Abstract
Recent advances in tumor immunology and cancer immunotherapy have generated significant interest in the field of immuno-oncology. With the promise of these advances comes an increasing need to train the next generation of scientists who will support ongoing basic and clinical research efforts in this field. At this time, however, there remains a documented underrepresentation of tumor immunology as a core content area in many undergraduate science curricula. This study introduces a novel pedagogical strategy that aimed to promote undergraduate student interest in tumor immunology in ways that support recent education guidelines published by the American Association of Immunologists, and it highlights the efficacy of this approach in enhancing student understanding of concepts relevant to the Cancer-Immunity Cycle. Using RNA-sequencing data obtained from clinical specimens catalogued in The Cancer Genome Atlas, students performed Kaplan–Meier survival analyses to identify Cancer-Immunity Cycle genes with prognostic significance. After correlating expression of such genes with tumor-infiltrating immune cell populations using a bioinformatic tool to deconvolute whole tumor-transcriptome data, students undertook an exercise that requires integration of course content and findings from the primary literature to generate hypotheses about the influence of genetic factors and immune cell types on the Cancer-Immunity Cycle and overall patient outcome. A pre-/post-project assessment instrument demonstrated the efficacy of this approach as a means of improving undergraduate student understanding of core cancer immunology concepts. This report describes these data and discusses potential ways in which the project can be adapted to extend its utility to broad and diverse student populations.
Similar content being viewed by others
Introduction
Advances in our understanding of tumor immunology over the last two and half decades have sparked a revolution in immuno-oncology, resulting in a paradigm shift that has transformed the landscape of cancer therapy. With immunotherapy being ushered to the forefront of a new age in cancer medicine, clinical outcomes for many patients have improved in ways that are unprecedented in the history of this disease. Indeed, as recently as 1999, it was reported that the estimated 1-year survival rate for advanced (stage III/IV) melanoma patients treated with dacarbazine, the only FDA-approved chemotherapeutic for this cancer, was a mere 27% [1]; today, the 5-year survival rate for this cancer now exceeds 50% for patients treated by combination immunotherapy with anti-CTLA-4 and anti-PD-1 immune checkpoint inhibitors [2]. Similar results have emerged in recent trials for various other cancer types, including lung cancer and renal cell carcinoma, where immunotherapy has significantly outperformed standard-of-care chemotherapy and targeted therapy regimens [3, 4].
Building on significant research efforts over the previous two-plus decades, the aforementioned and related successes in cancer immunotherapy were made possible only by improvements in our understanding of basic immunobiology and mechanisms of tumor immune evasion. This latter process in particular became recognized as so significant to cancer progression that it was included in 2011 as one of two new “Hallmarks of Cancer” in an update to Hanahan and Weinberg’s classic list of core tumor-promoting processes originally put forth at the turn of the century [5, 6]. The recognition of tumor immunity as a key regulator of cancer progression has in turn spawned an appreciation for several key events necessary to achieve successful anti-tumor immune reactivity. Dubbed “The Cancer-Immunity Cycle,” these 7 steps essential to immune recognition and eradication of tumor cells include (1) the release of cancer cell antigens (cancer cell death); (2) cancer antigen presentation by dendritic cells (DC) and other antigen-presenting cells (APC); (3) priming and activation of T lymphocytes by APC; (4) trafficking of T lymphocytes to tumors; (5) infiltration of T lymphocytes into tumors; (6) recognition of cancer cells by T lymphocytes; and (7) killing of cancer cells, which in turn releases additional tumor antigens to renew and amplify the cycle [7]. Ongoing research to better understand the multitude of factors that both positively and negatively regulate these aspects of The Cancer-Immunity Cycle remains a driving force in the field of immuno-oncology and continues to guide new advances in the development of immune-based therapeutics for cancer.
With the recognition that the immune system functions as a key regulator of cancer progression, the need to improve education efforts centered around tumor immunology has become increasingly apparent, not only as a means of effectively training the next generation of clinicians and research scientists in the fields of immunology and oncology but also as a means of promoting scientific literacy among those in the general population who will likely be impacted in the future, either directly or indirectly, by medical advances in these fields. In this regard, in its recently published guidelines for undergraduate immunology course design, the American Association of Immunologists (AAI) identified “Tumor Immunology” as one of fourteen topics recommended for inclusion as critical curricular content, with tumor immunosurveillance, tumor immune escape, and cancer immunotherapy being highlighted as foundational concepts that should be emphasized within this content category [8]. Importantly, these recommendations come at a time when recent survey analysis has documented tumor immunology as a topic for which little or no time is allocated by a significant proportion of undergraduate immunology instructors [9]. Additionally, AAI guidelines for undergraduate immunology course curricula also acknowledge the relevance of bioinformatics as a tool for studying immunologic concepts, including tumor immunology [8]. In this era of omics-based approaches to scientific inquiry, there is indeed a growing recognition that bioinformatics has become an essential aspect of undergraduate training in the natural sciences [10, 11], and it is therefore necessary to develop pedagogical strategies for incorporating bioinformatics into broad areas of undergraduate science curricula.
We and others have recently documented the pedagogical benefits of bioinformatics-based methods for improving undergraduate education on diverse cancer-related topics [12,13,14,15]. Many of these approaches take advantage of publicly available data from The Cancer Genome Atlas (TCGA), a collaborative program sponsored by the National Cancer Institute and the National Human Genome Research Institute that generated a comprehensive set of genomic, transcriptomic, epigenomic, and proteomic data from more than 20,000 tumor samples representing 33 distinct cancer types [16]. The study described herein documents the utility of TCGA RNA-sequencing data as a resource for a bioinformatics-based approach to improve undergraduate immunology student understanding of the Cancer-Immunity Cycle. That this approach effectively addresses documented needs in bioinformatics, immunology, and cancer education speaks to its broad applicability as a tool to enhance several important aspects of undergraduate science education.
Methods
Project Participants
Participants in this study were 2nd-, 3rd-, or 4th-year undergraduate students enrolled in a 4-credit, 300-level immunology lecture and laboratory course at Hampden-Sydney College, a private liberal arts college located in Virginia. A total of 35 students from two course sections were involved in the study. The course, which typically enrolls 14–18 students and is offered once every four academic semesters (every other year), includes three 50-min lecture sessions per week and a single 2.5-h laboratory period per week. Pre-requisites for enrollment in the course include a 100-level Principles of Biology lecture/laboratory course and a 200-level Genetics and Cell Biology lecture/laboratory course (each a total of 4 credits). This Immunology course is an upper-level elective that can be used to fulfill a major requirement for both the Biology Department and the Biochemistry & Molecular Biology (BCMB) Program; it may also be applied to the College’s Biology minor. Though it is open to non-majors, it is uncommon for such students to enroll in this course, and all participants across the two semesters spanned by this study were Biology or BCMB majors. Of the 35 participants in this study, 17 students were enrolled in the Spring 2020 semester, and 18 students were enrolled in the Spring 2022 semester. Students in both semesters received equivalent emphasis on lecture content, but only students enrolled in the Spring 2022 semester completed the bioinformatics-based project that is the focal point of this study. Though all students in this latter cohort were required to complete this project for the sake of equity in the course, participation in the pre- and post-lab assessment was strictly voluntary, as was the completion of an identical end-of-semester assessment for the Spring 2020 cohort not participating in the bioinformatics project under investigation. Despite being voluntary, the assessment instrument was completed by all students in each cohort, and all participants signed consent forms approved by the College’s Institutional Review Board prior to initiation of the study.
Study Design
Student participants from both semesters received equal emphasis on lecture content and had access to identical study materials throughout the semester. With respect to coverage of tumor immunology, each course section included 4 lecture class periods dedicated to this topic. For the Spring 2022 class cohort participating in the Cancer-Immunity Cycle bioinformatics project, students also analyzed next-generation RNA-sequencing data from TCGA over the course of the semester using the publicly available Tumor Immune Estimation Resource (TIMER). This web tool, which estimates tumor infiltration by six immune cell subsets (B cells, CD4 + T cells, CD8 + T cells, macrophages, neutrophils, and DC) via computational deconvolution of bulk whole transcriptome data acquired from heterogeneous tumor samples, is accessible at https://cistrome.shinyapps.io/timer/ [17]. In addition to inferring tumor infiltration by each of these immune cell types, this interface also offers modules for correlating specific immune cell infiltration in tumor tissue with various biological and clinical parameters, including gene expression levels, somatic mutations and copy number alterations, and patient survival. Together, these features allow users to integrate analysis of clinical, immunologic, and genomics data from diverse tumor types.
For this particular study, students were asked to choose a single cancer type of interest and interrogate TCGA RNA-sequencing data with TIMER for the purposes of evaluating how the expression level of specific genes relevant to each step of the Cancer-Immunity Cycle correlates with (1) immune cell infiltration of tumor tissue and (2) overall patient outcome. At the onset of the project, students were trained in the use of the TIMER platform and provided with step-by-step instructions for use of the necessary modules (see Appendix 1 of Online Resource 1). Students were also given a pre-defined list of 178 Cancer-Immunity Cycle signature genes (see Appendix 2 of Online Resource 1), as collated by Xu and colleagues during development of the Tracking Tumor Immunophenotype web server, an online platform that utilizes gene expression datasets to profile the status of the tumor immune microenvironment in the context of each step of the Cancer-Immunity Cycle [18]. Genes from this list were evaluated by students through Kaplan–Meier survival analysis in TIMER for the purposes of identifying specific genes whose expression levels carry prognostic significance for cancer patient outcome. For each gene assessed, patients were stratified into high- and low-expressor cohorts using various thresholds of expression to determine whether any cutoffs carried prognostic significance for overall patient survival. For instance, splitting patients at the 50% threshold divided the population analyzed into the top 50% expressors and bottom 50% expressors, whereas splitting patients at the 25% threshold compared only the top 25% expressors versus the bottom 25% expressors, thus excluding those patients whose range of gene expression fell within the middle 50th percentile. After identifying prognostic significance (either positive or negative) for expression of up to 5 genes within each Cancer-Immunity Cycle gene set, students then determined whether expression of such genes correlated with the presence of specific tumor-infiltrating immune cell populations, as estimated by TIMER. Upon compiling these survival and immune cell correlation data, students applied knowledge acquired from course content as well as PubMed literature searches to provide potential explanations for their findings, an exercise that required students to integrate their knowledge of gene and immune cell function with relevant steps of the Cancer-Immunity Cycle, all in the context of observed clinical outcomes for patients with the cancer type of interest.
Study Timeline
This semester-long project was conducted over the course of a 14-week term, with a project overview and data collection beginning during the first week of the semester. Work on the project occurred during some dedicated laboratory periods (or portions thereof) throughout the semester, as well as during independent, out-of-lab time. Students were encouraged to meet with the professor both during the laboratory time that was dedicated to this project as well as outside of the laboratory period during instructor office hours to discuss any questions related to their data and the corresponding gene functions, immune cell types, and Cancer-Immunity Cycle steps they were researching from the primary literature. A recommended timeline of student progress on this project is shown in Fig. 1, and a sample of the type of data summary that was collected from each student upon completion of the project is available in Appendix 3 of Online Resource 1. Students were provided with this sample data summary and the recommended timeline, the latter of which is constructed in such a way that data collection is the primary activity occurring over the first several weeks of the semester, thus providing sufficient time for students to be exposed to and master course content that is needed for the latter stages of the project, when primary literature work and integrated data analysis/interpretation is necessary. The timeline used in this study allows students to complete their work within 12 weeks, leaving a 2-week buffer that offers flexibility for faculty who wish to implement this project into their own curricula but who may deviate from this suggested timeline due to other course assignments.
Assessment
Students participating in the bioinformatics project described herein completed a pre- and post-project assessment instrument designed to evaluate improvements in their understanding of the Cancer-Immunity Cycle over the course of the semester. This assessment tool included a series of multiple choice and true/false questions about concepts pertaining to cancer immunology as well as a short answer question asking students to identify and describe the 7 steps of the Cancer-Immunity Cycle. Students from the Spring 2020 cohort not participating in this project also completed this same assessment at the end of the semester. In this way, the impact of the bioinformatics project on student learning was also evaluated by comparing assessment outcomes for students learning from lecture material alone versus those learning from lecture material in conjunction with the bioinformatics project under investigation.
Statistical Analysis
Unpaired t tests were used for comparative analysis of performance outcomes for the two distinct student cohorts. For analysis of pre- and post-project performance within the single cohort participating in the bioinformatics project, two-tailed paired t-tests were performed. Data are presented as the mean ± SD, and a value of p ≤ 0.05 (represented in graphs by *) was considered statistically significant. **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Results
A Bioinformatic Approach to Foster Student-centered Investigation and Understanding of the Cancer-Immunity Cycle
In order to increase student-centered, bioinformatics-driven learning opportunities in the context of a course-based research experience (CURE) that was designed to address documented needs in undergraduate immunology and cancer education, students in a 300-level Immunology course were engaged in a semester-long project aimed at improving their understanding of the Cancer-Immunity Cycle. Using TCGA RNA-sequencing data, students performed Kaplan–Meier survival analyses to evaluate the prognostic significance of RNA expression for individual genes known to have functional relevance to each of the 7 steps of the Cancer-Immunity Cycle. Expression of genes with prognostic significance was further analyzed for correlation with tumor-infiltrating immune cell subsets, whose presence within tumor tissue was inferred by computational deconvolution of bulk gene expression profiles using the TIMER web platform. The type of data collected by students through these analyses is highlighted in Fig. 2, where TAP1 gene expression in tumor tissue from the bladder urothelial carcinoma (BLCA) TCGA patient cohort is used as an example. As shown in Fig. 2a, elevated TAP1 gene expression in BLCA tumor tissue is a positive prognostic factor for patient survival, as determined by Kaplan–Meier survival analysis of patients stratified by gene expression level (top 10% expressors versus bottom 10% expressors in this case). Further analysis of this potentially clinically relevant gene within TIMER reveals that its expression level correlates negatively with tumor purity (Fig. 2b), suggesting that TAP1 expression in tumor tissue is likely attributable to other non-cancer cell populations within the heterogeneous tumor microenvironment. Indeed, TIMER estimation of tumor infiltration by six distinct immune cell subsets in the BLCA patient cohort reveals a statistically significant purity-corrected partial Spearman correlation between TAP1 gene expression and tumor infiltration by neutrophils, DC, CD8 + T cells, and CD4 + T cells (Fig. 2c). Based on data such as these, students then applied knowledge from course content and PubMed literature searches to engage in a hypothesis-building exercise designed to generate potential explanations for their findings. For instance, using the example data presented in Fig. 2, it is interesting to speculate that because the TAP1 gene encodes a protein involved in class I MHC antigen processing and presentation (activities with functional relevance to the “Cancer Antigen Presentation” step of the Cancer-Immunity Cycle), these data might collectively suggest that TAP1 expression by tumor-infiltrating neutrophils and DC enables these innate immune cell populations to present tumor antigen to CD8 + T cell populations that also infiltrate tumor tissue, in turn supporting the retention and activation of these cells within the tumor microenvironment and promoting more efficient anti-tumor immunity that correlates with improved patient survival. Students participating in the project were provided with examples of this type of integrative data interpretation and hypothesis building at the onset of the study in order to help guide consideration of their own data over the course of the project. Such examples are included in Appendix 3 of Online Resource 1.
Impact of Cancer-Immunity Cycle Bioinformatics Project on Student Learning
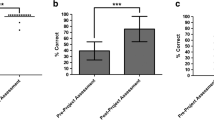
In order to evaluate baseline student knowledge of topics relevant to tumor immunology and assess the efficacy of this bioinformatics project as a pedagogical tool to promote student understanding of these concepts in the context of the Cancer-Immunity Cycle, a pre-/post-project assessment instrument was administered to students on the first day of class and upon completion of the project near the end of the semester. As shown in Fig. 3, baseline undergraduate knowledge of tumor immunology was limited. Indeed, although the majority of students entering the course successfully identified bacterial cells and viruses as potential targets of an immune response, nearly 1 in 4 students (22.3%) did not recognize cancer cells as potential immunologic targets at course outset (Fig. 3a). Moreover, even though some students did recognize the potential for immunity to cancer at the start of the semester, the majority of students were unable to offer any insight into mechanisms by which anti-tumor immunity is achieved. In this regard, when students were asked to describe the events necessary for initiating and maintaining a successful anti-tumor immune response, 61.1% of respondents were unable to identify any of the seven steps of the Cancer-Immunity Cycle, and at most, only two steps of the cycle could be described by any student (22.2% of respondents) prior to initiating the project described herein. (Fig. 3b). Importantly, however, students demonstrated significant gains in these and related content areas over the course of the semester. At the completion of the project, cancer cells were successfully identified as a potential immunologic target by 100% of respondents, and all students were able to identify and describe at least 6 of the 7 steps of the Cancer-Immunity Cycle (Fig. 3a, b). Analysis of pre-/post-assessment responses to a series of multiple choice and true/false questions on topics relevant to the Cancer-Immunity Cycle also revealed similar improvements in student knowledge of tumor immunology over the course of the semester (Fig. 3c). These documented gains in student learning were observed not only in terms of overall student performance on the assessment instrument but also in terms of student response accuracy for each question from the assessment tool (Fig. 3d). Moreover, all but one student (94.4% of respondents) demonstrated individual gains in performance outcomes on the assessment instrument over the course of the semester (Fig. 3e). Finally, these documented gains in student understanding of tumor immunology were not merely a reflection of their having received relevant instruction in this competency area by the time of course completion. A direct comparison of assessment outcomes for student cohorts from two distinct course sections, one in which the Cancer-Immunity Cycle bioinformatics project under investigation was implemented and one in which it was not, revealed specific learning advantages for students participating in the project (93.3% ± 10.8% assessment score for project participants versus 70.6% ± 15.2% assessment score for non-participants, p < 0.0001). Collectively, these data highlight the approach described herein as a useful pedagogical strategy for reinforcing course content and enhancing undergraduate student understanding of concepts relevant to tumor immunology (Fig. 3f).
Discussion
This study introduces a novel, bioinformatics-based approach for the student-centered investigation of specific genes and immune cell types that govern the Cancer-Immunity Cycle. Implemented in the context of an undergraduate laboratory classroom setting, this multi-week project not only exposes students to fundamental concepts in immunology, cancer biology, and anti-tumor immune regulation, but it also provides opportunities for students to gain experience with bioinformatic/computational tools for analyzing omics-level genetic sequencing data. Together with the demonstrated gains in student understanding of cancer immunology concepts achieved by this approach, these benefits support a number of documented needs in undergraduate science education [8, 10, 11].
Tumor immunology is a rapidly advancing field that has revolutionized cancer medicine over the last decade. Despite the promise of this discipline and the need to train and encourage future scientists to pursue research efforts in this field, however, students often do not receive significant exposure to this topic during early stages of their academic careers. To this point, a recent survey of faculty teaching semester-long undergraduate immunology courses found that over 70% of respondents dedicate little to no time to this topic in their classes [9]. Moreover, previous studies have shown that undergraduate students harbor several misconceptions about cancer, both in general and in the context of tumor immunity [13, 19,20,21]. The pre-project assessment described in this study supports these findings, documenting a poor baseline understanding of basic tenets of tumor immunology by upper-level undergraduate students and highlighting a failure of some such students to even recognize that cancer cells have the potential to be targeted by the immune system. Importantly, the pedagogical approach described herein promotes undergraduate student understanding of core concepts in tumor immunology by reinforcing over the course of the semester the relevance of key genes and immune cell populations that influence each step of the Cancer-Immunity Cycle, all in the context of clinically meaningful data associated with patient outcome. As highlighted in the post-project assessment and in comparative analyses of assessment outcomes for student cohorts participating, or not participating, in the project, many of the student learning gains reported in this study are directly attributable to engagement in this project.
Active and inquiry-based learning approaches have long been lauded as a means of increasing student engagement with course content. Particularly in the context of immunology, a field that builds on foundational knowledge from an array of STEM disciplines, strategies that foster students’ abilities to integrate concepts from genetics, cell biology, anatomy and physiology, and cancer biology, among others, are critical to provide students with opportunities for synthesizing knowledge in ways that reflect the interdisciplinary nature of this field [22]. Indeed, one of the strengths of the approach described herein is its focus on such integration of data, which fosters not only the synthesis of knowledge but also the development of critical research skills that include primary literature review and hypothesis building. Additionally, by employing bioinformatics-based analyses to meet these general STEM education goals, this pedagogical approach also contributes to efforts to meet the ever-expanding need for undergraduate training in omics-based sciences [23].
Although the primary focus of this study was to provide a tool for enhancing undergraduate student understanding of basic tumor immunology concepts, it is worth noting how this approach might be adapted to place greater emphasis on, and more advanced training in, both immunology and bioinformatics. With regard to the computational approach described herein, the original TIMER web platform employs a single algorithm to infer the presence of tumor-infiltrating immune cell populations. Its deconvolution method relies on assessment of tumor purity (estimated for each sample using DNA single-nucleotide polymorphism array data) as well as gene expression profiles of both bulk tumor biopsy tissue and an external reference dataset of purified immune cell populations [24]. Compared with other approaches for deconvolving immune cell populations within complex, heterogeneous tumor tissue, this TIMER algorithm is unique in factoring tissue specificity into its estimation of immune cell infiltration. However, it is limited by its capacity to infer only six immune cell types. In an effort to provide more comprehensive and robust estimation of tumor immune cell infiltration, a recently upgraded TIMER2.0 platform has also been released and offers particular advantages that may appeal to instructors of more advanced undergraduate/graduate courses in immunology [25]. Specifically, TIMER2.0 utilizes a particular R package that integrates six algorithms for estimating specific cell types within mixed populations. In addition to the original TIMER algorithm, these alternative algorithms include xCell, MCP-counter, CIBERSORT, EPIC, and quanTIseq, each of which has unique advantages. For instance, whereas TIMER can infer tumor infiltration by only six immune cell populations, the xCell algorithm is capable of estimating more diverse immune cell types, and both it and CIBERSORT can estimate intratumoral abundance of specific immune cell subsets and/or immune cell activation states (i.e., naïve, central memory, and effector memory CD8 + T cell populations can be parsed out from the bulk CD8 + T cell population inferred by TIMER). Depending on the desired application of the end user, these more refined estimates of specific immune cell subsets may be beneficial. Additionally, for bioinformatics courses designed to focus more heavily on the computational theory behind various mixed cell population deconvolution methods, TIMER2.0 offers a useful platform for side-by-side comparison of data output by distinct analytic methods. Of note, these types of computational analyses have become increasingly popular in cancer research, not only for providing basic insights into tumor immunobiology but also for identifying prognostic signatures that predict cancer patient response to immunotherapy [26]. Pedagogical strategies that offer training in the use of these methods are therefore becoming essential tools for preparing students who wish to pursue basic and clinical research careers in oncology.
Finally, the pedagogical approach described herein carries a number of advantages that extend beyond its documented benefits in cancer and immunology education. In various STEM disciplines, including immunology, project-based learning has been recognized as a strategy not only to promote student engagement [27, 28] but also to increase inclusivity and lessen inequities in science education [29]. This latter benefit is a particularly attractive feature of this bioinformatics project, which can be implemented without the significant costs of laboratory supplies and reagents that often limit the degree of experimental benchwork that can be accomplished in classroom settings. Moreover, though this project was implemented over the course of a 14-week semester at a liberal arts institution that maintains low course enrollments to promote student-faculty interaction, it is readily adaptable to significantly larger class sizes as well as independent research projects, and it can be modified to accommodate timelines that fit within an array of academic calendars followed by other institutions. It is also amenable to in-person, hybrid, and remote models of instruction, with internet access being the only requirement for implementation of the project. In light of the impact of the COVID-19 pandemic on both classroom instruction and independent research opportunities for students [30], this flexibility is beneficial for undergraduate coursework in several STEM disciplines, as well as for summer and academic-year research programs that have yet to return to full capacity due to ongoing concerns over the pandemic and that may therefore wish to expand this approach into more extensive independent research projects for students.
Data Availability
Primary data from individual responses to the assessment instrument described herein will be made available upon request. Individual respondents will remain anonymous. All other data and materials from this study are included in this report.
Code Availability
Not applicable.
References
Chapman PB, Einhorn LH, Meyers ML et al (1999) Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 17:2745–2751. https://doi.org/10.1200/JCO.1999.17.9.2745
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535–1546. https://doi.org/10.1056/NEJMoa1910836
Paz-Ares LG, Ramalingam SS, Ciuleanu TE et al (2022) First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 part 1 trial. J Thorac Oncol 17:289–308. https://doi.org/10.1016/J.JTHO.2021.09.010
Albiges L, Tannir NM, Burotto M et al (2020) Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5:e001079. https://doi.org/10.1136/ESMOOPEN-2020-001079
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. https://doi.org/10.1016/J.IMMUNI.2013.07.012
Porter E, Amiel E, Bose N et al (2021) American Association of Immunologists recommendations for an undergraduate course in immunology. ImmunoHorizons 5:448–465. https://doi.org/10.4049/IMMUNOHORIZONS.2100030
Bruns HA, Wisenden BD, Vanniasinkam T et al (2021) Inside the undergraduate immunology classroom: current practices that provide a framework for curriculum consensus. J Microbiol Biol Educ 22(1):1–8. https://doi.org/10.1128/JMBE.V22I1.2269
Wilson Sayres MA, Hauser C, Sierk M et al (2018) Bioinformatics core competencies for undergraduate life sciences education. PLoS One 13:e0196878. https://doi.org/10.1371/JOURNAL.PONE.0196878
Attwood TK, Blackford S, Brazas MD et al (2019) A global perspective on evolving bioinformatics and data science training needs. Brief Bioinform 20:398–404. https://doi.org/10.1093/BIB/BBX100
Hargadon KM (2016) A model system for the study of gene expression in the undergraduate laboratory. Biochem Mol Biol Educ 44:397–404. https://doi.org/10.1002/bmb.20958
Hargadon KM (2021) Using The Cancer Genome Atlas as a tool to improve undergraduate student understanding of cancer genetics and the hallmarks of cancer progression. J Cancer Educ. Online ahead-of-print. https://doi.org/10.1007/s13187-021-01962-y
Hankey W, Zanghi N, Crow MM et al (2020) Using The Cancer Genome Atlas as an inquiry tool in the undergraduate classroom. Front Genet 11:573992. https://doi.org/10.3389/FGENE.2020.573992
Taylor MD, Mendenhall B, Woods CS et al (2021) Online tools for teaching cancer bioinformatics. J Microbiol Biol Educ 22:e00167-e221. https://doi.org/10.1128/JMBE.00167-21
Hutter C, Zenklusen JC (2018) The Cancer Genome Atlas: creating lasting value beyond its data. Cell 173:283–285. https://doi.org/10.1016/j.cell.2018.03.042
Li T, Fan J, Wang B et al (2017) TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res 77:e108–e110. https://doi.org/10.1158/0008-5472.CAN-17-0307
Xu L, Deng C, Pang B et al (2018) TIP: a web server for resolving tumor immunophenotype profiling. Cancer Res 78:6575–6580. https://doi.org/10.1158/0008-5472.CAN-18-0689
Sariego J, Sariego LB, Matsumoto T et al (1992) Cancer knowledge and misconceptions among college undergraduates: a pilot study. J Cancer Educ 7:73–78. https://doi.org/10.1080/08858199209528146
Estaville L, Trad M, Martinez G (2012) University student understanding of cancer: analysis of ethnic group variances. J Cancer Educ 27:580–584. https://doi.org/10.1007/s13187-012-0356-x
Cocchio S, Bertoncello C, Baldovin T et al (2020) Awareness of HPV and drivers of HPV vaccine uptake among university students: a quantitative, cross-sectional study. Heal Soc Care Community 28:1514–1524. https://doi.org/10.1111/hsc.12974
Stranford SA, Owen JA, Mercer F, Pollock RR (2020) Active learning and technology approaches for teaching immunology to undergraduate students. Front Public Heal 8:114. https://doi.org/10.3389/FPUBH.2020.00114/BIBTEX
Goller CC, Srougi MC, Chen SH et al (2021) Integrating bioinformatics tools into inquiry-based molecular biology laboratory education modules. Front Educ 6:288. https://doi.org/10.3389/FEDUC.2021.711403/BIBTEX
Li B, Severson E, Pignon JC et al (2016) Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol 17:174. https://doi.org/10.1186/S13059-016-1028-7
Li T, Fu J, Zeng Z et al (2020) TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 48:W509–W514. https://doi.org/10.1093/NAR/GKAA407
Wang K, Patkar S, Lee JS et al (2022) Deconvolving clinically relevant cellular immune cross-talk from bulk gene expression using CODEFACS and LIRICS stratifies patients with melanoma to anti-PD-1 therapy. Cancer Discov 12:1088–1105. https://doi.org/10.1158/2159-8290.CD-21-0887
Asoodeh MH, Asoodeh MB, Zarepour M (2012) The impact of student - centered learning on academic achievement and social skills. Procedia - Soc Behav Sci 46:560–564. https://doi.org/10.1016/j.sbspro.2012.05.160
Connell GL, Donovan DA, Chambers TG (2016) Increasing the use of student-centered pedagogies from moderate to high improves student learning and attitudes about biology. CBE Life Sci Educ 15:ar3. https://doi.org/10.1187/cbe.15-03-0062
Riestra AM, Morales AJ, Mercer F (2019) Targeting the achievement gap: strategies toward removing inequities in undergraduate immunology education. Front Immunol 10:2906. https://doi.org/10.3389/FIMMU.2019.02906
Erickson OA, Cole RB, Isaacs JM et al (2022) “How do we do this at a distance?!” A descriptive study of remote undergraduate research programs during COVID-19. CBE Life Sci Educ 21:ar1. https://doi.org/10.1187/CBE.21-05-0125
Acknowledgements
Clinical data utilized in this project was generated and made publicly available by the TCGA Research Network: https://www.cancer.gov/tcga. The author also wishes to thank the undergraduate students who participated in this study.
Author information
Authors and Affiliations
Contributions
K. M. H. was solely responsible for the design, implementation, and reporting of data from this study.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Hampden-Sydney College Human Subjects Research Review Committee.
Consent to Participate
Informed consent to participate in this study was obtained from all undergraduate students involved in this assessment.
Consent for Publication
Informed consent for the reporting of assessment data was obtained from all undergraduate students participating in this study.
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13187_2022_2221_MOESM1_ESM.docx
Supplementary file 1 Detailed project description, including step-by-step instructions for the use of TIMER and an example summary with data interpretation (DOCX 728 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hargadon, K.M. A Bioinformatic Approach to Enhance Undergraduate Student Understanding of the Cancer-Immunity Cycle. J Canc Educ 38, 991–999 (2023). https://doi.org/10.1007/s13187-022-02221-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13187-022-02221-4