Abstract
Aloe vera (Aloe barbadensis Miller) cubes of 12.5 × 12.5 × 12.5 mm thick were osmosed for 4 h in sugar syrup of 30, 40 and 50°Brix concentration and temperatures of 30 and 50°C at constant syrup to fruit ratio of 5:1. Osmosed and unosmosed aloe vera samples were hot air dried at 50, 60, 70 and 80°C with constant air velocity of 1.5 m/s. The water loss, solid gain and convective drying behaviour were recorded during experiments. It was observed that water loss and solid gain ranged from 39.2 to 71.3 and 2.7 to 6.3%, respectively during osmo-drying. The moisture diffusivity varied from 2.9 to 8.0 × 10−9 m²/s and 2.7 to 4.6 × 10−9 m²/s during air drying of osmosed and unosmosed aloe vera samples, respectively. Drying air temperature and osmosis as pre-treatment affected the water loss, solid gain, diffusivity at −p ≤ 0.01
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aloe vera (Aloe barbadensis Miller), a traditional medicinal plant, is used in food, pharmaceutical and cosmetic industries (Grindlay and Reynolds 1986; Koga 1998). Aloe vera leaves are formed by a thick epidermis (skin) covered with cuticle surrounding the mesophyll, which can be differentiated into chlorenchyma cells and thinner walled cells forming the parenchyma (fillet). The parenchyma cells contain a transparent mucilaginous jelly, which is referred to as aloe vera gel. Gel contains 97–98% of water and more than 60% of dry matter is made up of polysaccharides (McAnalley 1993; Femenia et al. 1999). The gel is a colourless, odourless, hydrocolloid with several natural beneficial substances.
Aloe vera is an industrial crop and in the food industry it has been utilized for the preparation of health food drinks, beverages like tea, milk, ice-cream and confectionary (Seoshin et al. 1995). Aloe vera gel also finds application in cosmetic and toiletry industry for the preparation of creams, lotions, soaps, shampoos and facial cleaners (Grindlay and Reynolds 1986; Koga 1998). Reports credit that aloe has anti-tumor (Loadman and Christopher 2001) anti-diabetic (Beppu et al. 1993) and anti-tyrosine properties (Yagi et al. 1987) in addition to efficacy in healing wounds and burns (Chithra et al. 1998; Somboonwong et al. 2000) and treatment of gastric ulcers (Maze et al. 1997).
The potential use of aloe vera products often involves some type of processing, like heating, dehydration and grinding (Chang et al. 2006). Unfortunately, because of improper processing procedure aloe products contain very little or virtually no active ingredients (Ramachandra and Rao 2006), so it has become very important to evolve a better method of preservation for increasing the shelf life and maintaining the quality of aloe vera gel.
Osmotic dehydration is the process of water removal, with low energy consumption at low temperature. This provides minimum thermal degradation of nutrients due to low temperature water removal process (Shi et al. 1997). The osmotic process variables (pre-treatment, temperature, concentration of the solution, agitation, additives, immersion time) have been reported to have influence on mass transfer and on the product quality (Lerici et al. 1985; Rastogi and Raghavarao 1997; Erle and Schubert 2001; Rastogi et al. 2004). The osmotic process has received considerable attention as a pre-treatment so as to reduce energy consumption and improve food quality (Jayaraman and Das Gupta 1992; Karthanos et al. 1995). Besides reducing the drying time, the osmotic dehydration as a pre-treatment also inhibits enzymatic growing, retains natural colour and retains volatile aroma during the subsequent drying (Pokharkar et al. 1997).
Osmotic dehydration process has been studied for many fruits and vegetables, such as, apple, banana, carrot, cherry, citrus fruits, grape, guava and mango (Ponting et al. 1966; Flink 1980; Maguer 1988; Fito 1994; Ahmed and Choudhary 1995; Chaudhari et al. 2000). Also studies on dehydration of aloe vera was done by Simal et al. (2000), Chang et al. (2006), Vega et al. (2007) and Segovia et al. (2009). However no systematic attempt has been reported on osmo-air drying of aloe vera gel cubes. Therefore, the objective of the study was to investigate the effect of osmotic treatment on water loss (WL), solid gain (SG) and on convective drying behaviour of aloe vera gel.
Materials and methods
Aloe vera (Aloe barbadensis Miller) leaves were procured from Herbal Park, Rajasthan College of Agriculture, MPUAT, Udaipur. Fresh whole aloe vera leaves of 30 and 50 cm length from 3 to 4 year old plants were washed under tap water to remove adhering materials. The spikes, placed along the margins, were removed before slicing the leaves. The thick epidermis (or skin) was carefully separated from parenchyma (or gel fillet) using a stainless steel cutter. The fillets were cut into 12.5 × 12.5 × 12.5 mm cubes with the help of stainless steel cutter. Aloe vera gel cubes were freshly prepared on each day of experiment. Sugar syrup of desired concentration was prepared by dissolving required amount of sugar in tap water.
Osmotic dehydration
The solute used for osmotic dehydration was sugar. The sucrose concentrations selected for osmotic dehydration were 30, 40 and 50°Brix. The osmotic dehydration was carried out at 30 and 50°C for 4 h of immersion time at constant syrup to fruit ratio of 5:1. The osmotic dehydration experiments were carried out in a glass beaker placed in a hot water bath with thermostat-controlled heaters. After every 30 min interval, one glass beaker was removed from water bath and aloe vera samples were immediately rinsed with water and placed on absorbing paper to remove surface moisture. The samples were weighed and their moisture content was determined by using AOAC (1984) method. All the experimental measurements were replicated 4 times.
The WL and SG were calculated by using the following mass balanced equations (Lenart and Flink 1984). The WL was the net loss of water from aloe vera cubes at time (θ) on an initial mass basis.
The dry matter gain is related to solid gain (SG) and hence, the SG was the net gain in total solids by aloe vera cubes on the initial mass basis.
where, W θ = mass of aloe vera cubes after time θ, g, W i = initial mass of cubes, g, X θ = water content as a fraction of the weight at time ‘θ’, and X i = water content as a fraction of initial weight of cubes.
Air drying
The osmosed and unosmosed aloe vera cubes were dried in conventional hot air dryer at air temperatures of 50, 60, 70 and 80°C with constant air velocity of 1.5 m/s. The dryer was equipped with an electronic balance. The sample weight was continuously registered at 15 min interval for first 2 h of the experiments, afterwards the weights were noted at 30 min intervals till the sample attained constant weight. Approximately 150 g of sample was dried until final moisture content was attained for safe storage.
Moisture diffusivity during hot-air drying
In drying, diffusivity is used to indicate the flow of moisture. In falling rate period of drying, moisture is transferred mainly by molecular diffusion. Diffusivity is influenced by shrinkage, case hardening during drying, moisture content and temperature of material. The moisture diffusivity of the samples was estimated by using the simplified mathematical Fick’s second diffusion model. The solution of Fick’s second law in slab geometry, with the assumption that moisture migration was caused by diffusion, negligible shrinkage, constant diffusion coefficients and temperature was as follows (Crank 1975). For infinite plate shape (aloe vera cubes was considered to be infinite plate shape)
For long drying periods, Eq. 3 can be further simplified to only the first term of the series.
where, M R is the dimensionless moisture ratio, M the moisture content at any time (g H2O/g dry matter), M 0 the initial moisture content (g H2O/g dry matter), M e the equilibrium moisture content (g H2O/g dry matter), D eff the effective diffusivity (m2/s), H the half thickness of slab in sample (m), n the positive integer, t the Time (sec).
A general form of Eq. 4 could be written in semi-logarithmic form as follows.
The effective diffusivity is typically calculated by plotting experimental drying data in terms of ln (M R ) versus drying time. From Eq. 5, a plot of ln (M R ) versus the drying time gives a straight line with a slope of:
Result and discussion
Effect on water loss
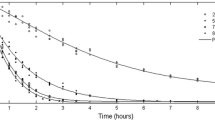
The WL at any concentration was affected by the temperature of the syrup (Fig. 1). It increased with increase in syrup temperature. This may be due to change in semi-permeability of cell membrane of the gel allowing more water to diffuse out in a shorter period. These finding were in conformation with the results of Ponting et al. (1966) and Farkas and Lazar (1969). However, higher temperature promoted faster WL through swelling and plasticizing of cell membranes, also faster water diffusion within the product and better water transfer characteristics on the product surface due to lower viscosity of osmotic medium. Rate of WL was rapid initially and decreased gradually with increase in time. This could be due to reducing concentration gradient of moisture between product and solution with time. When syrup temperature was increased from 30 to 50°C for 50°Brix syrup concentrations, WL increased from 66.9 to 71.3% after 4 h of osmotic dehydration causing approximately 4.5% point increment. Similarly for 40°Brix syrup concentration, the WL increased from 65.8 to 68.0% giving 2.2% point increment. Also for 30°Brix syrup concentration, the corresponding increase was 2.2%.
Figure 1 shows that a low temperature-low concentration condition (30°C–30°Brix) gives a low WL (63.1% after 4 h of osmosis) and high temperature-high concentration condition (50°C–50°Brix) gives a higher WL (71.3% after 4 h of osmosis). This indicates that WL can be increased by either increasing the syrup temperature or concentration of solution. Similar results have been reported for osmotic dehydration of onions by Sagar (2001). Such effects have also been reported in other fruits and vegetables (Karthanos et al. 1995; Ertekin and Cakaloz 1996; Pokharkar and Prasad 1998).
Effect on sugar gain
The increase in sugar concentration led to increase in SG (Fig. 1), because the concentration gradient between intracellular fluid and solute solution caused the osmotic pressure, which led to diffusion of water through semi-permeable membrane to achieve osmotic equilibrium (Sodhi et al. 2006). Figure 1 shows that a low temperature-low concentration condition (30°C–30°Brix) resulted into a low SG (4.0% after 4 h of osmosis) and a high temperature-high concentration condition (50°C–50°Brix) resulted into a higher SG (6.3% after 4 h of osmosis). The low temperature-high concentration condition 30°C–50°Brix resulted into a slightly lower SG of 4.4% after 4 h of osmosis than 50°C–30°Brix (5.0% after 4 h of osmosis) indicates a pronounced effect of temperature on SG. Similar results have been reported by Lazarides and Mavroudis (1995) with osmotic dehydration of apple slices in a temperature range of 20–50°C.
Effect on convective drying behavior of aloe vera
The moisture content of fresh and osmosed samples was 98.7 and 87.1 to 78.3% (wb). The final moisture content of osmotically dehydrated samples dried in a tray dryer was in the range of 12 to 20% (db). It could be evident from Fig. 2 that moisture content of aloe vera samples decreased exponentially with drying time under all drying conditions. Similar results were obtained by Vergara et al. (1997) for air drying of osmotically dehydrated apples. It took 5 h of drying time in tray dryer to reduce the moisture content to 17.3% (db) at 50°C drying temperature, whereas it took 4.5, 3.5 and 2.5 h of drying in tray dryer to reduce the moisture content to 16.3, 14.8 and 13.8% (db) at 60, 70 and 80°C air drying temperature, respectively.
As the sugar syrup concentration and temperature increased, the drying time also increased (Table 1). This may be due to accumulation of sugar on the surface of the product, which slows down the dehydration rate of aloe vera. In general, it was found that the complete drying of aloe vera cubes took place in the falling rate period and the constant rate period was totally absent. Drying rate was higher in the initial period of drying and subsequently it reduced with decrease in moisture content. Sugar concentration and drying air temperature have significant effect on drying time at 1% level (Table 2).
Moisture diffusivity of aloe vera cubes
The moisture loss data during air drying were analyzed and moisture ratios at various time intervals were determined. Moisture diffusivities were calculated from the slopes of these straight lines (Maskan et al. 2002; Doymaz 2004). The moisture diffusivity value of food material is affected by moisture content as well as temperature. At low moisture content the diffusivity was less than that of high moisture content (Table 1). Moisture diffusivity increased with drying air temperature in osmo-convective drying processes which is in accordance of the results reported by Rahman and Lamb (1990) and Pokharkar and Prasad (1998). The moisture diffusivity varied from 2.9 to 8.0 × 10−9 m²/s and 2.7 to 4.6 × 10−9 m²/s during hot air drying of osmosed and unosmosed aloe vera samples depending on the drying air temperature (Table 1). These values are within the general range of 10−08 to 10−12 m²/s for drying of food materials (McMinn and Magee 1999). Sugar concentration and drying air temperature had significant effect on moisture diffusivity at 1% level (Table 2).
Conclusion
In osmotic aloe gel dehydration, increase of sugar concentration and temperature of osmosis increased WL and SG. The WL from aloe gel was very rapid for the first 2 h of osmosis and reduced subsequently with duration of osmosis. As the sugar syrup concentration and temperature increased, the drying time also increased. Drying of aloe vera cubes occurred only in falling rate drying period. Constant rate drying period was absent throughout the drying process of aloe vera cubes dried under all drying air temperature. The moisture diffusivity varied in the range of 2.9 to 8.0 × 10−9 m²/s and 2.7 to 4.6 × 10−9 m²/s during air drying of osmosed and unosmosed aloe vera samples depending on the drying air temperature.
References
Ahmed J, Choudhary DR (1995) Osmotic dehydration of papaya. Indian Food Packer 49(4):5–11
AOAC (1984) Official methods of analysis. 14th edn, Association of Official Analytical Chemists, Arlington, Virginia, 22209, USA
Beppu H, Nagamura Y, Fujita K (1993) Hyglycaemic and antidiabetic effects in mice of Aloe arborescens Miller var. natalensis Berger. Phytotherapy Res 7:37–42
Chang LX, Wang C, Feng Y, Liu Z (2006) Effects of heat treatments on the stabilities of polysaccharides substances and barbaloin in gel juice from aloe vera Miller. J Food Eng 75:245–251
Chaudhari AP, Kumbhar BK, Narain M (2000) Effect of some process parameters on osmotic dehydration of papaya. J Inst Eng 81:59–63
Chithra P, Sajithlal GB, Chandrakasan G (1998) Influence of Aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J Exp Biol 36:896–901
Crank J (1975) The mathematics of the diffusion. Oxford University Press, London, pp 39–58
Doymaz I (2004) Effect of pre-treatments using potassium metabisulphide and alkaline ethyl oleate on the drying kinetics of apricots. Biosystems Eng 89:281–287
Erle U, Schubert H (2001) Combined osmotic and microwave-vacuum dehydration of apples and strawberries. J Food Eng 49:193–199
Ertekin FK, Cakaloz T (1996) Osmotic dehydration of peas: II. Influence of osmosis on drying behaviour and product quality. J Food Proc Preserv 20:105–119
Farkas DF, Lazar ME (1969) Osmotic dehydration of apple piece: effect of temperature and syrup concentration on rates. Food Technol 23(11):688–690
Femenia A, Sanchez ES, Simal S, Rosello C (1999) Compositional feature of polysaccharides from aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr Polymer 39:109–117
Fito P (1994) Modeling of vacuum osmotic dehydration of food. J Food Eng 22:115–117
Flink JM (1980) Dehydrated carrot slices: influence of osmotic concentration on drying behaviour and product quality. In: Linko P (ed) Food process engineering, Vol. 1. Academic, London, pp 412–418
Grindlay D, Reynolds T (1986) The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol 16:117–151
Jayaraman KS, Das Gupta DK (1992) Osmotic dehydration of fruits and vegetables: recent development in principles and techniques. Drying Technol 10:1–50
Karthanos VT, Kastaropoulus AE, Saravacos GD (1995) Air-drying behaviour of osmotically dehydrated fruits. Drying Technol 13(5–7):1503–1521
Koga T (1998) Cosmetic compositions containing aloe polysaccharides for inhibition of spots, darkening and wrinkles. Japanese Patent 1010 141
Lazarides HN, Mavroudis N (1995) Freeze/thaw effect on mass transfer rates during osmotic dehydration. J Food Sci 60:826–829
Lenart A, Flink JM (1984) Osmotic concentration of potato. Criteria for the end point of the osmotic process. J Food Sci Technol 19:45–63
Lerici CR, Pinnavaia G, Rosa M, Bartolucci L (1985) Osmotic dehydration of fruits: influence of osmotic agents on drying behavior and product quality. J Food Sci 50:1217–1226
Loadman PM, Christopher RC (2001) Separation methods for anthraquionone related anti-cancer drugs. J Chromatogr 764:193–206
Maguer ML (1988) Osmotic dehydration: review and future direction. Proc Int Symp Progress in Food Preservation Processes 1:283–309
Maskan A, Kaya S, Maskan M (2002) Hot air and sun drying of grape leather (pestil). J Food Eng 54:81–88
Maze G, Terpolilli RN, Lee M (1997) Aloe vera extract prevents aspirin-induced acute gastric mucosal injury in rats. Med Sci Res 25:765–766
McAnalley BH (1993) Process for preparation of aloe products. European Patent WO 89/06539
McMinn WAM, Magee TRA (1999) Principles, methods and applications of the convective drying of foodstuffs. Trans Inst Chem Eng 77(3):175–193
Pokharkar SM, Prasad S (1998) Mass transfer during osmotic dehydration of banana slices. J Food Sci Technol 35:336–338
Pokharkar SM, Prasad S, Das H (1997) A model of osmotic concentration of banana slices. J Food Sci Technol 34:230–232
Ponting JD, Watters GG, Forrey RR, Jackson R, Stanley WL (1966) Osmotic dehydration of fruits. Food Technol 20(10):125–128
Rahman MS, Lamb J (1990) Osmotic dehydration of pineapple. J Food Sci Technol 27:150–152
Ramachandra CT, Rao S (2006). Processing of aloe vera leaf gel: a focus on the present and innovative process technologies. In: Proc Int Conf Innovations in food and bioprocess technologies, AIT Pathumthami, Thailand, 12–14 Dec, p 358–377
Rastogi NK, Raghavarao KSMS (1997) Water and solute diffusion coefficients of carrot as a function of temperature and concentration during osmotic dehydration. J Food Eng 34:429–440
Rastogi NK, Nayak CA, Raghavarao KSMS (2004) Influence of osmotic pretreatment on rehydration characteristic of carrots. J Food Eng 65:287–292
Sagar VR (2001) Preparation of onion powder by means of osmotic dehydration and its packaging and storage. J Food Sci Technol 38:525–528
Segovia PG, Mognetti C, Bello AA, Monzo JM (2009) Osmotic dehydration of aloe vera (Aloe barbadensis Miller). J Food Eng 97:154–160
Seoshin Y, Lee KS, Lee JS, Lee CH (1995) Preparation of ghurt added with aloe vera and its quality characteristics. J Korean Soc Food Nutr 24:254–260
Shi JX, Maguer ML, Wang SL, Liptay A (1997) Application of osmotic treatment in tomato processing-effect of skin treatments on mass transfer in osmotic dehydration of tomatoes. Food Res Int 30(9):669–674
Simal S, Femenia A, Llull P, Rossello C (2000) Dehydration of aloe vera: simulation of drying curves and evaluation of functional properties. J Food Eng 43:109–114
Sodhi NS, Singh N, Komal (2006) Osmotic dehydration kinetics of carrots. J Food Sci Technol 43:374–376
Somboonwong J, Thanamittramanee S, Jariyapongskul A, Patumraj S (2000) Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J Med Assoc Thailand 83:417–425
Vega A, Uribe E, Lemus R, Miranda M (2007) Hot-air drying characteristics of Aloe vera (Aloe barbandensis Miller) and influence of temperature on kinetic parameters. Lebensm Wiss Technol 40:1698–1707
Vergara F, Amezaga E, Barcenas ME, Welti J (1997) Analysis of the drying processes of osmotically dehydrated apple using the characteristic curve model. Drying Technol 15:949–963
Yagi A, Kanbara T, Morinobu N (1987) Inhibition of mushroom tyrosinase by aloe extract. Planta Med 53:509–582
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pisalkar, P.S., Jain, N.K. & Jain, S.K. Osmo-air drying of aloe vera gel cubes. J Food Sci Technol 48, 183–189 (2011). https://doi.org/10.1007/s13197-010-0121-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-010-0121-2