Abstract
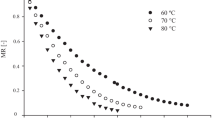
Low sugar, low fat, dry fruit and nut cereal bars without sugar were prepared using cereals, nuts, and sugar substitutes. The sorption characteristics of the bars prepared with sugar substitutes in comparison with that of sugar were studied by keeping the bars at water activity (aw) from 0.1 to 0.9. The sorption isotherms of low sugar bars were practically identical below aw of 0.5 but above aw of 0.5, a clear differentiation in the isotherms could be observed compared to that of sugar counterpart. A sharp increase in moisture content was observed in the bars prepared with alternative sweeteners, above aw 0.6, whereas a gradual increase in aw was observed in the case of bar prepared with sugar. The ERH (Equilibrium relative humidity) value for bar with sugar was 50 %, and for bars prepared with alternative sweeteners, it was about 60 %. Low sugar cereal bar prepared with sorbitol + maltitol (SM) syrup scored higher sensory quality compared to other product prepared with sorbitol + nutriose (SN) as the former retained softness and chewiness on storage. Thus, it was observed that bars with alternative sweeteners will be more stable as their ERH is closer to normal ambient conditions compared to that prepared with sugar.




Similar content being viewed by others
References
Agarwal KK, Clary BL (1971) Investigation in to the theories of desorption isotherms for rough rice and peanuts. J Food Sci 36:919–924
AOCS (2001) Official method of analysis and recommended practices of American oil chemists’ society, 4th edn, Ba 3–38, Rev. and Reapproved, 6th edn. American Oil Chemists Society, Champaign, p 3
Bala BK (1991) Physical and thermal properties of malt. Drying Technol 9(4):1091–1104
Bizot H (1984) F Escher. In: Jowitt R, Escher F, Escher F, Hallstrom B, Meffert HFT, Spiess WE, Vos G (eds) Physical properties of foods. Applied Science Publ, London, pp 27–41
Caurie MJ (1970) A new model for predicting safe storage moisture levels for optimum stability of dehydrated foods. J Food Technol 5:301–307
Chanasattru W, Decker EA, Mcclements DJ (2008) Impact of co solvents (polyols) on globular protein functionality; ultrasonic velocity, density, surface tension and solubility. Food Hydrocolloids 22(8):1475–84
Chinachoti P, Steinberg MP (1986) Moisture hysteresis due to amorphous sugar. J Food Sci 51(2):453–455
Chirife J, Iglesias HA (1978) Equation for fitting water sorption isotherms of foods-1. A review. J Food Technol 13:159–174
Duncan DB (1955) New multiple range and multiple F test. Biometrics 11:1–42
Hossain MD, Bala BK, Hossain MA, Mondol MRA (2001) Sorption isotherms and heat of sorption of pineapple. J Food Engg 48:103–107
Hossain SA, Pal PK, Sarkar PK, Patil GR (2002) Moisture sorption characteristics of dudh churpi, a traditional milk product in India. Nahrung 46(3):136–140
Labuza TP (1968) Sorption phenomenon in foods. Food Technol 22:263–272
Labuza TP, Tannenbaum SR, Karel M (1970) Water content and stability oflow moisture and intermediate moisture foods. Food Technol 24:543–550
Li Y, Szlachetka K, Chen P, Lin X, Ruan R (2008) Ingredient characterization and hardening of high-protein food bars: An NMR state diagram approach. Cereal Chem 85(6):780–6
Liu X, Zhou P, Tran A, Labuza TP (2009) Effects of polyols on the stability of whey proteins in intermediate-moisture food model systems. J Agric Food Chem 57(6):2239–45
Mcmahon DJ, Adams SL, Mcmanus WR (2009) Hardening of high-protein nutrition bars and sugar/polyol-protein phase separation. J Food Sci 74:E312–E321
Mizrahi S, Karel MJ (1977) Moisture transfer in a packaged product in isothermal storage: extrapolating data to any package-humidity combination and evaluating water sorption isotherms. J Food Preservation 1:225–234
Nadeem M, Rehman S, Anjum FM, Murtaza MA, Mueen-ud-din G (2012) Development characterization, and optimization of protein level in date bars using response surface methodology. The Sci world J doi: 1100/2012/518702
Ozligen S (2011) Influence of chemical composition and environmental conditions on the textural properties of dried fruits. Czech J Food Sci 29(5):539–547
Padmashree A, Sharma GK, Srihari KA, Bawa AS (2012) Development of shelf stable protein rich composite cereal bar. J Food Sci Technol 49(3):335–341
Stefan J (2003) Made at last. Prepared foods, Sept. Avaliable from: http://www.preparedfoods.com/CDA/Archives?Issue=65117. Accessed Oct 24, 2008.
Stone H, Sidel JL (2004) Sensory evaluation practices, 3rd edn. Elseiver, London
Tavakolipour H, Ashtari AK (2008) Estimation of moisture sorption isotherms in Kerman pistachio nuts. J Food Process Eng 31(4):564–582
Wilkinson C, Dijksterhius GB, Minekus M (2000) From food structure to texture. Trends. J Food Sci Technol 11(12):442–450
Wolf W, Speiss WEL, Jung G, Weisser H, Bizot H, Duckworth RB (1984) The water-vapour sorption isotherms of microcrystalline cellulose (MCC) and of purified potato starch. Results of a collaborative study. Food Engg 3:51–73
Acknowledgments
The authors thank Dr. B. R. Lokesh, Head, Lipid Science and Traditional Foods department and Dr. G. Venkateswara Rao, Director, CFTRI, for their keen interest in the work and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pallavi, B.V., Chetana, R., Ravi, R. et al. Moisture sorption curves of fruit and nut cereal bar prepared with sugar and sugar substitutes. J Food Sci Technol 52, 1663–1669 (2015). https://doi.org/10.1007/s13197-013-1101-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1101-0