Abstract
Iron deficiency anemia (IDA) is a global health concern that is affecting all age groups significantly. Among many of the existing methods, the fortification of foods with iron salts is the best and most cost-effective strategy for targeting large-scale populations to provide nutritional security. The fortification of foods with iron salts is a challenging task because most iron complexes (ferrous sulfate, ferrous chloride) used in fortification are highly water-soluble, which impart unacceptable organoleptic changes in food vehicles and also causes gastrointestinal problems. However, insoluble iron salts (ferric pyrophosphate) do not cause unacceptable taste or color in food vehicles but low bioavailable. Nanosized iron salts can overcome these concerns. The particle size of iron salts has been reported to play an important role in the absorption of iron. Reduction in the particle size of iron compounds increases its surface area, which in turn improves its solubility in the gastric juice leading to higher absorption. Nanosized iron compound produces minimal organoleptic changes in food vehicles compared to water-soluble iron complexes. Thus nanosized iron salts find potential applications in food fortification to reduce IDA. This paper focuses on providing a complete review of the various iron salts used in IDA, including their bioavailability, the challenges to food fortification, the effects of nanosized iron salts on IDA, and their applications in food fortification.

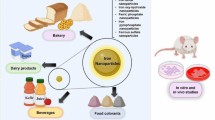
Graphic abstract
Fortification of foods with water-soluble Fe salts imparts unacceptable organoleptic changes in food vehicle and adverse impact on health. However, insoluble iron salts do not cause unacceptable taste or color in food vehicles but low bioavailable. Using Nano-sized iron compound produces minimal organoleptic changes in food vehicles compared to changes produced by water-soluble iron complexes, improves Fe absorption in the gastrointestinal tract and does not cause any health issues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is viewed as a global public health problem in which the number of red blood cells or their oxygen-carrying capacity is insufficient to meet physiological requirements of the body. The severity of anemia varies by age group, sex categories, altitude, smoking habits, and during the gestation period. In 2015, the World Health Organization (WHO) estimated that approximately 1.62 billion (24.8%) people of the world’s population are anemic. It impacts all stages of the life cycle. Still, higher cases of anemia have a 38% (32.4 million) in pregnant ladies, 29% (496 million) in non-pregnant women, and 43% (273 million) in children (Stevens et al. 2013). Anemia may have multiple causes, but according to WHO (2015), 50% of the cases of anemia are due to insufficient iron (Fe) intake (Fig. 1a). So, anemia and iron deficiency are often used synonymously. Other nutritional deficiencies can also cause anemia, including deficiencies of vitamins B12, B6, A, C, D, and E, folate, riboflavin, copper, and zinc (Wieringa et al. 2016). Heavy menstrual losses, parasite infections hookworms, roundworms, acute and chronic infections, malaria, cancer, tuberculosis, and HIV can also lower blood hemoglobin (Hb) concentrations (Chaparro and Suchdev 2019). Anemia is expressed as a challenge by many countries despite the implementation of several schemes for decades. In compliance, WHO has included anemia as the 2nd global goal of nutrition target for the year 2025, targeting to 50% reduction of anemia in girls and reproductive-age women. In March 2018, the Government of India launched the Anemia Mukt Bharat Abhiyan, which aims to reduce the prevalence of anemia among children, adolescents, and women of reproductive age (15–49 years) by three percentage points between the year 2018 and 2022.
Iron deficiency anemia and health concerns
The main biological function of iron is oxygen transport, as it forms part of the haem nucleus in the proteins hemoglobin and myoglobin. However, small quantities of iron participate in more than 200 enzymatic systems that are essential for cellular functions, including energy utilization by cells, DNA, RNA, and protein synthesis, as well as participating in a redox reaction, which is beneficial for exchange between ferrous (Fe+2) and ferric (Fe+3) states. Iron participates in enzyme systems such as those involved in neurotransmitter metabolism, vitamin D activation, collagen metabolism, and cholesterol catabolism. Therefore, iron is essential for oxygen transport and storage and many other metabolic functions related to growth, muscular activity, immunity, bone strength, and the nervous system (Toxqui and Vaquero 2015; Blanco-Rojo and Vaquero 2019).
Although there is an equilibrium between iron loss and absorption but several milligrams of iron loss in a day cannot be overcome by the gut's absorption capacity and cause iron deficiency, and once iron stores deplete, iron deficiency anemia ensues (Rockey, 2010; Camaschella, 2019). Symptoms, physiological and pathological disorders that can promote IDA are shown in Fig. 1b. Children aged 0–5 years, women of childbearing age, and pregnant women are particularly at risk. Several chronic diseases are frequently associated with iron deficiency anemia (chronic kidney disease, chronic heart failure, cancer, and inflammatory bowel disease) (Lopez et al. 2016). Obesity-associated inflammation is tightly linked to iron deficiency (Aigner et al. 2014). A small study was carried out in 20 obese premenopausal females to examine the relationship between obesity and anemia and found that after weight loss, iron status improved (Tussing‐Humphreys et al. 2010). In contrast, excess iron is another vicinity of the subject since it has been related to several diseases, such as cirrhosis, cardiovascular disease, type II diabetes, and cancer (Raju and Venkataramappa 2018).
Diagnosis of iron deficiency anemia
Hemoglobin concentration is often used as an indicator of IDA, which varies with age, sex, and physiological status. In iron deficiency, hemoglobin concentration is < 13 g/dL in adult men, < 12 g/dL in non-pregnant, and < 11 g/dL in reproductive-age women. The more severe stage of anemia is defined as hemoglobin < 8 g/dL in men and non-pregnant women, < 7 g/dL if pregnant. Meanwhile, measurement of serum transferrin and ferritin saturation, soluble transferrin receptor (STfR), and STfR-ferritin index are more accurate than classical hemoglobin tests in the IDA evaluation. In iron homeostasis, hepcidin plays a key role, so it is identified as a new diagnostic and therapeutic target (Lopez et al. 2016).
Possible link between iron deficiency anemia and COVID-19
According to World Health Organization (WHO), severe acute respiratory syndrome coronavirus- 2 (SARS-CoV-2) continues to wreak havoc all over the world, which affects all age groups but chances of infection is high in the geriatric population, pregnant women, and people with pre-existing medical conditions (IDA, diabetes and cardiovascular disease). While there is no particular medicine or vaccine for COVID-19 till now, it will be necessary to take preventive measures and immunity booster (Arshad et al. 2020). Iron and immunity are closely linked with each other as it is the fundamental element for the development of the immune system. Inadequate intake of iron (Fe) may lead to suppressed immunity by affecting innate, T cell-mediated, and adaptive antibody responses. Beneficial effects induced by iron includes enhances neutrophil function such as increase myeloperoxidase activity (MPO) and possibly paired with intracellular bacteriocidal activity, which strengthens the body’s defense system against infections. Iron also helps increase T-lymphocyte counts, natural killer cell activity, and help in the production of macrophage inhibition factor and interleukin-2, which are essential to immunity development (Fig. 1c) (Ward et al. 2011). Therefore, it may be hypothesized that supplementation of iron could boost the immunity against COVID-19.
Dietary factor responsible for iron deficiency
Iron is an essential mineral present naturally in many foodstuffs in two forms: haem and non-haem iron. Iron absorption generally starts from the first part of the intestine (duodenum) by diverse mechanisms according to the form of iron present in food. Animal food such as meat, liver, seafood, or black pudding is the only source that provides heam iron, and its absorption is unaffected by dietary factors (Thompson and Amoroso 2011). Plant-based foodstuff contains non-haem iron complex that commonly exists in two oxidative states ferrous or ferric form. Most non-haem iron is present in the ferric (Fe3+) form, which has low bioavailability and solubility (Han, 2011). Non-beam iron is digested in the digestive system by the action of pepsin enzymes and HCl and exhibits different absorption rates (2–10%) depending on the presence of iron enhancers and inhibitors in food. Commonly, ascorbic acid (Vitamin C), folic acid, β-carotenoids, organic acids, and amino acids rich in the peptide are enhancers of iron absorption that can reduce ferric (Fe3+) to ferrous (Fe2+) from (Hurrell and Egli 2010). By contrast, antinutritional factors such as phytic acid, polyphenols, calcium (Ca), magnesium(Mg), phosphorus(P), and casein have a negative impact on iron absorption (Martınez-Navarrete et al. 2002). Vegetarian diets have a large number of antinutritional factors that reduce the bioavailability of iron. So, IDA can be eradicated by fortifying food and food product with iron salts.
Strategies for eradicating iron fortification
Several methods have been used to eliminate IDA for the last several years, but an effective method is needed that runs for a long-time and cost-effective. In this context, food and food products fortified with iron are the most common and cost-effective strategies to alleviate iron deficiency among humans being (García-Bañuelos et al. 2014). Recently, dual and quadruple fortification strategies have been used for ensuring the delivery of multiple micronutrients such as iron and iodine. For fortification, those food products have been selected as the food carrier, which is widely and commonly consumed by the target groups. According to WHO, the most common staple food (salt, sugar, flours, rice, oil, and dairy products) can be selected as a carrier for food fortification to control iron deficiency. However, fortification of food and food products with iron (Fe) salt that is more bioavailable, does not alter the characteristics of the food and cost effective is a challenge (Hurrell, 2002). This paper focuses on providing a complete review of the various iron salts used, including bioavailability, the challenges of food fortification, the effects of nano-iron salts on IDA, their applications in food fortification, and safety concerns of iron nanoparticles.
Iron fortificant for food fortification
In the design of iron-fortified food, it is necessary to select a suitable combination of food vehicle and iron fortificant. Numerous iron salts are available to enrich foodstuffs with iron. Table 1 shows some iron salts that are commonly used in food and food products fortification. Based on solubility, iron salts are categorized into three types (a) high water-soluble, (b) poorly water-soluble but soluble in dilute acid, (c) water-insoluble but poorly soluble in acid. Generally, the high water-soluble iron compound is more bioavailable except ferrous chloride (Preedy et al. 2013). Ferrous chloride is very quickly oxidized just after solubilization which easily observed by the rapid appearance of orange coloring. It explains why ferrous chloride less bioavailable as predicted by its solubility. In the human metabolic process, form of iron fortificant (Fe2+, Fe3+) plays an important role in iron absorption. Water-soluble iron compound affects the taste, color, and overall acceptability of food products (Preedy et al. 2013; Ward and Crichton 2016). So, the biggest concern is to find an iron compound that is stable, more bioavailable, not alter pH and organoleptic properties of food.
Challenges in iron fortification
According to food technologists, iron is the most challenging micronutrient to produce fortified food because the reaction rate of the iron with the different active substances of the food matrix is very high, and it may produce adverse organoleptic changes. Many iron complexes had been tried and incorporated into food vehicles to obtain ideal iron fortificant according to the needs of the anemic population (Dary and Hurrell 2006). However, these problems were not yet completely solved. The problem is related to its chemistry. In food fortification, two forms of iron are commonly used: ferrous and ferric. Both forms of iron have empty d-block and tried to obtain a stable position, so they form complexes with anthocyanins, flavonoids, tannins, and phenolic compounds present in food. Due to this reaction, several common problems formed that is an adverse impact on texture and color, metallic aftertaste, off-flavor due to peroxidation of lipids, degradation of vitamins (ascorbic acid and carotenoid), and reduce the absorption of iron, as shown in Fig. 2 (Mellican et al. 2003; Mehansho 2006). Tolkien et al. (2015) studied the impact of ferrous sulphate on human health. The result shows that ferrous sulphate severely affects the GI tract and natural microflora of the gut in all the population groups investigated. Chelation-redox modulation-based technology may overcome metallic aftertaste but then marked enhancement of colon cancer risk is seen in rodent models using chelates of iron(III) citrate or iron(III) EDTA. Allen (2002) used amino acid–chelated ferrous bis-glycinate as an iron source in whole maize meal and found that the bisglycinate has higher redox potential and subsequently greater tendency to cause lipid oxidation which causes adverse organoleptic changes. Iron(III) pyrophosphate and reduced iron are water-insoluble and more suitable for food fortification because they do not alter organoleptic properties of food but poorly bioavailable. Haem iron also attempted to prevent IDA but was not proved to be safe, as excessive intake causes colorectal and prostate cancer (Blanco-Rojo and Vaquero 2019). These types of problems significantly limit the use of iron salts to prevent IDA. So, researchers are very active in finding ideal iron fortificant that added in sufficient quantity, adequately absorbed, stable, and does not alter the appearance or taste of the food vehicle.
Nanotechnology for iron deficiency anemia
As stated earlier, food fortification is the most cost-effective strategy for reducing the prevalence of iron deficiency in the population group without dependency on pharmaceutical tablets or capsules. As alternatives to the soluble iron salts, which might be highly bioavailable but lead to an undesirable flavor, color, as well as affects the gastrointestinal tract, insoluble iron salts can be used which include elemental iron, ferric pyrophosphate and ferric phosphate. Insoluble iron salts do not impart sensory changes to the food matrix. Although the poor solubility of iron salt in gastric acids leads to lower bioavailability and thereby reduces its nutritional value (Hurrell 2002). Figure 3 shows the important factors that play a role in developing an effective iron food fortification technique, and nanotechnology can fulfill all its criteria. Over the past few decades, nanotechnology has increasingly been considered as to be attractive technology that has revolutionized the medical, agricultural, and food sectors. Typically the length of the synthesized nanoparticle is between 1–100 nm (Chaudhry et al. 2008). In the food industry, nanoparticles have been used as a food additive, anti-caking agents, carriers for smart delivery of nutrients, antimicrobial agents, fillers for improving mechanical strength and durability of the packaging material (McClements and Xiao 2017; Shukla et al. 2017). Among many nanotechnology applications, one is to increase nutrient bioavailability and delivery of minerals, nutrients, molecules, and drugs to their target. Researchers found that when some of the materials are prepared in nanometer size, their bioavailability increases (Shafie et al. 2016; Foujdar et al. 2020). For these details, food technologists and researchers are now involved in employing nanotechnology, which is emergent as a promising strategy for the efficient iron food fortification and delivery of drugs for the treatment of anemia. This statement has been validated by the findings of Verma et al. (1977) that decreasing the particle size of elemental iron powder by 50–60% to a mean particle size of 7–10 mm increases iron absorption by 50% in rats. Harrison et al. (1976) did another study using five commercial samples of ferric orthophosphate based on particle size distribution (1–15 µm) and solubility in 0.1 N HCl(11.6–63.4%), result data showed that the finer and higher solubility in HCl has more relative bioavailability value (RBV). In the case of Fe(III) pyrophosphate, Hurrell et al. (2004) reported that by decreasing the particle size of ferric pyrophosphate from 8 to 4 μm, absorption of iron increased 2–4 times in adults. These studies prove that particle size reduction is an effective strategy for improving iron bioavailability. Particle size reduction techniques increase the surface area of iron compound which improves its solubility in the gastric acid leading to higher absorption.
Metabolic pathway of the iron nanoparticles
A major barrier to the treatment of IDA via conventional water-soluble iron fortification is adverse effects on the gastrointestinal tract, harmful effects to the oesophagus, and the microflora present in the gut. These problems are arises due to redox cycling of water-soluble iron fortificant. Oral administration of conventional iron supplements only small part is absorbed in the upper intestinal tract and the remaining reaches the low tract where reacts with hydrogen peroxide and superoxide producing free radicals and unfavorable effects. Nano-sized iron salts are considered safer, better tolerated in the gut lumen and more bioavailable than conventional iron fortificant. Reduction in the particle size of iron compounds saves oesophagus and gastrointestinal tract from the harsh effects and increases the surface area of iron salts, improving its solubility in the gastric juice leading to higher absorption (Shukla et al. 2017). Figure 4 gives a diagrammatic representation of the metabolic pathway of the iron nanoparticle’s, which involved the whole physiology of iron nanoparticle from intake up to storage and recycling. In brief, iron present in the duodenum as ferric(Fe3+) form that will be reduced to the ferrous(Fe+2) state by Dcyt B(Duodenal cytochrome B) enzyme that is ferrireductase, then reduced ferrous(Fe+2) transported across the enterocyte apical membrane by the divalent metal transporter1 (DMT1). Fenton reaction is the source of free ferrous ion in enterocytes, which need to be converted or export immediately. So, reduced ferrous(Fe+2) is released at the enterocytes serosal membrane via ferroportin (export protein) and oxidized to the ferric(Fe+3) form by hephaestin protein. Apotransferrin bound to ferric and makes a complex called iron-transferrin, and transported to the site where it is required (Shubham et al. 2020).
Types of iron nanoparticle used to prevent IDA
Table 2 shows various types of iron nanoparticles such as iron oxide, ferric phosphate, iron oxo hydroxide and ferric pyrophosphate that used for the treatment of IDA rather than the frequently used iron salts. These nanoparticles are synthesized mainly by the co-precipitation method, flame spray pyrolysis, or titration method. The shapes of nanoparticles (NPs) exert a remarkable impact on their properties. TEM, XRD and zeta potential analysis help in determination of the shape, size and nature (crystalline or amorphous) of nanoparticles (Ali et al. 2016). So, the properties of nanoparticles depend on the method of synthesis and their preparation conditions.
Iron oxide nanoparticle (IONPs)
Iron oxide nanoparticles is the most investigated nanoparticles due to their unique properties, such as superparamagnetism, surface-to-volume ratio, greater surface area, easy separation methodology, biocompatibility and bioavailability. It can be prepared by physical, chemical, and biological methods. Figure 5a showed the synthesis of iron oxide nanoparticle in which co-precipitation of iron salt with a hydroxide base. The co-precipitation method is the most common, well-known conventional iron oxide synthesis method (Ali et al. 2016). Synthesized iron oxide nanoparticles can be encapsulated with a polymer such as dextran, polyethylene glycol (PEG), starch, and chitosan to increase stability. Iron oxide nanoparticles have a wide range of applications, one of which is a supplement for the patient having anemia (Arami et al. 2015). Elshemy (2018) conducted a study to investigate the effect of IONPs (0.4 mg/kg body weight per 10 days) in male albino rats via drinking water in the treatment of IDA. The result shows that IONPs significantly increases red blood cells (RBCs) count, hemoglobin concentration, red blood cells indices (mean cell volume and mean corpuscular Hb concentration), ferritin, hematocrit (Hct), transferrin saturation (TS) and total iron binding capacity (TIBC) compared to FeSO4 group. It may be due to nanosized iron oxide increased the bioavailability and absorption rate. These effects matched with Hashem et al. (2018), who designed in vivo study in female wistar rats to study the effect of iron oxide nanoparticle in the treatment of IDA and the result was compared with frequently used ferrous sulfate. The study shows that spherical-shaped Fe3O4 nanoparticles with an average size in the range of 65.95–295.3 nm significantly increase RBCs compared to ferrous sulfate groups. These results revealed that iron oxide nanoparticles proved as an effective supplement for the treatment of iron deficiency anemia.
Effect of enhancer on IONPS
Vitamins C, Vitamin B9 (folic acid) and vitamin B3 (nicotine) enhance iron absorption. To study the impact of enhancer on iron fortificant, Mahmoud and Helmy (2016) conducted a study in which iron oxide nanoparticle coated with a mixture of vitamins such as vitamin B9, B3, and L- ascorbic acid. This coated mixture feed-in an iron-deficient rat for one week. Animal trials studies reveal that introducing a single dose of iron oxide- vitamin nano-composites containing 2.57 mg elemental iron/kg rat body weight is sufficient to correct the Hb level from 2.73 mmol/L to 9.06 mmol/L within four days and cure anemia without any apparent toxicity in comparison with ferric chloride treated group. It can be used as a new formula for food fortification in the treatment of IDA.
IONPs as food fortificant
Food fortification is an efficient technology to fight against the disease, so the fortification of food and dairy products with iron salts is deemed efficient in preventing IDA.
Dairy product
Yogurt is the most popular fermented dairy product with higher nutritional properties, low cost, fantastic taste, and higher shelf-life than milk. It can be used as a suitable vehicle for incorporating the iron complex to treat anemic population. Garcés et al. (2020) develop magnetic yogurts by incorporating iron oxide nanoparticles. In this planning, IONPs is synthesized by the co-precipitation method and grafting NPs to the mixture of Lactobacillus acidophilus and Streptococcus thermophilus bacteria. Magnetic yogurts were prepared by adding bacterial coated iron nanoparticles to fresh milk at a temperature around (40–42 °C).TEM images reveals that most of the magnetic nanoparticles were attached to the bacterial exopolysaccharides (EPSs). The capacity of bacteria to ferment the milk was not affected by the presence of nanoparticles’ (NPS). The magnetic yogurts show a new concept in functional foods and the perfect vehicle for the treatment of IDA as well as used to cure injury of the digestive system that causes by hyperthermia.
To study the effect of Fe/Zn nanoparticle on bioavailability and color change in commercially available food products (Banana and chocolate milk, and cassis red currant yogurt), Hilty et al. (2009) synthesized iron and zinc containing nano-sized compounds by flame spray pyrolysis (FSP). The new nanostructured iron powders produced minimal color changes when added to dairy products containing chocolate or fruit compared to the changes produced by water-soluble iron. Thus, these synthesized nanostructured powders can also be used for food and dairy products fortification without organoleptic changes to prevent iron and zinc deficiency.
Cereal products
Biscuit is one of the globally consumed baked products with higher calorific value and low cost, and due to low moisture content, its shelf life is also high. So biscuits are considered the right carrier for mineral fortification and nutritional improvement (Manley and Vir 2011). Salaheldin and Regheb (2016) synthesize ascorbic acid coated spherical shape iron oxide (Fe3O4) nanoparticles with a size range of 20 ± 2 nm. Biscuits fortified with three levels of IONPs (10, 30, and 60 mg/kg iron) and result compared with iron(III) chloride fortified biscuits. The result finding shows that as increasing the concentration of vitamin C coated iron oxide nanoparticles promoted the growth rate, increased the nutritional quality of protein, enhance the erythropoiesis process as well as increases Hb concentration and RBC count without any toxicological symptom and no mortality. Iron-fortified biscuit shows complete recovery from the anemic state within five weeks. The present result recommends the use of 10 mg/kg nano iron-fortified biscuits for mild IDA and the 30 mg/kg level for more severe ones to control IDA.
Ferric phosphate nanoparticle
Commercially available ferric phosphate (FePO4) is a white-colored, insoluble iron salt with low nutritional value (Hallberg et al. 1989). However, nanosized ferric phosphate is high bioavailable and almost the same sensory score as control food sample. Hilty et al. (2010) fortified chocolate and banana milk with nanosized FePO4/Zn3(PO4)2. These nanoparticles are synthesized by flame aerosol technology with a specific surface area of approximately 190 m2g−1. The sensory analysis shows that Fe/Zn nanoparticle cause less colour change in reactive food matrices than conventional iron fortificants.
Flame spray pyrolysis (FSP) is a quick, dry, and flexible process for synthesizing nanoparticles with high specific surface area (SSA) and low production fees. Therefore Rohner et al. (2007) used the flame spray pyrolysis method to synthesize ferric phosphate nanoparticle in which precursor Fe(III)-acetylacetonate, and tributylphosphate were dissolved in xylene at an iron and phosphorus ion concentration of 0.2 mol/L each. The bioavailability of nanosized ferric phosphate was evaluated by the hemoglobin (Hb) repletion method. This study shows that when the size of ferric phosphate (FePO4) is reduced to the nanoscale, its bioavailability increased and almost similar to ferrous sulphate. In histological examinations and thiobarbituric acid reactive substances (TBARS) analyses, there were no indications of toxicity or necrosis found.
Previous studies were not adequately investigated the potential adverse effects of Fe(III)phosphate nanoparticle on human cell line. So, Von Moos et al. (2017) investigated the toxicological effects of different nanosized ferric phosphate (FePO4) on human cell lines such as HCECs, HT29 (colon cell), and HT29-MTX. Study shows that it does not induce cytotoxicity and oxidative stress to cells. Furthermore, Fe(III)PO4 nanoparticles enriched diets feeding to rats did not show any toxicity signs, not gather in tissues although when the amount of nanoparticle was 100 times higher than the daily requirement of iron for women.
Ferric pyrophosphate nanoparticle
Iron(III) pyrophosphate is creamish white water insoluble iron salt which does not impart sensory changes to the food matrix and used in food fortification to meet recommended dietary allowance (RDA) of iron. The bioavailability of ferric pyrophosphate is low due to the low solubility even at high pH. Hurrell (2002) first time reported that its bioavailability is about 30–50% of ferrous sulfate. As stated earlier that particle size is an essential factor for iron absorption in the GI tract. Fidler et al. (2004) reported that when the particle size of iron(III) pyrophosphate was about 0.3 mm, the relative bioavailability was 82% in human, whereas Moretti et al. (2006) reported 62% relative bioavailability when particle size of ferric pyrophosphate was 0.77 mm. Therefore Srinivasu et al. (2015) synthesized iron (III) pyrophosphate nanoparticles by titration method in which FeCl3 was titrated against Na4P2O7 solution in the presence of ethane-1,2-diol at 65 °C. Vigorous stirring is done to prevent the accumulation of the particles (Fig. 5b). The bioavailability of ferric pyrophosphate nanoparticle was assessed by in vivo study in rats by nourishing them with Fe4(P2O3)3 nanoparticle rich diet. The hemoglobin regeneration efficiency was calculated to determine the relative bioavailability of ferric pyrophosphate. The relative bioavailability of ferric pyrophosphate nanoparticle was found to be 103.2% with respect to ferrous sulfate. Histopathological studies of different organs did not show any toxic symptoms. However, iron nanoparticles have a high specific gravity (1.5 or higher) so when nanoparticles were dispersed in a liquid product such as milk, whose specific gravity is around 1.028–1.032, the nanoparticle is suspended in a short period, thereby adversely affecting the stability and appearance of the liquid products. So, Marshman and Velikov (2009) used whey protein, gum arabic as stabilizer to stabilized iron (II/III) pyrophosphate nanoparticles. The synthesis was done by titration method in which 1% whey protein isolate was added to sodium pyrophosphate solution and titrated it against ferric chloride. The synthesized biopolymer stabilized iron nanoparticle can be used to fortify a liquid food product without precipitation or changing the organoleptic properties of food products.
Iron oxo hydroxide
Iron oxo-hydroxide nanoparticles are an iron complex of iron, oxygen, and hydrogen, which have potential to treat IDA population. They are more bioactive than conventional iron salts, and their structure is similar to ferritin. Ferritin is a protein that stores iron. Pereira et al. (2014) synthesized 5–10 nm size of Fe(III) oxo-hydroxide and made a similar structure like ferritin core by adding tartrate and adipinic acid. Animal and cellular models were used to check cellular uptake and utilization of iron oxo-hydroxide nanomaterial. Results showed that iron-oxo-hydroxide nanomaterial with adipinic and tartrate acid was 80% relative bioavailable to FeSO4 in humans, and in a rat model, relative bioavailability is equal to ferrous sulfate. Unlike ferrous sulfate, it does not affect natural microbiota and not accumulate in the intestinal tract. Thus, adipinic and tartaric acid-coated iron-oxo-hydroxide nanoparticle is non-toxic and more efficient than the commonly used iron salts.
Isotopic rich iron oxo hydroxide nanoparticles in formula milk
In the neonate, the major source of iron (Fe) is maternal milk. If maternal milk is not available or sufficient, formula milk fortified with essential trace elements (e.g. Fe, Zn) are used (American association, 1999). There is also a fact that maternal milk has low iron content (0.4 mg/L) and is not sufficient to fulfill daily iron requirements, so the neonate is fed formula milk. Fernández-Menéndez et al. (2018) for the first time, check the efficiency of tartaric acid-coated 57Fe oxo-hydroxide nanoparticles for formula milk fortification. For this purpose, iron enriched powder of 57Fe was firstly dissolved with concentrated hydrochloric acid, and then the resulting solution was added to sodium tartrate solution in a ratio of 2:1(Fe:tartrate ratio 2:1). Sodium chloride was added to the solution until the pH reached 7.4. The mixture was then centrifuged and precipitate was washed by ultrafiltration by using a filter of 3000 Da cut-off. The NPs were then left to dry for at least 24 h at 45 °C under vacuum. The effectiveness of 57Fe(III) oxo-hydroxide nanoparticle has been examined in two-week-old Wistar rats having 16 μg iron nanoparticles/g milk powder. It was found that serum level and red blood cell count (RBCs) increased with feeding formula milk containing 57Fe(III)-NPs. On statistical analysis, synthesized nanoparticles were not showing much differences comparing with commonly used ferrous sulfate at the same dose. This study demonstrates the successful application of 57Fe-NPs in formula milk, and it is an efficient option that can fulfil the iron requirement in a neonate with higher bioavailability than that of the commonly used iron salts (FeSO4).
Hybrid iron nanoparticle
β -lactoglobulin amyloid fibrils are protein aggregates. It is used as a reductant, and recently used for the synthesis of gold crystal. β-lactoglobulin fibrils act as a reducing agent, so when mixed with ferric form, it converts into ferrous form that is more bioavailable. Using this technique, Shen et al. (2017) discovered a new β-lactoglobulin fibrils–iron nanoparticle hybrid material for use in iron fortification. Hybrid material made of biodegradable amyloid fibril and iron nanoparticle. Figure 5c shows the synthesis of iron-BLG fibril. In brief, amyloid fibril was formed by denaturing native β-lactoglobulin protein by heating at 90 °C in pH 2 for 5 h. Obtained amyloid fibril mixed with iron chloride solution in the presence of sodium borohydride (NaBH4), iron nanoparticles were nucleated onto the fibrils. New iron nanoparticles were stable and did not clump together in foods and drinks. In vivo analysis showed that new supplement was easily digested and high bioavailable without changing the organoleptic properties of food vehicles. Toxicological studies in rats show that iron BLG fibril nanocomposites did not show toxicity and necrosis in cells. Therefore, these iron amyloid nanofibrils can be used to fortify different types of solid and liquid food products without affecting their sensory qualities.
Toxicity of iron nanoparticle
INPs have many safety concerns, especially with oral exposure or as a fortificant. So, there is a need to study all parameters of toxicity and clinical practice. In vivo and in vitro toxic studies of iron nanoparticles usually need long-term investigations and monitoring for months or even years because it prolonged circulated in the body after degradation (Arami et al. 2015). Different pharmacokinetics and degradation rates of the iron nanoparticle and their coating molecules make the toxicity studies more complicated. Table 3 summarizes the recent research work that has proposed toxicity of iron nanoparticles for treatments of IDA. Gastrointestinal effects such as nausea, vomiting, abdominal pain, diarrhea and constipation were among the most frequent adverse effects reported in a clinical study. Overall, study found that orally intake of iron nanoparticles is a safe way for drug delivery at a low amount. However, some investigators still believe that the possible long-term safety effects of these INPs have not been fully evaluated.
Conclusion and future prospects
Anemia is a serious global health problem that affects all age group but most prevalent in young children and pregnant women. Iron fortification of staple foods can be an efficient and long-term strategy to improve the iron level of the populace without changing their dietary habit, but adding iron to foods that are well absorbed by the body is a challenge. Conventional fortificants (FeSO4, FeCl3) caused gastrointestinal problems, black stool, and other health-related issues and changed the organoleptic properties of food. Nanosized iron salts have the potential to overcome the problem caused by the use of water-soluble iron complexes. Synthesis of iron complexes in nanosize increases iron bioavailability, and problems like unacceptable colour, flavour, metallic taste, and rancidity in food vehicles by using water-soluble iron salts are solved. Thus, the nanosized iron powder plays an important role in the fortification of food and food products for the treatment of IDA. Still, there is concern about their potential toxic effect. Due to their small size, nanoparticles can cross physiological barriers, damage cells or tissues and exposure to nanoparticles might lead to health hazards. Therefore, various technical aspects such as characterization, safety assessments, and the impact of iron nanoparticles in the rheology of food should be studied for their long-term applicability.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- IDA:
-
Iron deficiency anemia
- TEM:
-
Transmission electron microscopy
- XRD:
-
X-ray diffraction
- RBV:
-
Relative bioavailability value
- NPs:
-
Nanoparticles
- IONPs:
-
Iron oxide nanoparticles
References
Aigner E, Feldman A, Datz C (2014) Obesity as an emerging risk factor for iron deficiency. Nutrients 6(9):3587–3600
Ali A, HiraZafar MZ, ulHaq I, Phull AR, Ali JS, Hussain A (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9:49
Allen LH (2002) Advantages and limitations of iron amino acid chelates as iron fortificants. Nutr Rev 60(suppl-7):S18–S21
American Academy of Pediatrics Committee on Nutrition (1999) Iron fortification of infant formulas. Pediatrics 104:119–123
Arami H, Khandhar A, Liggitt D, Krishnan KM (2015) In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev 44:8576–8607
Arshad MS, Khan U, Sadiq A, Khalid W, Hussain M, Yasmeen A, Rehana H (2020) Coronavirus disease (COVID-19) and immunity booster green foods: a mini review. Food Sci Nutr 8(8):3971–3976
Blanco-Rojo R, Vaquero MP (2019) Iron bioavailability from food fortification to precision nutrition. A review. Innov Food Sci Emerg Technol 51:126–138
Camaschella C (2019) Iron deficiency. Blood 133(1):30–39
Chamorro S, Gutiérrez L, Vaquero MP, Verdoy D, Salas G, Luengo Y, Teran FJ (2015) Safety assessment of chronic oral exposure to iron oxide nanoparticles. Nanotechnology 26:205101
Chaparro CM, Suchdev PS (2019) Anemia epidemiology, pathophysiology, and etiology in low-and middle-income countries. Ann N Y Acad Sci 1450(1):15
Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Watkins R (2008) Applications and implications of nanotechnologies for the food sector. Food Addit Contam 25:241–258
Thompson B, Amoroso L (eds) (2011) Combating micronutrient deficiencies: food-based approaches. CABI, Willingford
Dary O, Hurrell R (2006) Guidelines on food fortification with micronutrients. Geneva, Switzerland, World health organization, food and agricultural organization of the United Nations
Elshemy MA (2018) Iron oxide nanoparticles versus ferrous sulfate in treatment of iron deficiency anemia in rats. Egypt J Vet Sci 49:103–109
Feng J, Liu H, Zhang L, Bhakoo K, Lu L (2010) An insight into the metabolic responses of ultra-small superparamagnetic particles of iron oxide using metabonomic analysis of biofluids. Nanotechnology 21(39):395101
Fernández-Menéndez S, Peixoto RRA, Fernández-Colomer B, Romero MC, Sanz-Medel A, Fernández-Sánchez ML (2018) Searching for enhanced iron fortification of formula milk via nanoparticles and isotope pattern deconvolution. Spectrochim Acta Part B 148:165–171
Fidler MC, Walczyk T, Davidsson L, Zeder C, Sakaguchi N, Juneja LR, Hurrell RF (2004) A micronised, dispersible ferric pyrophosphate with high relative bioavailability in man. British J Nutr 91:107–112
Foujdar R, Chopra H, Bera M, Chauhan A, Mahajan P (2020) Effect of probe ultrasonication, microwave and sunlight on biosynthesis, bioactivity and structural morphology of punica granatum peel’s polyphenols-based silver nanoconjugates. Waste Biomass Valoriz 12(5):2283–2302
Garcés V, González A, Sabio L, Sánchez-Arévalo CM, Gálvez N, Dominguez-Vera JM (2020) Magnetic and golden yogurts. Food as a potential nanomedicine carrier. Materials 13(2):481
García-Bañuelos ML, Sida-Arreola JP, Sánchez E (2014) Biofortification-promising approach to increasing the content of iron and zinc in staple food crops. J Elementol 19:3
Hallberg L, Rossander-Hulthen L, Gramatkovski E (1989) Iron fortification of flour with a complex ferric orthophosphate. Am J Clin Nutr 50:129–135
Han O (2011) Molecular mechanism of intestinal iron absorption. Metallomics 3(2):103–109
Harrison BN, Pia GW, Clark GA, Fritz JC (1976) Selection of iron sources for cereal enrichment. Cereal Chem 53:78–84
Hashem F, Nasr M, Ahmed Y (2018) Preparation and evaluation of iron oxide nanoparticles for treatment of iron deficiency anemia. Int J Pharm Pharm Sci 10:142
Hilty FM, Teleki A, Krumeich F, Büchel R, Hurrell RF, Pratsinis SE, Zimmermann MB (2009) Development and optimization of iron-and zinc-containing nanostructured powders for nutritional applications. Nanotechnol 20:475101
Hilty FM, Arnold M, Hilbe M, Teleki A, Knijnenburg JT, Ehrensperger F, Zimmermann MB (2010) Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat Nanotechnol 5:374
Hurrell RF (2002) Fortification: overcoming technical and practical barriers. J Nutr 132:806S-812S
Hurrell R (2004) How to ensure adequate iron absorption from iron-fortified food. Nutr Rev 60:S7–S15
Hurrell R, Egli I (2010) Iron bioavailability and dietary reference values. Am J Clin Nutr 91(5):1461S-1467S
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L (2016) Iron deficiency anaemia. The Lancet 387(10021):907–916
Mahmoud MBM, Helmy SHA (2016) Novel formula of iron based nanocomposites for rapid and efficient treatment of iron deficiency anemia. U.S. Patent Application No. 20160022733
Manley D, Vir S (2011) Manley’s technology of biscuits, crackers and cookies. Woodhead Publishing Limited
Marshman CE, Velikov KP (2009) Iron fortified food product and additive. U.S. Patent Application No. 20090061068
Martınez-Navarrete N, Camacho MM, Martınez-Lahuerta J, Martınez-Monzó J, Fito P (2002) Iron deficiency and iron fortified foods—a review. Food Res Int 35:225–231
McClements DJ, Xiao H (2017) Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci Food 1:1–13
Mehansho H (2006) Iron fortification technology development: new approaches. J Nutr 136:1059–1063
Mellican RI, Li J, Mehansho H, Nielsen SS (2003) The role of iron and the factors affecting off-color development of polyphenols. J Agric Food Chem 51:2304–2316
Moretti D, Zimmermann MB, Wegmüller R, Walczyk T, Zeder C, Hurrell RF (2006) Iron status and food matrix strongly affect the relative bioavailability of ferric pyrophosphate in humans. Am J Clin Nutr 83:632–638
Pereira DI, Bruggraber SF, Faria N, Poots LK, Tagmount MA, Aslam MF, Powell JJ (2014) Nanoparticulate iron (III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomed Nanotechnol Biol Med 10:1877–1886
Preedy VR, Srirajaskanthan R, Patel VB (2013) Handbook of food fortification and health. Concepts Pub Health Appl. https://doi.org/10.1007/978-1-4614-7076-2
Raju K, Venkataramappa SM (2018) Primary hemochromatosis presenting as type 2 diabetes mellitus: a case report with review of literature. Int J Appl Basic Med Res 8:57
Rockey DC (2010) Occult and obscure gastrointestinal bleeding: causes and clinical management. Nat Rev Gastroenterol Hepatol 7:265–279
Rohner F, Ernst FO, Arnold M, Hilbe M, Biebinger R, Ehrensperger F, Zimmermann MB (2007) Synthesis, characterization, and bioavailability in rats of ferric phosphate nanoparticles. J Nutr 137:614–619
Salaheldin TA, Regheb EM (2016) In-vivo nutritional and toxicological evaluation of nano iron fortified biscuits as food supplement for iron deficient anemia. J Nanomed Res 3(1):00049
Shafie EH, Keshavarz SA, Kefayati ME, Taheri F, Sarbakhsh P, Vafa MR (2016) The effects of nanoparticles containing iron on blood and inflammatory markers in comparison to ferrous sulfate in anemic rats. Int J Prev Med. 7(1):117
Shen Y, Posavec L, Bolisetty S, Hilty FM, Nyström G, Kohlbrecher J, Mezzenga R (2017) Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat Nanotechnol 12:642
Shubham K, Anukiruthika T, Dutta S, Kashyap AV, Moses JA, Anandharamakrishnan C (2020) Iron deficiency anemia: a comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci Tech 99:58–75
Shukla A, Dasgupta N, Ranjan S, Singh S, Chidambram R (2017) Nanotechnology towards prevention of anaemia and osteoporosis: from concept to market. Biotechnol Biotec Eq 31:863–879
Srinivasu BY, Mitra G, Muralidharan M, Srivastava D, Pinto J, Thankachan P, Thomas TS (2015) Beneficiary effect of nanosizing ferric pyrophosphate as food fortificant in iron deficiency anemia: evaluation of bioavailability, toxicity and plasma biomarker. RSC Adv 5:61678–61687
Stevens GA, Finucanen MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Nutrition Impact Model Study Group (2013) Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 1(1):e16–e25
Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ (2015) Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PloS one 10(2):e0117383
Toxqui L, Vaquero MP (2015) Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients 7:2324–2344
Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Holterman AXL, Galvani C, Ayloo S, Vitello J, Braunschweig C (2010) Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity 18(10):2010–2016
Verma RS, Motzok I, Chen SS, Rasper J, Ross HU (1977) Effect of storage in flour and of particle size on the bioavailability of elemental iron powders for rats and humans. J Assoc off Anal Chem 60(4):759–765
Von Moos LM, Schneider M, Hilty FM, Hilbe M, Arnold M, Ziegler N, Naegeli H (2017) Iron phosphate nanoparticles for food fortification: biological effects in rats and human cell lines. Nanotoxicology 11:496–506
Ward RJ, Crichton RR (2016) Iron: properties and determination. Encycl Food Health. https://doi.org/10.1016/B978-0-12-384947-2.00403-7
Ward RJ, Crichton RR, Taylor DL, Della Corte L, Srai SK, Dexter DT (2011) Iron and the immune system. J Neural Transm 118:315–328
WHO (2015) The global prevalence of anaemia in 2011. Geneva
Wieringa FT, Dahl M, Chamnan C, Poirot E, Kuong K, Sophonneary P, Sinuon M, Greuffeille V, Hong R, Berger J, Dijkhuizen MA (2016) The high prevalence of anemia in cambodian children and women cannot be satisfactorily explained by nutritional deficiencies or hemoglobin disorders. Nutrients 8(6):348
Yang L, Kuang H, Zhang W, Aguilar ZP, Xiong Y, Lai W, Xu H, Wei H (2015) Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale 7(2):625–636
Acknowledgements
The authors acknowledge Department of Dairy Science and Food Technology, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi for the support in the work related to this review article.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
The manuscript was written through equal contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors agree to publish.
Ethics approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, A., Chauhan, A.K. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: a review. J Food Sci Technol 59, 3319–3335 (2022). https://doi.org/10.1007/s13197-021-05184-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05184-4