economic value of shells of the freshwater snail B. bengalensis, apart from its value as cheap protein source.
Abbreviations
- AAS:
-
Atomic absorption spectroscopy
- ANN:
-
Artificial neural network
- SEM:
-
Scanning electron microscope
- BSD:
-
Bellamya shell dust
- FT-IR:
-
Fourier transformed infrared
- EDX:
-
Energy dispersive X-ray
- BCF:
-
Bio-concentration factor
References
Ahmad MF, Haydar S, Bhatti AA, Bari AJ (2014) Application of artificial neural network for the prediction of biosorption capacity of immobilized Bacillus subtilis for the removal of cadmium ions from aqueous solution. Biochem Eng J 84:83–90
Al-Anber ZA, Matouq MAD (2008) Batch adsorption of cadmium ions from aqueous solution by means of olive cake. J Hazard Mater 151:194–201
Arvand M, Pakseresht MA (2012) Cadmium adsorption on modified chitosan coated bentonite: batch experimental studies. J Chem Technol Biotechnol 88(4):572–578
Asuquo ED, Martin AD (2016) Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: characterisation, kinetic and isotherm studies. J Environ Chem Eng 4:4207–4228
Azouaou N, Sadaoui Z, Mokaddem H (2008) Removal of cadmium from aqueous solution by adsorption on vegetable wastes. J Appl Sci 8:4638–4643
Bernard A (2008) Cadmium and its adverse effects on human health. Indian J Med Res 128:557–564
Blazquez G, Martin-Lara MA, Tenorio G, Calero M (2011) Batch biosorption of lead(II) from aqueous solutions by olive tree pruning waste: equilibrium, kinetics and thermodynamic study. Chem Eng J 168:170–177
Boro J, Deka D, Thakur AJ (2012) A review on solid oxide derived from waste shells as catalyst for biodiesel production. Renew Sustain Energy Rev 16:904–910
Brooks BW, Stanley JK, White JC, Turner PK, Wu KB, La Point TW (2004) Laboratory and field responses to cadmium: an experimental study in effluent-dominated stream mesocosms. Environ Toxicol Chem 23:1057–1064
Cavas L, Karabay Z, Alyuruk H, Dogan H, Demir GK (2011) Thomas and artificial neural network models for the fixed bed adsorption of methylene blue by a beach waste, Posidonia oceanea (L.) dead leaves. Chem Eng J 171(2):557–562
Çelekli A, Bozkurt H (2011) Bio-sorption of cadmium and nickel ions using Spirulina platensis: kinetic and equilibrium studies. Desalination 275:141–147
Çelekli A, Geyik F (2011) Artificial neural networks (ANN) approach for modeling of removal of Lanaset Red G on Characontraria. Bioresour Technol 102:5634–5638
Çelekli A, Birecikligil SS, Geyik F, Bozkurt H (2012) Prediction of removal efficiency of Lanaset Red G on walnut husk using artificial neural network model. Bioresour Technol 103:64–70
Çelekli A, Bozkurt H, Geyik F (2013) Use of artificial neural network and genetic algorithms for prediction of sorption of an azo–metal complex dye onto lentil straw. Bioresour Technol 129:396–401
Çelekli A, Bozkurt H, Geyik F (2016) Artificial neural network and genetic algorithms for modeling of removal of an azo dye on walnut husk. Desalin Water Treat 57:15580–15591
Cui L, Wu G, Jeong T (2010) Adsorption performance of nickel and cadmium ions onto brewer’s yeast. Can J Chem Eng 88:109–115
Dahiya S, Tripathi RM, Hegde AG (2008) Biosorption of lead and copper from aqueous solutions by pre-treated crab and arca shell biomass. Bioresour Technol 99:179–187
Ding Y, Jing D, Gong H, Zhou L, Yang X (2012) Biosorption of aquatic cadmium (II) by unmodified rice straw. Bioresour Technol 114:20–25
Du Y, Lian F, Zhu L (2011) Biosorption of divalent Pb, Cd, Zn on aragonite and calcite mollusk shells. Environ Pollut 159:1763–1768
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem 57A:385–470
Ghaedi AM, Vafaei A (2017) Application of artificial neural networks for adsorption removal of dyes from aqueous solution: a review. Adv Colloid Interface Sci 245:20–39
Ghodbane I, Nouri L, Hamdaoui O, Chiha M (2008) Kinetic and equilibrium study for the sorption of cadmium (II) ions from aqueous phase by eucalyptus bark. J Hazard Mater 152:148–158
Gifford S, Dunstan RH, O’Connor W, Koller CE, MacFarlane GR (2006) Aquatic zooremediation: deploying animals to remediate contaminated aquatic environment. Trends Biotechnol 25:60–65
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1(1):22
Guo X, Zhang S, Shan XQ (2008) Adsorption of metal ions on lignin. J Hazard Mater 151(1):134–142
Hetzer A, Daughney CJ, Morgan HW (2006) Cadmium ion biosorption by the thermophilic bacteria Geobacillus stearothermophilus and G. thermocatenulatus. Appl Environ Microbiol 72:4020–4027
Ho YS, McKay G (1999) Pseudo-second order model for sorption process. Process Biochem 34:451–465
Ho YS, Ofomaja AE (2006) Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem Eng J 30:117–123
Hossain A, Aditya G (2013) Cadmium biosorption potential of shell dust of the fresh water invasive snail Physa acuta. J Environ Chem Eng 1:574–580
Hossain A, Bhattacharyya SR, Aditya G (2015) Biosorption of cadmium from aqueous solution by shell dust of the freshwater snail Lymnaealuteola. Environ Technol Innov 4:82–91
Jana BB, Das S (1997) Potential of freshwater mussel (Lamellidens marginalis) for cadmium clearance in a model system. Ecol Eng 8:179–193
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23:783–792
Kaplan DL (1998) Mollusc shell structure: novel design strategies for synthetic materials. Curr Opin Solid State Mater Sci 3:232–236
Khan RA, Choudhury S (1984) The population and production ecology of a freshwater snail, Bellamya bengalensis (Lamarck) (Gastropoda: Viviparidae) in an artificial lake of Calcutta, India. Bull Zool Surv India 5:59–76
Khataee AR, Dehghan G, Zarei M, Ebadia E, Pourhassan M (2011) Neural network modelling of biotreatment of triphenylmethane dye solution by a green macroalgae. Chem Eng Res Des 89:172–178
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Liu Y, Sun C, Xu J, Li Y (2009) The use of raw and acid pretreated bivalve mollusk to remove metals from aqueous solutions. J Hazard Mater 168:156–162
Maghsoudi M, Ghaedi M, Zinali A, Ghaedi AM, Habibi MH (2015) Artificial neural network (ANN) method for modeling of sunset yellow dye adsorption using zinc oxide nanorods loaded on activated carbon: kinetic and isotherm study. Spectrochim Acta A Mol Biomol Spectrosc 134:1–9
Markou G, Mitrogiannis D, Muylaert K, Çelekli A, Bozkurt H (2016) Biosorption and retention of orthophosphate onto Ca(OH)2-pretreated biomass of Phragmites sp. J Environ Sci 45(49–5):9
Nouri L, Ghodbane I, Hamdaoui O, Chiha M (2007) Batch sorption dynamics and equilibrium for the removal of cadmium ions from aqueous phase using wheat bran. J Hazard Mater 149:115–125
Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour Technol 97(18):2321–2329
Pena-Rodriguez S, Fernandez-Calvino D, Novoa-Munoz JC, Nunez-Delgado A, Fernandez-Sanjurjo MJ, Alvarez-Rodriguez A (2010) Kinetics of Hg(II) adsorption and desorption in calcined mussel shells. J Hazard Mater 180:622–627
Perez-Garrido C, Fernandez-Diaz L, Pina CM, Prieto M (2007) In situ AFM observations of the interaction between calcite surfaces and Cd-bearing aqueous solutions. Surf Sci 601:5499–5509
Pino GH, deMesquita LMS, Torem ML, Pinto GAS (2006) Biosorption of cadmium by green coconut shell powder. Miner Eng 19:380–387
Prieto M, Cubillas P, Fernandez-Gonzalez A (2003) Uptake of dissolved Cd by biogenic and abiogenic aragonite: a comparison with sorption onto calcite. Geochim Cosmochim Acta 67:3859–3869
Purkayastha D, Mishra U, Biswas S (2014) A comprehensive review on Cd(II) removal from aqueous solution. J Water Process Eng 2:105–128
Ramos RL, Jacome LAB, Rodriguez IA (2005) Adsorption of cadmium (II) from aqueous solution on natural and oxidized corncob. Sep Purif Technol 45:41–49
Reddy HKD, Seshaiah K, Reddy AVR, Rao MM, Wang MC (2010) Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: equilibrium and kinetic studies. J Hazard Mater 174:831–838
Sari A, Tuzen M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J Hazard Mater 164:1004–1011
Semerciöz AS, Göğüş F, Çelekli A, Bozkurt H (2017) Development of carbonaceous material from grapefruit peel with microwave implemented-low temperature hydrothermal carbonization technique for the adsorption of Cu (II). J Clean Prod 65:599–610
Sen TK, Mohammod M, Maitra S, Dutta BK (2010) Removal of Cadmium from aqueous solution using castor seed hull: a kinetic and equilibrium study. Clean Soil Air Water 38:850–858
Shanmugaprakash M, Sivakumar V (2013) Development of experimental design approach and ANN-based models for determination of Cr(VI) ions uptake rate from aqueous solution onto the solid biodiesel waste residue. Bioresour Technol 148:550–559
Sharma M, Kaushik A, Kaushik CP (2011) Biosorption of reactive dye by waste biomass of Nostoc lincia. Ecol Eng 37:1589–1594
Sinha RK, Herat S, Tandon PK (2007) Phytoremediation: role of plants in contaminated site management. In: Singh N, Tripathi RD (eds) Environ. Biorem. Technol. Springer, New York, pp 315–330
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48
Tanzifi M, Hossein SH, Kiadehi AD, Olazar M, Karimipour K, Rezaiemehr R, Ali I (2017) Artificial neural network optimization for methyl orange adsorption onto polyaniline nano-adsorbent: kinetic, isotherm and thermodynamic studies. J Mol Liq 244:189–200
Tanzifi M, Yaraki MT, Kiadehi AD, Hosseini SH, Olazar M, Bhati AK, Agarwal S, Gupta VK, Kazemi A (2018) Adsorption of Amido Black 10B from aqueous solution using polyaniline/SiO2 nanocomposite: experimental investigation and artificial neural network modeling. J Colloid Interface Sci 510:246–261
Tao T, Yuan Z, XiaonaH Wei M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicol Environ Saf 81:55–64
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105:121–142
Wang FY, Wang H, Ma JW (2010) Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—bamboo charcoal adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—bamboo charcoal. J Hazard Mater 177:300–306
Witek-Krowiak A, Szafran RG, Modelski S (2011) Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 265:126–134
Witek-Krowiak A, Chojnacka K, Podstawczyk D, Dawiec A, Pokomeda K (2014) Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour Technol 160:150–160
Wu Y, Wen Y, Zhou J, Dai Q, Wu Y (2012) The characteristics of waste Saccharomyces cerevisiae biosorption of arsenic(III). Environ Sci Pollut Res 19:3371–3379
Yang Y, Wang G, Wang B, Li Z, Jia X, Zhou Q, Zhao Y (2011) Biosorption of Acid Black 172 and Congo Red from aqueous solution by non-viable Penicillium YW 01: kinetic study, equilibrium isotherm and artificial neural network modelling. Bioresour Technol 102:828–834
Yasemin B, Zek T (2007) Removal of heavy metals from aqueous solution by sawdust adsorption. J Environ Sci 19:160–166
Zhang C, Shan B, Tang W, Zhu Y (2017) Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour Technol 238:352–360
Zheng L, Dang L, Yi X, Zhang H (2010) Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk. J Hazard Mater 176:650–656
Acknowledgements
The authors are thankful to the respective Heads, Department of Zoology, Department of Chemistry, and Department of Environmental Science, the University of Burdwan, Burdwan, West Bengal, India for the facilities provided. We thankfully acknowledge the anonymous reviewers for their thoughtful comments and kind suggestions to enrich this manuscript. We express our gratitude and regards to Prof. Enrico Drioli for his kind cooperation in revising the earlier version of the manuscript. AH thankfully acknowledges the financial assistance provided Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aditya, G., Hossain, A. Valorization of aquaculture waste in removal of cadmium from aqueous solution: optimization by kinetics and ANN analysis. Appl Water Sci 8, 68 (2018). https://doi.org/10.1007/s13201-018-0712-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0712-z