Abstract
Uptake of decision aids (DAs) in daily routine is low, resulting in limited knowledge about successful DA implementation at a large scale. We assessed implementation rates after multi-regional implementation of three different prostate cancer (PCa) treatment DAs and patient-perceived barriers and facilitators to use a DA. Thirty-three hospitals implemented one out of the three DAs in routine care. Implementation rates for each DA were calculated per hospital. After deciding about PCa treatment, patients (n = 1033) completed a survey on pre-formulated barriers and facilitators to use a DA. Overall DA implementation was 40%. For each DA alike, implementation within hospitals varied from incidental (< 10% of eligible patients receiving a DA) to high rates of implementation (> 80%). All three DAs were evaluated positively by patients, although concise and paper DAs yielded higher satisfaction scores compared with an elaborate online DA. Patients were most satisfied when they received the DA within a week after diagnosis. Pre-formulated barriers to DA usage were experienced by less than 10% of the patients, and most patients confirmed the facilitators. Many patients received a DA during treatment counseling, although a wide variation in uptake across hospitals was observed for each DA. Most patients were satisfied with the DA they received. Sustained implementation of DAs in clinical routine requires further encouragement and attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (Pca) is the most common malignancy diagnosed in men in the western world. In the case of localized prostate cancer, patients are typically required to choose between multiple equivalent treatment options. Although survival perspectives with each treatment are similar, treatment procedures and risk for side effects vary, and many patients have poor understanding of these differences between treatments [1]. Therefore, clinical guidelines concerning localized Pca suggest a shared patient-doctor decision to incorporate patient preferences and values into the treatment decision [2,3,4,5]. Decision aids (DAs) have been developed to assist patients and care providers with shared decision-making (SDM) [6].
Evidence for the beneficial effects of applying DAs is widely available and shows that patients have better knowledge of the treatment options, and are more aware of their personal preferences and values [7]. As a consequence, DAs help patients to take a more active role in the decision-making process [8]. So far, most DA trials, including those related to Pca treatment, focused on determining the DA effects, with limited attention for implementation aspects [7, 9]. Many DA trials took place within a single institution or location, and even if the absolute number of the DAs distributed was known, their relative reach within the targeted patient population often remained unknown [10, 11]. Moreover, uptake of DAs in daily routine, outside of clinical trials, is low, resulting in limited knowledge about successful DA implementation at a large scale [7, 12,13,14,15,16].
After distribution of the DA to eligible patients, the next step in implementation is actual DA use by patients. Patient-perceived barriers and facilitators related to DA usage have been studied more extensively [12, 17,18,19,20,21]. Common barriers against DA usage from the patients’ perspective are insufficient trust in the DA quality or its benefits, the DA being unpractical in use, inadequate timing (e.g., the DA being offered too late after diagnosis), or inadequate explanation of how to use the DA. Patient-perceived facilitators include that the DA is practical in use, and that the presented information is complete and trusted [12, 17,18,19,20,21].
This study was conducted by the Joint Implementation Prostate cancer Patient-centered care (JPPPA) consortium, consisting of three DA research groups that each developed a DA for Pca patients. With the current implementation study, we aimed to investigate the implementation rate of these three DAs in routine Pca care in The Netherlands, and aimed to identify possible barriers and facilitators from the patients’ perspective.
Patients and Methods
The Three Decision Aids
Each of the three DAs involved was developed according to the International Patient Decision Aids Standards (IPDAS) and contained information about the disease, treatment options, and (dis) advantages of all options based on (inter) national guidelines and international literature [22]. Patients, urologists, and radiation oncologists were involved in the development and review process of the DAs [23,24,25]. In each DA, the same choice options were presented: surgery, brachytherapy, and external beam radiotherapy, as well as the option of active surveillance. The DAs varied in their format and length. DA1 was a concise booklet (14 pages), DA2 was an even more concise (in diagram style with short explanations) booklet or online DA (by patient choice), and DA3 was an elaborate online DA with values clarification exercises (VCEs). The DA format coincided with the intended moment of use. DA1 and DA2 could be incorporated in clinical consultation, or used at home, while DA3 was, by design, supposed to be used outside of consultations. The characteristics of the DAs are presented in Table 1. Detailed descriptions of the separate trials investigating the DA effects have been published earlier [23,24,25,26].
Setting and Participants
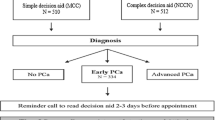
Thirty-three hospitals (out of a total of 90 hospitals in The Netherlands) implemented one of the three DAs in treatment counseling. Each DA was implemented in a specific region of The Netherlands (DA1 East, 8 hospitals; DA2 North-West, 16 hospitals; DA3 South, 9 hospitals). Per DA, hospitals were recruited to participate based on convenience (e.g., distance); the allocation of DAs to hospitals was not randomized. The DAs were handed out to patients newly diagnosed with localized Pca. For all 3 DAs, patients were eligible to participate if they had the possibility to choose between at least two treatments covered by the DA. The assessment of whether the DA was applicable (e.g., eligibility for at least two treatments covered by the DA) was done by the patient’s urologist. Actual distribution of the DA was done by either the urologist or a specialized nurse, depending on what best fitted with existing local care pathways. After the treatment decision was made, but before treatment started, patients received a questionnaire to evaluate receipt and usage of the DA. All data were collected between July 2013 and June 2016. Research protocols from each DA group were reviewed by their respective local institutional ethics committees, which each provided a waiver for further ethical assessment.
Outcome Measures
Our primary outcome measure was the implementation rate. This rate was calculated by the proportion of patients who received a DA compared with the estimated total number of eligible Pca patients per hospital during the period the DA was implemented. Since the total number of eligible patients was not prospectively registered in a structured manner in all participating hospitals, an estimation was based on hospital-specific registry data of the 6 years prior to the current project, retrieved from the Netherlands Cancer Registry.
After a treatment decision was made, a questionnaire was used to collect self-reported data about patient’s demographic variables (age, marital status, having children, and educational level). Evaluation measures consisted of DA distribution procedures (e.g., “Who presented the DA to you?”), DA user-friendliness (e.g., “Did it occur fonts were too small?”), and a 24-item list of barriers and facilitators for DA use (e.g., “I had insufficient trust in the DA”) based on literature [21] (items presented in Table 3). All three DA groups used the same questionnaires to evaluate DA use in order to enable combined data analyses.
Data Analysis
Descriptive questionnaire data are presented as means (Ms) with standard deviations (SDs) for continuous variables, and frequencies and percentages for categorical variables. Comparisons between DAs for continuous variables were made with analyses of variance (ANOVA) and Bonferroni post-hoc tests and with chi-square tests for categorical variables. Statistical analyses were conducted with SPSS 22.0 (Statistical Package for Social Sciences, Chicago, IL). Tests were two-sided and considered statistically significant if p < .05.
Results
During the study period, 908 newly diagnosed Pca patients received a DA out of an estimated total of 2285 eligible patients, resulting in an overall implementation rate of 40%. With each DA, high implementation levels (> 80%) were achieved in 1 or 2 hospitals, whereas for the other hospitals, implementation varied considerably (2–80%). The highest average implementation was achieved with the concise paper DA1 (60%); average implementation levels for DA2 and DA3 were comparable (34–35%). Implementation rates across hospitals for each DA are presented in Fig. 1.
Out of the 908 patients who received a DA, 673 patients agreed to complete the post-decision questionnaire evaluating DA use (response 74%). Compared with participants from both other DA groups, participants from DA3 were slightly younger and more often highly educated (Table 2). Mean PSA and Gleason scores were lower for participants from DA3, but the same distribution across categories was found between DA groups (Table 2).
Most participants indicated that they received the DA from their urologist (n = 478, 71%) and perceived that the urologist is the most suitable person to hand out a DA (n = 511, 76%; Table 3). However, of the participants who received the DA from a nurse (n = 192, 29%), 60% considered the nurse to be the most suitable person for this (data not shown). Most participants (n = 573, 85%) perceived that they received sufficient explanation about the DA, regardless of DA type, care provider handing out the DA (urologist versus nurse), or moment of receipt (Table 3). Almost all participants who used DA1 or DA2 were satisfied with the DA format (99 and 96%), but for the online DA3, a considerable proportion of participants (n = 67, 21%) would have preferred a paper format (Table 3). Overall, satisfaction with the online DA3 was lower compared with DA1 and DA2 (Table 3).
Barriers against DA usage were reported by less than 10% of the participants, regardless of which DA they received (Table 3). Differences found between DAs were related to format (unpractical, insufficiently adjusted to personal preferences) or subjective evaluations (no confidence, expected no benefit). Overall, most barriers were reported for the elaborate online DA3.
Facilitators for DA use were reported by a large majority of participants (Table 2). For all DAs, more than 80% of participants found the DA pleasant to use and well organized and were confident in the DA quality. Overall, facilitators were reported mostly by respondents who used the most concise DA (DA2) and least by patients who used DA3. A full overview of the responses to perceived barriers and facilitators for all DA formats is presented in Table 3.
Discussion and Conclusion
Discussion
Many DA initiatives struggle to get structurally embedded in clinical routine, despite ample evidence revealing the benefits of using DAs when making medical decisions [7, 12]. At the onset of a multi-regional implementation initiative of three new Pca treatment DAs in Dutch clinical practice, a consortium was formed to jointly measure implementation rates and patient evaluations (i.e., barriers and facilitators from the patients’ perspective) from these three DAs. Overall, 40% of eligible Pca patients received a DA. For all DAs alike, implementation was quite successful (implementation rate > 80%) in a limited number of hospitals, whereas uptake varied widely at other sites (2–80%). Overall, patient evaluations were supportive of implementation of each DA; however, the online DA3 was evaluated as having the least facilitators.
The format of the implemented DAs as well as their level of information density varied [23,24,25]. DA1 and DA2 could be incorporated in clinical consultation, or used at home, while DA3 was, by design, supposed to be used outside of consultations. Despite the variation between DAs, implementation results showed the same variation between hospitals with each DA, and successful implementation (> 80%) was only achieved in a limited number of hospitals. Increasing the number of hospitals for implementation, as DA2 was implemented at 16 hospitals, compared with 8 and 9 hospitals for DA1 and DA3, did not result in more hospitals with successful implementation. This could suggest that for each DA, support was present in some hospitals prior to the start of implementation, and that for upscaling implementation, more structural encouragement and monitoring of implementation progress are needed in hospitals where the baseline support (in terms of care providers attitude or available resources) for DAs is lower.
When patient-perceived barriers were reported, most were related to DA characteristics (unpractical, unadjusted to needs) or expectations (no confidence, expected no benefits or reduction of uncertainty). Although overall report of barriers was low, barriers were reported most often for the online, elaborate DA3, and least for the very concise hybrid DA2. However, both DAs achieved similar implementation rates that were lower than the concise paper DA (DA1). This finding seems inconsistent with previous studies concluding that web-based DAs are the most promising modality for improving implementation [27, 28]. However, care providers have also shown hesitance towards online tools [29, 30]. Future research is needed to gain a deeper understanding of how the benefits of online tools, such as tailoring to patient information needs and enabling interactive VCEs, can be balanced with patients’ apparent preference for a more concise, paper format. One solution might be to provide concise, paper add-ons to online tools, which can be introduced during consultation and may enhance the user-friendliness of online tools.
The joint implementation efforts by the JIPPA consortium may have contributed to raising national awareness for SDM in both urology and oncology in The Netherlands. Many care providers have been introduced to the DA and to the principles of SDM, and during the course of the projects, consortium members contributed to national Pca treatment guidelines with a section on SDM and DAs (www.oncoline.nl). Therefore, the study in itself increased awareness for SDM and the existence of DAs and educated many teams in using DAs in clinical routine. However, it may also have caused a barrier, as clinical practice was unclear about which DA should be applied, and what the differences between the available DAs entailed. To the best of our knowledge, no earlier studies have reported (national) implementation rates for Pca DAs, and comparability to other DA implementations studies is difficult to interpret as they were aiming at different patient populations (e.g., women with breast cancer, or orthopedic patients) and settings (e.g., screening decisions often include the general practitioner) [10, 11, 31]. Further research is needed to determine if having different types of DA can help implementation since patients and care providers can select the DA they prefer most, or that the variety in available DAs hinders implementation since each DA has its specific characteristics and usability aspects that require training. Moreover, future research could study if specific DA characteristics have an effect on implementation rates, by randomizing distribution of different DA types across hospitals.
A strength of the current study was that we were able to investigate implementation of three DAs by using a similar questionnaire at a similar point in time. As a consequence of studying three different DAs, sample size and number of participating hospitals were higher than most previous Pca DA studies [9, 32]. Eventually, one in three Dutch hospitals was exposed to one of the three DAs. Hospitals from different levels (academic and non-academic) and from different regions were included in the study, increasing the generalizability of our findings.
A limitation of the current study is that the implementation rate was calculated based on actual receivers of a DA as proportion of an estimation of the total number of eligible patients. Since the number of patients eligible for study inclusion was not systematically registered by the hospitals included in our studies, we relied on the hospital-specific retrospective cohorts of PCa patients from the cancer registry. This ensured the sample was determined via the same method for every hospital. However, since the total number of patients eligible for DA receipt was estimated, this entailed that no information was available about patient characteristics from those patients who were possibly eligible but were not offered a DA. In particular, in hospitals with low implementation rates, a selection bias could have occurred if only patients were included who favored DA use. Another limitation is that the implementation period was not exactly simultaneous for all three DAs. Implementation of DA1 started almost a year ahead of DA2 and DA3. Moreover, a previous version of DA1 was studied in an earlier trial, which could have helped achieving the higher overall implementation of DA1 [25]. Furthermore, each participating hospital was linked to one of the three regions, and consequently implemented its respective DA. Possibly, some patients or care providers could have been more supportive of another DA and overall DA uptake would have been higher if all formats would be matched according to patient or care providers’ preferences. For example, one patient might benefit more from an elaborate DA, while for another patient, optimal understanding and satisfaction are reached with a concise DA [33,34,35,36]. Finally, no information was available from patients who received, and possibly also used, a DA but did not consent to participate in the survey study.
Patient evaluations from the three DAs in the current study were all favorable towards implementation. To further understand the observed differences in implementation rates between hospitals, future steps towards sustained DA use should include further investigation into barriers at the level of care providers and organizational barriers.
Conclusion
Overall implementation rate of the DAs in clinical routine was 40%. A wide variation in uptake across hospitals was observed for each DA. Most patients were satisfied with the DA they received, and only few barriers of usage were perceived by patients. Offering an online-only DA led to less patient-reported facilitators compared with a paper-only or hybrid DA.
Practice Implications
Patients appeared to be satisfied with each DA format. Sustained implementation of DAs in clinical routine requires further encouragement and attention, and could require a tailored distribution approach per hospital site [37].
References
van Stam M-A, van der Poel HG, van der Voort van Zyp JRN, Tillier CN, Horenblas S, Aaronson NK, van der Voort van Zyp JRN, Tillier CN, Horenblas S, Aaronson NK, Ruud Bosch JLH (2018) The accuracy of patients’ perceptions of the risks associated with localised prostate cancer treatments. BJU Int 121:405–414
Resnick MJ, Koyama T, Fan K-H, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL, Stroup AM, van Horn RL, Penson DF (2013) Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 368(5):436–445
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, Yamamoto T, Mamedov A, Loblaw A (2015) Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 33(3):272–277
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol 65(1):124–137
Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS et al (2007) Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 177(6):2106–2131
Stiggelbout AM, Van der Weijden T, De Wit M, Frosch D, Légaré F, Montori VM et al (2012) Shared decision making: really putting patients at the centre of healthcare. BMJ. 344:e256
Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB et al Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017(4)
Stacey D, Samant R, Bennett C (2008) Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin 58(5):293–304
Violette PD, Agoritsas T, Alexander P, Riikonen J, Santti H, Agarwal A et al (2015) Decision aids for localized prostate cancer treatment choice: systematic review and meta-analysis. CA Cancer J Clin 65(3):239–251
Sepucha KR, Simmons LH, Barry MJ, Edgman-Levitan S, Licurse AM, Chaguturu SK (2016) Ten years, forty decision aids, and thousands of patient uses: shared decision making at Massachusetts General Hospital. Health Aff 35(4):630–636
Mangla M, Cha TD, Dorrwachter JM, Freiberg AA, Leavitt LJ, Rubash HE et al. (2018) Increasing the use of patient decision aids in orthopaedic care: results of a quality improvement project. BMJ Qual Saf 27(5):347–354
Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C et al (2013) “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak 13(2):S14
Wyatt KD, Branda ME, Anderson RT, Pencille LJ, Montori VM, Hess EP, Ting HH, LeBlanc A (2014) Peering into the black box: a meta-analysis of how clinicians use decision aids during clinical encounters. Implement Sci 9(1):26
Elwyn G, Lloyd A, Joseph-Williams N, Cording E, Thomson R, Durand M-A, Edwards A (2013) Option grids: shared decision making made easier. Patient Educ Couns 90(2):207–212
Elwyn G, Légaré F, Weijden T, Edwards A, May C (2008) Arduous implementation: does the normalisation process model explain why it’s so difficult to embed decision support technologies for patients in routine clinical practice. Implement Sci 3(1):57
Härter M, van der Weijden T, Elwyn G (2011) Policy and practice developments in the implementation of shared decision making: an international perspective. Z Evid Fortbild Qual Gesundheitswes 105(4):229–233
O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P (2006) Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract 12(2):174–181
Silvia KA, Ozanne EM, Sepucha KR (2008) Implementing breast cancer decision aids in community sites: barriers and resources. Health Expect 11(1):46–53
Joseph-Williams N, Elwyn G, Edwards A (2014) Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 94(3):291–309
Légaré F, Witteman HO (2013) Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff 32(2):276–284
Légaré F, Ratté S, Gravel K, Graham ID (2008) Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns 73(3):526–535
Volk RJ, Llewellyn-Thomas H, Stacey D, Elwyn G (2013) Ten years of the international patient decision aid standards collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak 13(2):S1
Cuypers M, Lamers RE, Kil PJ, The R, Karssen K, van de Poll-Franse LV, de Vries M (2017) A global, incremental development method for a web-based prostate cancer treatment decision aid and usability testing in a Dutch clinical setting. Health Informatics J. https://doi.org/10.1177/1460458217720393
Al-Itejawi HHM, van Uden-Kraan CF, Vis AN, Nieuwenhuijzen JA, Hofstee MJA, van Moorselaar RJA et al (2016) Development of a patient decision aid for the treatment of localised prostate cancer: a participatory design approach. J Clin Nurs 25(7–8):1131–1144
van Tol-Geerdink JJ, Willem Leer J, Weijerman PC, van Oort IM, Vergunst H, van Lin EN, Alfred Witjes J, Stalmeier PF (2013) Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU Int 111(4):564–573
Cuypers M, Lamers RED, Kil PJM, van de Poll-Franse LV, de Vries M (2015) Impact of a web-based treatment decision aid for early-stage prostate cancer on shared decision-making and health outcomes: study protocol for a randomized controlled trial. Trials. 16(1):1–10
Hoffman AS, Llewellyn-Thomas HA, Tosteson ANA, O’Connor AM, Volk RJ, Tomek IM, Andrews SB, Bartels SJ (2014) Launching a virtual decision lab: development and field-testing of a web-based patient decision support research platform. BMC Med Inform Decis Mak 14:112
Wantland JD, Portillo JC, Holzemer LW, Slaughter R, McGhee ME (2004) The effectiveness of web-based vs. non-web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res 6(4):e40
Elwyn G, Rix A, Holt T, Jones D (2012) Why do clinicians not refer patients to online decision support tools? Interviews with front line clinics in the NHS. BMJ Open 2(6):e001530
Schroy PC, Mylvaganam S, Davidson P (2014) Provider perspectives on the utility of a colorectal cancer screening decision aid for facilitating shared decision making. Health Expect 17(1):27–35
Hersch J, Barratt A, Jansen J, Irwig L, McGeechan K, Jacklyn G, Thornton H, Dhillon H, Houssami N, McCaffery K (2015) Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet 385(9978):1642–1652
Adsul P, Wray R, Spradling K, Darwish O, Weaver N, Siddiqui S (2015) Systematic review of decision aids for newly diagnosed patients with prostate cancer making treatment decisions. J Urol 194(5):1247–1252
Alden DL, Friend J, Schapira M, Stiggelbout A (2014) Cultural targeting and tailoring of shared decision making technology: a theoretical framework for improving the effectiveness of patient decision aids in culturally diverse groups. Soc Sci Med 105:1–8
Fagerlin A, Zikmund-Fisher BJ, Smith DM, Nair V, Derry HA, McClure JB et al (2010) Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat 119(3):613–620
van Weert JCM, van Noort G, Bol N, van Dijk L, Tates K, Jansen J (2011) Tailored information for cancer patients on the Internet: effects of visual cues and language complexity on information recall and satisfaction. Patient Educ Couns 84(3):368–378
Jensen JD, King AJ, Carcioppolo N, Davis L (2012) Why are tailored messages more effective? A multiple mediation analysis of a breast cancer screening intervention. J Commun 62(5):851–868
Politi MC, Adsul P, Kuzemchak MD, Zeuner R, Frosch DL (2015) Clinicians’ perceptions of digital vs. paper-based decision support interventions. J Eval Clin Pract 21(2):175–179
Acknowledgments
This study is part of a larger research project on the implementation of decision aids for prostate cancer in three regions in The Netherlands (the JIPPA study), funded by CZ, a Dutch not-for-profit health insurance company. We thank Dr. Linda van Mierlo from CZ for her efforts in bringing the three research groups together and for the useful input and critical feedback we received during the progress of this project. We are also grateful to all the patients and staff of the hospitals that participated in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cuypers, M., Al-Itejawi, H.H.M., van Uden-Kraan, C.F. et al. Introducing Decision Aids into Routine Prostate Cancer Care in The Netherlands: Implementation and Patient Evaluations from the Multi-regional JIPPA Initiative. J Canc Educ 35, 1141–1148 (2020). https://doi.org/10.1007/s13187-019-01572-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13187-019-01572-9