Abstract
The main object of this research is to assess the water quality of Shatt Al-Arab River and its suitability for various purposes near power plants (Hartha and Najibia) through physical and chemical analysis [temperature, pH, EC, Cl−, Na+, K+, Ca+2, Mg+2, HCO3−, NO3−, SO −24 , Fe+, total alkalinity, total hardness, biological oxygen demand (BOD5), NH4+, and NO2−] using water quality index (WQI), organic pollution index (OPI), sodium adsorption ratio (SAR), and percentage of sodium ion (Na%) during the dry season (August, 2016) and the wet season (January, 2017). WQI of Shatt Al-Arab falls under very poor quality during summer season, while it ranges from very poor quality to unsuitable for drinking purposes during winter season. There is a clear effect of power plants on water quality. Hartha and Najibia power plants contribute to the deterioration of water quality by increasing the percentage ratio of WQI near these plants by 13.22 and 9.69%, respectively, compared to the north sites of these plants during summer season. The percentage ratios of increased WQI near Hartha and Najibia power plants compared to the north sites of these plants are 17.93 and 15.92%, respectively, during winter season. Water quality of Shatt Al-Arab falls under a high level of organic pollution during the summer and winter seasons. There is a slight effect by the power plants on the OPI. Hartha and Najibia power plants contributed to the change of the OPI by 10% compared to the north site of Hartha power plant. According to the comparison between the SAR values which represent the suitability of water for serve irrigation purposes and SAR values of Shatt Al-Arab, all sites lie in the first class (excellent). According to Na+%, the type of surface water in the studied area lies in good class during winter season and permissible class during summer season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is one of the most important natural sources for the continuation of life. Fresh water is a source of life in various environments, especially in the arid and semi-arid regions like Iraq. Rivers are considered to be the most important source of fresh water, which is the main source of water for drinking, agriculture, and industry. Over the past decade, widespread water quality degradation in inland water systems has been reported due to the rapid development of industries, agriculture, and urbanization (Vié et al. 2009). The hydrological system of the rivers and its quality are subject to continuous changes due to the construction of dams, reservoirs, and industrial structures. Prevailing local conditions such as the climate and quality of the rocks lead to a change in water quality from one region to another. Surface water quality has become an important and sensitive issue in many countries, due to concern that fresh water will be a scarce resource in the future, and therefore, the water quality monitoring program is essential for the protection of freshwater resources (Pesce and Wunderlin 2000). Water systems monitoring programs play an important role in monitoring water quality, because it is necessary to determine the degree of contamination and the effect of water quality on its use for different purposes (Almeida et al. 2007).
WQI aims to understand the overall state of the water quality and has been applied to both the surface water and groundwater quality evaluation around the world since the past few decades (Sanjib Kumar and Chakrabarty 2007; Khwakaram et al. 2012; Ravikumar et al. 2013; Bhutiani et al. 2014; Kirubakaran et al. 2015; Yaseen et al. 2015; Puri et al. 2015; Krishan et al. 2016; Bora and Goswami 2017; Shah and Joshi 2017; Wagh et al. 2017; Kangabam et al. 2017). The main object of WQI development is to convert a complex set of water quality data into clear and useful information which enables decision-makers to make the decision easily about the state of the water source (Akoteyon et al. 2011; Balan et al. 2012). WQI helps to give one value to the water quality from a source of physical and chemical parameters by converting the list of parameters and their concentrations to a single value, which in turn provides a broad explanation of water quality and suitability for different purposes such as drinking, irrigation, and industrial. (Abbasi and Abbasi 2012). There are three stages in order to calculate the WQI (U.S. EPA 2009): (1) measure the individual indicators of water quality, (2) convert the measurements to a subindex to represent them on a common scale, and (3) compile the values of the individual subindex into an overall WQI value.
There are several studies conducted on the water quality of the Shatt Al-Arab Canal. Abdullah (2013) presented a study to evaluate the heavy metal pollution index (HPI) and metal index (MI) for evaluating the source of heavy metal; the mean HPI is equal to (8.33), and this value is less than the critical value of pollution index (100). The result of the MI indicates that the river is pure with respect to heavy metal pollution. Moyel (2014) used principal component analysis (PCA) and cluster analysis (CA) for the assessment of the water quality data set. The results show that PCA and CA techniques are useful tools for identifying important stations and parameters for monitoring the surface water quality. Moyel and Hussain (2015) conducted a study to assess the suitability of the water quality for various purposes such as aquatic life, drinking water supplies, and irrigation uses. The results of this paper showed that the deterioration of the water quality is due to the decrease in the discharge of fresh water from the Tigris and Euphrates rivers, the decrease in annual rainfall rates, and an advancing salt wedge from the Arabian Gulf. Yaseen et al. (2016) presented a study for evaluating the factors affecting the change of river discharge value and the deterioration of water quality. The results showed that the high salinity in the Shatt Al-Arab is due to natural factors, including climate change, domestic waste water in the canal, and others related to the management of water resources and the policies of neighboring countries.
The current study aims to evaluate the quality of the Shatt Al-Arab River near the power plants (Hartha and Najibia) and the effect of these plants on the water quality. As it is known, the electric power plants draw large quantities of water for the cooling purposes and then return this water to the waterway after increasing its temperature and being polluted with chemical compounds. Also, the pollution came from discharging of industrial wastes and chemical materials used for treatment of inlet water. The object of this study is to assess the water quality near the power plants (Hartha and Najibia) through physical and chemical analysis using WQI and organic pollution index (OPI) during the dry season (DS) (August, 2016) and the wet season (WS) (January, 2017).
Description of the study area
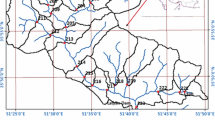
Shatt Al-Arab is a river in Basrah Province, southern Iraq. It is located between longitude lines (47°30′ and 48°30′) and latitude lines (30°00′ and 30°30′). Rainfall usually starts in October and lasts until May; the maximum rainfall value is obtained in January, while its vanished during the summer. Basrah Province is one of the hottest cities on the planet during the summer season, with temperature exceeding 45 °C in July (Al-Aboodi 2016). Winter frost is not unknown. However, the climate is healthy and acceptable. Relative humidity may exceed 90%, due to the location of Basrah City near the Arabian Gulf. Shatt Al-Arab River is formed by the confluence of the Euphrates and the Tigris River in Qurna City, north of Basrah Province. The width of this river ranges from about 232 meters in center of Basrah City to 800 meters in its mouth, and the length of this river is about 200 km. Many factories and industrial plants have been established on the banks of the Shatt Al-Arab, and these industrial factories consume large quantities of water for industrial purposes and cooling processes. There are two power plants (Hartha and Najibia). Hartha power plant is located on the west bank of the Shatt Al-Arab River, where it is located about 28 km from the center of Basrah Province as shown in Fig. 1. It consists of four thermal units; the capacity of this unit is 200 MW/h. This power plant consumes about 74,000 m3/h of water. For studying the effect of Hartha power plant on water quality indices, three sites were selected for water sampling: the first one is located in front of the power plant (S1), the second one located (1000 m) north of the power plant (S2), and the third is located (1000 m) south of the power plant (S3). Najibia power plant is located on the west bank of the Qarmat Ali River, where it is located about 10 km from the center of Basrah Province. It consists of four thermal units; the capacity of this unit is 240 MW/h. This power plant consumes about 34,000 m3/h of water. For studying the effect of Najibia power plant on water quality indices, three sites were selected for water sampling: the first one is located in front of the power plant (S4), the second one located (1000 m) north of the power plant, (S5) and the third is located at the confluence of Shatt Al-Arab River and Qarmat Ali River (S6). The water quality of the river is related to water discharge because it is related to the water levels, the amount of hydraulic gradient, and the velocity of water current. The change in climatic conditions in the river basin, which is characterized by low rainfall and increased evaporation, as well as the growth of water projects such as dams and reservoirs in neighboring countries (Turkey, Syria, and Iran), led to a decrease in discharge rate from 919 m3/s during 1977 to 38 m3/s during 2015. All parts of the Shatt Al-Arab are affected by the tide phenomenon. This phenomenon affects the quality of water as the salinity of the river increases during the tide, which reaches the north of Faw city.
Sample preparation and analytical procedures
Six sites were selected to collect water samples from the Shatt Al-Arab River during the DS (August, 2016) and the WS (January, 2017). Plastic bottles were used to collect water samples, which were fully filled and stored in a refrigerated box until they were transferred to the laboratory. Transparent and dark Winkler bottles of 250–300 ml are used to collect water samples taking into account not to leave air bubbles. Water temperature was measured using a mercury thermometer, and electrical conductivity (EC) and pH were measured in situ using a Lovipond multi-meter model Sensodirect 150. BOD5 was measured according to the APHA (2005) method. Ca+2, Mg+2, and total hardness (TH) were measured by ethylenediaminetetraacetic acid complexometric titration. Flame photometry has been used for measuring the concentration of Na+, K+ and silver nitrate titrate has been used for measuring the concentration of Cl−. Method of barium sulfate turbidity has been used for measuring the concentration of SO −24 . Total dissolved solids (TDS) are measured using temperature-controlled oven. Alkalinity (TA) and HCO3− is measured by titration method. PO4− and NO2− concentration were measured by molybdate ascorbic acid method and cadmium reduction method, respectively; also NO3− concentration has been measured by cadmium reduction method. NH4+ is measured by spectrophotometric method. Fe+ was measured by spectrophotometry and atomic absorption spectrometry. The overall water quality data of Shatt Al-Arab River were compared with WHO (2011) guidelines for drinking water, in addition to aquatic life criteria endorsed by CCME (2007) as shown in Tables 1 and 2.
The sodium adsorption ratio (SAR) is determined in this paper. It is an irrigation water quality parameter used in the management of sodium-affected soils. Also, percentage of Na+ is calculated in this research for studying the suitability of Shatt Al-Arab water for irrigation purposes.
It is important to find sodium concentration in the water when using this water for irrigation purposes; sodium has a bad effect on soil structure. For evaluating the suitability of water for irrigation purposes, the SAR is calculated as follows:
In the other direction, most of the specifications confirmed that the percentage of Na+ for irrigation purposes should not exceed 50–60 in order to avoid its deleterious effects on soil. Water is considered unsuitable for irrigation when percentage of sodium exceeds 60. Percentage of Na+ can be calculated using the following formula:
Water quality index (WQI)
WQI is one of the most important and powerful tools for providing water quality information to the consumers and decision-makers. It provides a clear picture for the surface water and groundwater quality for different purposes uses; also WQI is defined as a classification that reflects the combined effect of different water quality parameters (Sahu and Sikdar 2008). Fourteen physical and chemical parameters (pH, EC, TDS, Cl, Na, K, Ca, Mg, HCO3, NO3, SO4, Fe, TA, and TH) were selected according to their importance in water quality as shown in Table 3. The following steps are used for calculating WQI.
-
1.
The weight of each chemical parameter (w i ) was determined according to its impact on primary health and its relative importance for drinking purposes (Table 3). The highest weight was assigned to 5 that have significant effects on water quality such as NO3 and TDS. The minimum weight is two for parameters that are considered harmless for example (Mg, Ca, K, and TA).
-
2.
Calculate the relative weight (Wi) of each parameter using Eq. 3.
-
3.
The (q i ) of each parameter is calculated by dividing the concentration of parameter on its standard value according to the guidelines laid down by BIS (1998) using Eq. 4.
-
4.
The quality subindex for each parameter (SIi) is calculated by multiplying the relative weight of the parameter by its (q i ) using Eq. 5.
-
5.
WQI is calculated by summing all over quality subindex for each parameter using Eq. 6.
where \(W_{i}\) is the relative weight, \(w_{i}\) is the weight of each chemical parameter, and \(n\) is the number of parameters.
where \(q_{i}\) is the quality rating, \(V_{n}\) is the actual amount of nth parameter present, \(V_{i}\) is the ideal value of parameter (\(V_{i}\) = 0, except for pH (\(V_{i}\) = 7)), and \(V_{\text{s}}\) is the Indian drinking water standard (BIS 1998) for each chemical parameter.
where \({\text{SI}}_{i}\) is the sub-index of ith parameter.
Organic pollution index (OPI)
Domestic wastewater and industrial wastewater contain a large amount of organic matters in various forms. The degradation of organic compounds in water requires large amounts of dissolved oxygen; therefore, oxygen imbalance occurs. The decomposition of organic matter leads to the deterioration in water quality and death of fish and other aquatic life due to lack of oxygen. Oxygen consumed by organic matters is used to estimate the content of organic material in water indirectly, and biochemical oxygen demand (BOD5) is one of the effective indicators to estimate the amount of organic matter. Several types of wastewater sources such as domestic, hospitals, laboratories, fuel stations and power plants, this wastewater is contaminated with toxic substances and organic matter, which leads to the phenomenon of eutrophication. The phenomenon of eutrophication leads to a decrease in the level of oxygen in water and loss of biological diversity (Vousta et al. 2001). Four parameters (BOD5, NH4, NO2, and PO4) are selected for evaluating OPI (Guasmi et al. 2010). For evaluating the effect of power plants (Hartha and Najibia) on OPI in Shatt Al-Arab River, two sites are selected for sampling water: the first one is located (1000 m) north of the Hartha power plant (S2), while the second one is located at the confluence of Shatt Al-Arab River and Qarmat Ali River (S6) during the DS (August, 2016) and the WS (January, 2017) (see Tables 1 and 2).
Results and discussion
The seasonal quality of Shatt Al-Arab River is shown in Tables 1 and 2 during summer season (August, 2016) and the winter season (January, 2017). The quality parameters were compared with WHO (2011) guideline for drinking purposes, in addition to aquatic life standards submitted by CCME (2007). Physical and chemical parameters are related with water temperature value. The solubility of soluble gases in water (e.g., oxygen, carbon dioxide, etc.), biological and microbial activity in water, non-ionic ammonia, salinity, and pH are subject to changes in water temperature (Rubio-Arias et al. 2013). The increase in the temperature of water near the power plants is due to the discharge of hot water after the cooling process inside the plant. There is an increase in the temperature of water up to 30% during summer season and up to 45% during winter season in front of plant compared with other sites. The migration, reproduction, and effectiveness of fish are closely related to the temperature of the water because the fish are cold blooded and take on the temperature of their surroundings (Larnier et al. 2010).
The permissible value of pH in natural water is usually between 6.5 and 9 as shown in WHO (2011) guidelines for drinking water, in addition to aquatic life criteria endorsed by CCME (2007). pH is closely related to water solubility and chemical form, and has a significant impact on aquatic life activities. All studied sites fall within the acceptable range, where the water quality of the river tends to be slightly alkaline.
Conductivity is using as indirect prediction for the total concentration of ions in water. The reason for increasing the electrical conductivity (EC) near the power plants is due to the use of large amounts of water for cooling purposes, resulting high concentration of salts due to evaporation. TDS in water originate from natural sources, wastewater, urban runoff, agricultural and industrial wastewater, and chemical materials used in the water treatment process. TDS concentration is an alternative expression for the sum of cations and anions in water. Therefore, the total TDS test provides a qualitative measure of the amount of dissolved ions. The TDS of water increase with increasing the level of ions concentration such as Cl−, Na+, K+, Ca+2, Mg+2, HCO3−, and SO −24 . The effect of power plants on these ions is low or slight (the highest increase for the concentration of Ca+2 about 39.3% in DS, while increase for the concentration of K+ about 33.3% in WS). There are other reasons for increasing concentration of salts near the power plants in addition to the impact of these plants as mentioned earlier: freshwater input discharge in the upper stream of the Shatt Al-Arab River from Tigris and Euphrates River tends to decrease specially at the end of summer season and starting of winter season. This decline in fresh water flow during this period, combined with the intrusion of a salt wedge from the Arabian Gulf, has led to an increased concentration of these parameters, particularly in terms of TDS. The diversion of the Karun River across Iranian territory, which was an important source of fresh water entering the Shatt Al-Arab, has reduced the ability of rivers to act as natural barriers to the intrusion of the salt wedge front from the Arabian Gulf. Increasing temperatures and hot climate especially in summer season lead to increase evaporation and concentration of salts in the River. According to comparison between the acceptable value of TDS specified by WHO (2011) guidelines for drinking water and TDS stations value during DS and WS, all TDS values exceeded the standard limit.
Cl− is present in both freshwater and saline, which is essential element of life. Cl− in the environment comes from sodium chloride or other chloride salts such as magnesium chloride, calcium chloride, and potassium chloride. The reason for the presence of chlorides in surface water is also wastewater or sewage pollution. Cl− also comes from primary treatment of inlet water with ferric chloride which is used as a coagulant in flocculation and sedimentation process. There are two main reasons for the increased concentration of chloride ion near power plants: the first one is the progress of the saline water front from the Arabian Gulf due to the reduction of river discharge in upstream, and the second one is the increase in the evaporation process in locations close to the power plants, leading to a high concentration of ions. There are some sites that did not exceed the limits allowed by the specifications, but the greatest number of examined sites exceeded the range limits by WHO (2011) in addition to aquatic life criteria endorsed by CCME (2007).
The major cations and anions such as Na+, K+, Ca+2, Mg+2, HCO3−, and SO −24 were measured during summer and winter season as shown in Tables 1 and 2. It is clear that the concentration of Na+, Ca+2, HCO3−, and SO −24 remained dominant in the Shatt Al-Arab River during dry and WS. Calcium and magnesium exist in surface water and groundwater mainly as carbonates and bicarbonates. The main source of magnesium is the flow of sewage and minerals generated from soil erosion (Ramesh and Seetha 2013). Calcium and magnesium, along with bicarbonate, carbonate, sulfate, and other species, contribute to the hardness of natural water. Calcium concentration in surface water is usually less than 15 (mg/l) and it can rise to 100 (mg/l) where this water passes through carbonate-rich rocks (Nirmala et al. 2012). Magnesium concentrations range from 1 to more than 50 (mg/l) depending on the types of rocks within the watershed (Nirmala et al. 2012). It is clear that the concentrations of both ions (Ca+2 and Mg+2) in Shatt Al-Arab River have exceeded permissible limits. It is obvious that the concentration of Na+ was the most dominant than other cations (> 200 mg/l), making the water unsuitable for domestic use (BIS 1998). A higher concentration of Na+ in the surface water is attributed to the sewage pollution. SO −24 is one of the major anions occurring in natural water. WHO (2011) has prescribed 500 mg/l as the desirable limit for drinking water. All sites did not exceed the desirable limit of SO −24 for drinking water during dry and WS. There is a slight increase in major ion concentrations near power plants and this increase is attributed to the cooling process by power plants, hot water discharge to the river, and high evaporation due to high water temperature, such as the increase of concentration for Na+, Ca+2, and SO −24 near the Hartha power plant compared with the north site by 30.6, 39.3, and 4.6%, respectively.
Nitrate concentrations of up to 1700 (µg/l) have been reported for all tested sites of Shatt Al-Arab River where municipal wastewater flows into surface water. A large amount of nitrates in drinking water causes methemoglobinemia in bottle-fed infants. WHO has recommended the guideline value for drinking water of 11,000 (µg/l). The concentration of Fe+ was found to be high in Shatt Al-Arab water samples collected from different six sampling sites. Variation in iron in collected water sample was 3.80 mg/l near to the Hartha power plant during WS to 1.4 mg/l north of Najibia power plant during DS. The main source of Fe+ is ferric chloride and corrosion from power plants.
Natural alkalinity is transferred to water sources mainly by the salts of weak acids such as carbonate, bicarbonate, silicate, borax, phosphate, and humic and fulvic acid salts. A few industrial effluents, such as calcium hydroxide from the cement plant, and sodium hydroxide from soap manufacturing, contribute to water alkalinity. Alkalinity is not reported for harmful drinking water but generally 100 mg/l is desirable for drinking water (Nirmala et al. 2012). In this study, none of the alkaline samples were analyzed in the specified range and therefore required appropriate treatments prior to use.
In this study, all samples from the six sites have hardness greater than 600 mg/l. Hardness during DS (summer) is greater than WS (winter) which may be attributed to high rates of evaporation and low water level. WHO (2011) has prescribed 500 mg/l as the desirable limit for drinking water. Therefore, none of the hardness samples were analyzed in the specified range and therefore required appropriate treatments before to supply.
After comparing Table 4 which represents the suitability of water for serve irrigation purposes depending on SAR values with Table 5 which represents SAR values of Shatt Al Arab, all sites lie in the first class (excellent).
The values of Na% in Shatt Al-Arab River lie between 25.54 and 48.64% during DS and WS as shown in Table 6, and after its comparison with Table 7 one may suggest that the type of surface water in the studied area lies in good class during winter season and permissible class during summer season.
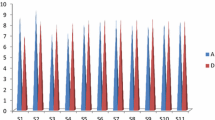
The values of the WQI of Shatt Al-Arab River at six tested sites during dry and wet season are presented in Table 8. The variation of WQI with respect to location of tested site during DS and WS is illustrated in Fig. 2.
It can be seen that the Shatt Al-Arab River has WQI ranging from 204.44 at the north of Hartha power plant (S2) to 282.25 at the confluence of Shatt Al-Arab River and Qarmat Ali River (S6) during summer season, while it has WQI ranging from 236.01 at the south of Hartha power plant (S3) to 385.02 at the confluence of Shatt Al-Arab River and Qarmat Ali River (S6) during winter season. The WQI was classified according to the scale suggested by Ramakrishnaiah et al. (2009) and Mohanty (2004) as shown in Table 9. Based on these classifications, the water quality of Shatt Al-Arab falls under very poor quality during summer season, while the water quality of Shatt Al-Arab ranges from very poor quality to unsuitable for drinking use during winter season. There is a clear effect by the power plants on the quality of Shatt Al-Arab water. It is possible to note the deterioration of water quality of Shatt Al-Arab water by increasing the WQI near the power plants. The percentage ratios of increased WQI near Hartha and Najibia power plants compared to the north sites of these plants are 13.22 and 9.69%, respectively, during summer season. The percentage ratios of increased WQI near Hartha and Najibia power plant compared to the north sites of these plants are 17.93 and 15.92%, respectively, during winter season.
OPI was developed by classifying the quality parameters (BOD5, NH4, NO2, and PO4) into five classes (Table 10). Depending on parameters values, the class of each parameter is determined. The average of all classes is calculated and then compared with tabulated value (Table 11).
Four parameters (BOD5, NH4, NO2, and PO4) are selected for evaluating OPI. For determining the effect of power plants (Hartha and Najibia) on OPI of Shatt Al-Arab River, two tested sites are selected for sampling water: the first one is located (1000 m) north of the Hartha power plant (S2), and another one is located at the confluence of Shatt Al-Arab River and Qarmat Ali River (S6). The variation of OPI with respect to location of tested site during DS and WS is illustrated in Table 12. According to the classifications of OPI as shown in Table 11, the water quality of Shatt Al-Arab falls under a high level of organic pollution during summer and winter season. There is a slight effect by the power plants on the OPI of Shatt Al-Arab River. There is a change in the level of organic pollution of control stations (S2 and S6) which is equaled to 10% during WS.
Conclusion
There is an increase in water temperature of up to 30% during the summer season, and up to 45% during winter season in front of power plants compared to other sites. The pH values of all tested sites fall within the acceptable range, where the water quality of the river tends to be slightly alkaline. Based on the comparison between TDS values of all tested sites in Shatt Al-Arab River and the acceptable value of TDS specified by WHO (2011) guidelines for drinking water, all TDS values exceeded the standard limit. The values of Cl− concentration for greatest number of tested sites exceeded the range limits for drinking water purposes and aquatic life criteria. The concentrations of ions (Ca+2, Mg+2, and Na+) have exceeded permissible limits, making the water unsuitable for domestic use. Both alkalinity and hardness exceeded the permissible limits and therefore required appropriate treatments prior to use. According to the comparison between the SAR values which represents the suitability of water for irrigation purposes and SAR values of Shatt Al-Arab, all sites lie in the first class (excellent). According to Na+%, the type of surface water in the studied area lies in good class during winter season and permissible class during summer season. WQI of Shatt Al-Arab falls under very poor quality during summer season, while it ranges from very poor quality to unsuitable for drinking purposes during winter season. There is a clear effect of power plants on water quality. Hartha and Najibia power plants contribute to increasing the percentage ratios of WQI by 13.22 and 9.69%, respectively, during summer season compared to the north sites of these plants. The percentage ratios of increased WQI near Hartha and Najibia power plants compared to the north sites of these plants are 17.93 and 15.92%, respectively, during winter season. Water quality in the Shatt Al-Arab falls under a high level of organic pollution during the summer and winter. There is a slight effect by the power plants on the OPI.
References
Abbasi T, Abbasi S (2012) Water quality indices. Elsevier, Amsterdam
Abdullah EJ (2013) Quality assessment for Shatt Al-Arab River using heavy metal pollution index and metal index. J Environ Earth Sci 3(5):114–120
Akoteyon IS, Omotayo AO, Soladoye O, Olaoye HO (2011) Determination of water quality index and suitability of urban river for municipal water supply in Lagos, Nigeria. Eur J Sci Res 54(2):263–271
Al-Aboodi AH (2016) Estimation of monthly mean reference evapotranspiration using gene expression programming. Kufa J Eng 8(1):37–50
Almeida CA, Quintar S, Gonzalez P, Mallea MA (2007) Influence of urbanization and tourist activities on the water quality of the Potrero de los Funes River (San Luis-Argentina). Environ Monit Assess 133:459–465
Balan IN, Shivakumar M, Madan Kumar PD (2012) An assessment of groundwater quality using water quality index in Chennai, Tamil Nadu, India. Chron Young Sci 3(2):146–150
Benkhedda B, Belguermi A, Tayeb A, EL-Amine BM, Fethi D, Youssouf M (2014) Organic pollution assessment and biological quality of the River Oued Rhiou (Algeria). Int J Sci Basic Appl Res 18(1):33–44
Bhutiani R, Khanna DR, Kulkarni DB, Ruhela M (2014) Assessment of Ganga river ecosystem at Haridwar, Uttarakhand, India with reference to water quality indices. Appl Water Sci 6(2):107–113
Bora M, Goswami DC (2017) Water quality assessment in terms of water quality index (WQI): case study of the Kolong River, Assam, India. Appl Water Sci 7(6):3125–3135
Bureau of Indian Standards (BIS) (1998) Drinking water specifications (revised 2003), IS: 10500
CCME (Canadian Council of Ministers of the Environment) (2007) For the protection of aquatic life 2007. In: Canadian Environmental Quality Guidelines, 1999, Canadian Council of Ministers of the Environment, 1999, Winnipeg
CCME (Canadian Council of Ministers of the Environment) (2011) Chloride Ion. Scientific criteria document, Canadian Council of Ministers of the Environment, Winnipeg
Guasmi I, Kherici-Bousnoubra H, Kherici N, Hadji F (2010) Assessing the organic pollution of surface water of Medjerda watershed (NE Algeria). Environ Earth Sci 60(5):985–992
Kangabam RD, Bhoominathan SD, Kanagaraj S, Govindaraju M (2017) Development of a water quality index (WQI) for the Loktak Lake in India. Appl Water Sci 7:2907–2918
Khwakaram AI, Majid SN, Hama NY (2012) Determination of water quality index (wqi) for Qalyasan stream in Sulaimani city/Kurdistan region of Iraq. Int J Plant Anim Environ Sci 2(4):148–157
Kirubakaran M, Ashokraj C, Colins Johnny J, Anjali R (2015) Groundwater quality analysis using WQI and GIS techniques: a case study of Manavalakurichi in Kanyakumari District, Tamilnadu, India. Int J Innov Sci Eng Technol 2(11):341–347
Krishan G, Singh S, Singh RP, Ghosh NC (2016) Water quality index of groundwater of Haridwar District, Uttarakhand, India. Water Energy Int 58(10):55–58
Larnier K, Roux H, Dartus D, Croze O (2010) Water temperature modeling in the Garonne River (France). Knowl Manag Aquat Ecosyst 398:04
Mohanty SK (2004) Water quality index of four religious ponds and its seasonal variation in the temple city Bhuvaneshwar. In: Kumar A (ed) Water pollution. APH Publishing Corporation, New Delhi, pp 211–218
Moyel MS (2014) Assessment of water quality of the Shatt Al-Arab River, using multivariate statistical technique. Mesop Environ J 1(1):39–46
Moyel MS, Hussain NA (2015) Water quality assessment of the Shatt al-Arab River, Southern Iraq. J Coast Life Med 3(6):459–465
Nirmala B, Suresh Kumar BV, Suchetan PA, Shet Prakash M (2012) Seasonal variations physico chemical characteristics of ground water samples of Mysore City, Karanataka, India. Int Res J Environ Sci 1(4):43–49
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Córdoba city (Argentina) on Suquía River. Water Res 34:2915–2926
Puri PJ, Yenkie MKN, Rana DB, Meshram SU (2015) Application of water quality index (WQI) for the assessment of surface water quality (Ambazari Lake). Eur J Exp Biol 5(2):37–52
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the Groundwater in Tumkur Taluk, Karnataka State India. J Chem 6:523–530
Ramesh K, Seetha K (2013) Hydrochemical analysis of surface water and groundwater in tannery belt in and around Ranipet, Vellore district, Tamil Nadu, India. Int J Res Chem Environ 3(3):36–47
Ravikumar P, Mehmood MA, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3(1):247–261
Rubio-Arias H, Ochoa-Rivero JM, Quintana RM, Saucedo-Teran R, Ortize-Delgado RC, Rey-Burciaga NI (2013) Development of water quality index (WQI) of an artificial aquatic ecosystem in Mexico. J Environ Prot 4:1296–1306
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata wetlands, West Bengal, India. Environ Geol 55(4):823–835
Sanjib Kumar DAS, Chakrabarty D (2007) Limnological survey of three tropical water reservoirs in Eastern India. Acta Bot Malacitana 32:5–16
Shah KA, Joshi GS (2017) Evaluation of water quality index for River Sabarmati, Gujarat, India. Appl Water Sci 7(3):1349–1358
U S EPA (2009) Environmental impact and benefits assessment for final effluent guidelines and standards for the construction and development category. Office of Water, Washington, DC. EPA- 821-R-09-012
Vié J-C, Hilton-Taylor C, Stuart SN (2009) Wildlife in a changing world—an analysis of the 2008 IUCN red list of threatened species. IUCN, Gland
Vousta D, Manoli E, Samara C, Sofoniou M, Starits I (2001) A study of surface water quality in Macedonia, Greece: speciation of nitrogen and phosphorus. Water Air Soil Pollut 129(1–4):13–32
Wagh V, Panaskar D, Mukate S, Lolage Y, Muley A (2017) Groundwater quality evaluation by physicochemical characterization and water quality index for Nanded Tehsil, Maharashtra, India. In: Proceedings of the 7th international conference on biology, environment and chemistry, IPCBEE, vol 98
WHO (World Health Organization) (2011) Guidelines for drinking water quality, 4th edn. WHO, Geneva. ISBN 978-92-4-154815-1
Wilcox LV (1995) Classification and use of irrigation waters. U.S. Dept. of Agriculture, Washington
Yaseen S, Pandit AK, Shah JA (2015) Water quality index of fresh water streams feeding Wular Lake, in Kashmir Himalaya, India. Int J Water Resour Environ Eng 7(4):50–57
Yaseen BR, Al Asaady KA, Kazem AA, Chaichan MT (2016) Environmental impacts of salt tide in Shatt Al-Arab-Basra/Iraq. J Environ Sci Toxicol Food Technol 10(1):35–43
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Aboodi, A.H., Abbas, S.A. & Ibrahim, H.T. Effect of Hartha and Najibia power plants on water quality indices of Shatt Al-Arab River, south of Iraq. Appl Water Sci 8, 64 (2018). https://doi.org/10.1007/s13201-018-0703-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0703-0