Abstract
Background
Risk prediction models can help identify individuals at high risk for type 2 diabetes. However, no such model has been applied to clinical practice in eastern China.
Aims
This study aims to develop a simple model based on physical examination data that can identify high-risk groups for type 2 diabetes in eastern China for predictive, preventive, and personalized medicine.
Methods
A 14-year retrospective cohort study of 15,166 nondiabetic patients (12–94 years; 37% females) undergoing annual physical examinations was conducted. Multivariate logistic regression and least absolute shrinkage and selection operator (LASSO) models were constructed for univariate analysis, factor selection, and predictive model building. Calibration curves and receiver operating characteristic (ROC) curves were used to assess the calibration and prediction accuracy of the nomogram, and decision curve analysis (DCA) was used to assess its clinical validity.
Results
The 14-year incidence of type 2 diabetes in this study was 4.1%. This study developed a nomogram that predicts the risk of type 2 diabetes. The calibration curve shows that the nomogram has good calibration ability, and in internal validation, the area under ROC curve (AUC) showed statistical accuracy (AUC = 0.865). Finally, DCA supports the clinical predictive value of this nomogram.
Conclusion
This nomogram can serve as a simple, economical, and widely scalable tool to predict individualized risk of type 2 diabetes in eastern China. Successful identification and intervention of high-risk individuals at an early stage can help to provide more effective treatment strategies from the perspectives of predictive, preventive, and personalized medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a common chronic disease, with considerably high rates of morbidity and mortality [1], and researchers estimate that there were 451 million diabetic patients (5.5% of the global population) worldwide in 2017 and that these numbers will increase to 693 (10.9%) million by 2045 [2]. In addition, an estimated 374 million people suffer from impaired glucose tolerance (IGT) [3]. According to the World Health Organization (WHO), in 2019, an estimated 1.5 million people died from diabetes directly and 2.2 million people died from hyperglycemia in 2012. More than 90% of diabetes is type 2 diabetes (T2DM) [4, 5], and its clinical symptoms are usually not so outwardly apparent. Therefore, the disease may not be diagnosed until several years after its onset, after complications have already appeared. Hence, diagnostic delay is a critical factor that contributes to overall disease controllability and risk for complications [6]. The white paper of the ‘European Association for Predictive, Preventive and Personalized Medicine (PPPM)’ (EPMA) mentions that a key step in the prevention of T2DM is the identification of high-risk individuals [7]. Although genetic structure may partially determine an individual’s response to environmental changes, the main drivers of the global type 2 diabetes epidemic are increases in obesity, sedentary lifestyles, high-calorie diets, and the aging of the population [8], and there is strong evidence that type 2 diabetes can be prevented through simple lifestyle changes [9, 10]. This evidence also provides a basis for identifying high-risk individuals for whom to implement early lifestyle interventions in order to prevent type 2 diabetes altogether.
Risk prediction models may also be able to contribute to the decision-making process involved in the clinical management of patients. Health care interventions or lifestyle changes can target those who are at increased risk for type 2 diabetes or for poor prognoses. Although a variety of early prediction models have already been constructed for type 2 diabetes [11,12,13], they have yet to be applied in clinical practice due to their lack of predictive accuracy and successful data validation. Furthermore, the predictive value of various risk prediction models may not carry over from one population to another. At present, the amount of related research in China is limited, and there is no research on eastern China specifically.
Therefore, this study takes a new approach and constructs a precise personalized type 2 diabetes predictive model based on repeated medical examination data from residents of eastern, which can be used to guide early lifestyle intervention that can enable predictive, preventive, and personalized medicine in future.
Methods
Data
The physical examination records used in this study come from the database of the Health Management Center, Drum Tower Hospital Affiliated to Nanjing University Medical School, Nanjing, Jiangsu, China. The data include the physical examination records of 60,740 individuals from 2006 to 2020. Participants provided written informed consent to use their data for the study, and this research protocol was approved by the Nanjing Drum Tower Hospital Institutional Review Board.
Data preprocessing
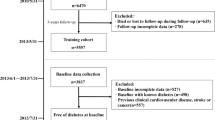
This study first selected individuals who had repeated physical examination data for 2 years or more. According to the information that most individuals had in their records, this study selected 31 items to include in the study: gender, age, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), alanine transaminase (ALT), creatinine (CREA), triglycerides (TG), cholesterol (CHOL), HDL, LDL, glucose (GLU), hemoglobin A1c (HbA1c), lymphocyte (LY), granulocyte (GR), percentage of granulocyte (GR%), monocytes (MO), percentage of monocytes (MO%), eosinophil (EOS), percentage of eosinophil (EOS%), basophil (BA), percentage of basophil (BA%), mean corpuscular hemoglobin (MCH), HB, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), red blood cell volume distribution width (RDW), blood platelets (PLT), red blood cell (RBC), and white blood cell count (WBC). Individuals with incomplete data on these items were excluded from the study. After these exclusions, there were 15,166 nondiabetic patients. During the follow-up period of 1 to 14 years, 623 (4.1%) individuals developed type 2 diabetes, which defined as a fasting blood glucose level ≥ 7.0 mmol/l, glycosylated hemoglobin ≥ 6.5% or self-reported type 2 diabetes. According to whether the last physical examination showed diabetes, data from the first physical examination data of all individuals were divided into a nondiabetic group (no diabetes) and a new-onset diabetes (new diabetes) group.
Statistical analysis
In this study, RStudio (https://www.rstudio.com) was used to carry out the statistical analysis. The characteristics of all participants were expressed as the mean (SD) for continuous variables and the frequency (percentage) for categorical variables. One-way analysis of variance and Kruskal–Wallis test were used to analyze the difference between the continuous variables with normal distributions and skewed distributions, and the chi-square test was performed to help analyze categorical variables (Table 1).
Additionally, the study further used least absolute shrinkage and selection operator (LASSO) regression to reduce high-dimensional data, and selected features with non-zero coefficients in the LASSO regression model as the most useful candidate predictors for type 2 diabetes. The study then combined the candidate predictors with multivariate logistic regression analysis, and the OR (95% CI) and P value of each candidate predictor variable were calculated in order to predict each patient’s possible diagnosis. Finally, the study established a nomogram of the type 2 diabetes prediction model based on these predictor variables.
Furthermore, this study evaluated the performance differentiation, calibration, and clinical validity of the nomogram. First, a calibration curve was plotted to evaluate the calibration ability of the type 2 diabetes nomogram prediction model. The area under the ROC curve (AUC) using 500 bootstrap resampling was plotted in order to quantify its discriminative performance. Second, a decision curve was drawn to evaluate the nomogram’s clinical validity. Subtracting the proportion of false-positive results from the proportion of true positive results, and then, weighing the relative risks of false-positive and false-negative results, the net benefit of each decision was obtained.
Results
Baseline characteristics
From 2006 to 2020, 15,166 individuals who went to the health management center of the hospital for physical examination were nondiabetic individuals at the first physical examination. Among them, 623 (4.1%) individuals were diagnosed with type 2 diabetes at some point during the next 1 to 14 years. According to whether the last physical examination had diabetes, the first physical examination data of all individuals was divided into the nondiabetic group (no diabetes) and the new-onset diabetes (new diabetes) group. Baseline characteristics of the study individuals in the two groups are reported in Table 1.
Character selection and development of an individualized prediction model
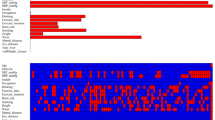
For the recorded characteristics of population statistics, diseases, and therapeutic features, the study effectively reduced 31 features to 10 potential predictors (Fig. 1a and b) that have non-zero coefficients in the Lasso regression model. The 10 potential predictors were gender, BMI, ALT, CREA, CHOL, HDL, Glu, MCHC, WBC, and age (Table 2). A nomogram for predicting the 14-year risk of type 2 diabetes was developed based on these 10 independent predictors, as shown in Fig. 2.
Feature selection using a LASSO binary logistic regression model. a The optimal parameter (lambda) in the LASSO model was selected by five-fold cross-validation. Plot binomial deviation versus log (lambda). A dashed vertical line is drawn at the optimal value by using the smallest criterion (left dashed line) and one standard error of the smallest criterion (1-SE criterion) (right dashed line). The minimum criterion refers to one of all lambda values to obtain the mean of the minimum target parameter. The 1-SE criterion refers to the lambda value of the simplest model within the minimum criterion variance. b LASSO coefficient curve for 31 features. Coefficient distribution plots were generated for the log(lambda) series. Plot vertical lines at the values chosen using five-fold cross-validation where the best lambda results in 10 features with non-zero coefficients for building the predictive model
Nomogram for predicting 14-year risk of type 2 diabetes in non-diabetic individuals. To estimate an individual’s 14-year risk of type 2 diabetes, first find the corresponding value on each variable axis of the nomogram, and then draw a vertical line upward to get the corresponding points, and find the corresponding points for each variable. Finally, adding the points of all variables gives the total points of the individual, and based on the total points, a vertical line is drawn downward to obtain the 14-year risk of type 2 diabetes for the individual
The performance of the nomogram in the cohort study
The calibration curve for predicting the risk line diagram of the risk line chart of future type 2 diabetes patients showed good consistency in the queue (Fig. 3a). Using an internally validated bootstrap sampling method, we found the AUC of the nomogram to be 0.865 (95% CI: 0.847–0.883), which indicates that the model has good predictive capabilities (Fig. 3b).
Calibration and ROC curves of the nomogram for 14-year type 2 diabetes risk. a Calibration curves of the nomogram for 14-year type 2 diabetes risk. The x-axis represents the predicted 14-year risk of type 2 diabetes. The y-axis represents the actual diagnosed type 2 diabetes. Diagonal dashed lines represent perfect predictions from the ideal model. The solid line represents the performance of the nomogram, where the dashed line closer to the diagonal represents better prediction. b ROC curves of the nomogram for 14-year type 2 diabetes risk. The AUC of the nomogram is 0.865 (95% CI: 0.847–0.883) using bootstrap resampling (times = 500). ROC: receiver operating characteristics curves; AUC: area under curve
Decision curves for the nomogram
Decision curve analysis (DCA) of the 14-year type 2 diabetes risk prediction nomogram model is shown in Fig. 4. The abscissa is the threshold probability, and the ordinate is the net profit rate after subtracting the pros and cons. The two straight lines in the figure represent the two extreme cases. The horizontal line indicates that all participants being considered free of type 2 diabetes, and thus the net benefit is 0 when no intervention is performed. The slashes indicate the net benefit when all participants are considered to have type 2 diabetes and all received the intervention. The model curve is thus compared to these two lines. The farther the model curve is from these two lines, the better the clinical benefit of the nomogram. For example, at a risk threshold of 10%, the net benefit is about 2%.
Decision curve analysis of the nomogram for 14-year type 2 diabetes risk. The horizontal line indicates that all participants were considered free of type 2 diabetes, and when no intervention was performed, the net benefit was 0. The slashes represent the net benefit when all participants were considered to have type 2 diabetes and all received the intervention. The further the model curve is from these two lines, the better the clinical value of the nomogram
Discussion
PPPM is a holistic strategy in healthcare that aims to predict individual susceptibility, provide targeted prevention, and create personalized treatments [14]. T2DM is usually treated after disease onset, which is a very delayed approach from a PPPM perspective [15]. In an effort to remedy this, in this study, we developed a simple, quantifiable, and clinically beneficial nomogram to predict the 14-year risk of T2DM in nondiabetic populations of eastern China. Both the calibration curve and AUC values indicate that the nomogram had good calibration and has good predictive capabilities. In addition, the DCA shows that the nomogram has good clinical application value. The predictive model can help to predict the incidence of T2DM, and early intervention may help at-risk individuals avoid the occurrence of T2DM-related complications.
The prevalence of T2DM continues to rise [1], and effective prevention of T2DM is essential to reduce the overall impact of this disease. Identifying high-risk groups through risk prediction methods and intervening early can help reduce the psychological stress experienced by patients, can help enhance their confidence in following a healthy lifestyle, and can help improve their quality of life. Such intervention can also delay disease progression and reduce the risk of life-long complications. In the past few decades, various predictive models have been developed to predict the occurrence of T2DM. Well-known examples include the Finnish Diabetes Risk Score [16], Australian Type 2 Diabetes Risk [17], QRISK[18], and Framingham Offspring (FOS) Risk [19]. Most T2DM prediction models use logistic [20,21,22] or Cox regression [23,24,25] and use carriage return and automatic direction forward selection, backward elimination, or step-by-step procedures.
In this study, the nomogram first constructs a multi-factor regression model (LASSO regression and logistic regression), and assigns each value level of each influencing factor according to the degree of contribution of each influencing factor in the model to the outcome variable (the size of the regression coefficient). It then assigns a score to each value level of each influencing factor and sums these scores to obtain a total score. Finally, the predicted value of the individual outcome event is calculated through the function conversion relationship between the total score and the probability of the outcome event. Simply put, the nomogram transforms complex regression equations into a visual graph, making the results of the predictive model more readable and possibly better facilitating patient evaluation. The intuitive and easy-to-understand characteristics of the nomogram have gradually garnered it more and more attention and application in both medical research and clinical practice [26, 27]. In addition, all previous T2DM risk prediction studies conducted in China have established T2DM risk scores of integer points or segment values, and the nomograms can provide more accurate and personalized risk predictions due to the use of continuous values. This is in line with EPMA. The same point of view is that individualization should become a general social trend in the field of medicine and healthcare [28].
At present, nomogram has been previously studied in the field of T2DM in China [29,30,31], but existing nomograms cannot be extended from one region to another. In particular, there has never been a nomogram study in eastern China. Compared to existing nomogram studies for other regions, this study has the highest AUC value and the longest follow-up time (14 years) used so far. Furthermore, risk factors included in this nomogram were gender, BMI, ALT, CREA, CHOL, HDL, Glu, MCHC, WBC, and age, which provide greater detail than the existing studies. Among them, gender, BMI, HDL, GLU, and age have been also included in other predictive models [29,30,31]. In addition, the studies have also shown that impaired liver function is associated with T2DM [32]. And CREA [33], WBC [34], and MCHC [35] are also related to T2DM. Therefore, the application of these parameters in the model appears to be well-founded. Finally, the calibration curve shows that the nomogram is well-calibrated, and the AUC shows its statistical accuracy. However, accuracy does not necessarily mean that it has value in clinical applications. For this reason, we also conducted a decision curve analysis, and this showed that the nomogram indeed has good clinical utility.
Although this model’s predictive results are good, a key limitation of this study is that the risk of T2DM was predicted based only on laboratory data, and did not include factors such as diet, exercise, or genetics that have been shown to be closely related to T2DM because these data were not collected. High-risk groups are typically in an intermediate physical state between ideal health and disease, also known as suboptimal health status (SHS). As a subclinical stage of chronic disease, from the perspective of PPPM, early identification of SHS is of great significance for targeted prevention and individualized treatment of T2DM [36]. The most widely used SHS screening tool is the Suboptimal Health Status Questionnaire 25 (SHSQ-25), but we also did not collect responses to this questionnaire in this study. Another limitation is that the diagnosis of T2DM could only rely on fasting blood glucose, glycosylated hemoglobin, and medical history from physical examination data because the physical examination population could not perform the standard OGTT test for the diagnosis of T2DM. In addition, this study did not divide into two groups for internal verification and external verification.
Diabetes is a metabolic disease caused by the interaction of genetic and environmental factors [37], and epigenetic modifications have been shown to be associated with its pathogenesis [38]. Epigenetics affects life activities through DNA methylation, histone acetylation, and methylation modification, and its changes also affect the occurrence and development of diabetes. In addition, environmental factors can significantly increase the risk of T2DM by affecting DNA methylation and histone modification [39]. In view of the fact that the metabolic processes and interactions of life activities and healthy cells, including the occurrence of various human diseases, depend on changes in the molecular structure of polysaccharides, glycomics (the study of the molecular structure of polysaccharides) is used in the field of biomedicine. Genetic and other factors influence glycosylation and, in turn, whether glycoproteins trigger anti- or pro-inflammatory responses [40]. This is associated with various diseases such as biological aging, metabolic syndrome, and coronavirus disease 2019 (COVID-19) [41,42,43,44]. Immunoglobulin G fragment crystallizable (IgG Fc) glycosylation has potential as a biomarker for T2DM [40], and the integration of glycomics with other biomarkers may offer further hope for future T2DM PPPM. In the future, our group intends to adopt a comprehensive glycomics strategy to study the changes in the blood glycosylation of T2DM patients, which may help us to understand the complex physiological changes of T2DM and provide better PPPM.
Expert recommendations
As suggested in the “PPPM in Diabetes Mellitus” EPMA white paper from 2012 [7], treatment measures focused on prevention and early identification methods deserve due consideration, and a central component of prevention strategies is identification of individuals at risk for diabetes mellitus. This study constructed a T2DM prediction model in line with the recommendations in the “patient-specific modeling” paper of the EPMA that focused on research and development for the predictive and preventive potential of new IT tools for in vitro and in vivo diagnostics and the consequent evaluation and implementation of these tools in daily healthcare. The model constructed in this study can predict individuals at high risk of T2DM, and personalized medical guidance for these high-risk individuals can be furnished to primary can providers to help prevent T2DM. In subsequent work, we aim to include other data (such as OGTT and eating habits) in the model with the goal of further optimizing it.
Conclusion
In conclusion, this study is based on a relatively large sample size and the longest yet reported follow-up time of 14 years to predict the risk factors for T2DM. We developed a nomogram prediction model for the occurrence of T2DM in the nondiabetic population of eastern China within this 14-year sample period. This nomogram may therefore be able to be used as a simple, economical, and widely scalable tool to predict personalized 14-year risk of T2DM in eastern China, thereby providing strategies for treatment from a PPPM perspective.
Data availability
All requests for data are made upon request, and access to relevant data can be requested through the corresponding author.
Change history
24 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13167-022-00297-y
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98.
Lin X, Xu Y, Pan X, Xu J, Ding Y, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, et al. Incidence of type 1 and type 2 diabetes in adults aged 30–49 years: the population-based registry in the province of Turin. Italy Diabetes Care. 2005;28:2613–9.
Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32:1119–20.
Cavan D. Why screen for type 2 diabetes? Diabetes Res Clin Pract. 2016;121:215–7.
Golubnitschaja O, Costigliola V. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive Preventive and Personalised Medicine. EPMA J. 2012;3:14.
Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–51.
Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:543–51.
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7.
Wu H, Yang S, Huang Z, He J, Wang X. Type 2 diabetes mellitus prediction model based on data mining. Inform Med Unlocked. 2018;10:100–7.
Arellano-Campos O, Gómez-Velasco DV, Bello-Chavolla OY, Cruz-Bautista I, Melgarejo-Hernandez MA, et al. Development and validation of a predictive model for incident type 2 diabetes in middle-aged Mexican adults: the metabolic syndrome cohort. BMC Endocr Disord. 2019;19:41.
Zou Q, Qu K, Luo Y, Yin D, Ju Y, et al. Predicting diabetes mellitus with machine learning techniques. Front Genet. 2018;9:515.
Golubnitschaja O, Watson ID, Topic E, Sandberg S, Ferrari M, et al. Position paper of the EPMA and EFLM: a global vision of the consolidated promotion of an integrative medical approach to advance health care. EPMA J. 2013;4:12.
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23.
Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725–31.
Chen L, Magliano DJ, Balkau B, Colagiuri S, Zimmet PZ, et al. AUSDRISK: an Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust. 2010;192:197–202.
Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880.
Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, et al. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–74.
Wang A, Chen G, Su Z, Liu X, Liu X, et al. Risk scores for predicting incidence of type 2 diabetes in the Chinese population: the Kailuan prospective study. Sci Rep. 2016;6:26548.
Hippisley-Cox J, Coupland C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ. 2017;359:j5019.
Miyakoshi T, Oka R, Nakasone Y, Sato Y, Yamauchi K, et al. Development of new diabetes risk scores on the basis of the current definition of diabetes in Japanese subjects [Rapid Communication]. Endocr J. 2016;63:857–65.
Heianza Y, Arase Y, Hsieh SD, Saito K, Tsuji H, et al. Development of a new scoring system for predicting the 5 year incidence of type 2 diabetes in Japan: the Toranomon Hospital Health Management Center Study 6 (TOPICS 6). Diabetologia. 2012;55:3213–23.
Lim NK, Park SH, Choi SJ, Lee KS, Park HY. A risk score for predicting the incidence of type 2 diabetes in a middle-aged Korean cohort: the Korean genome and epidemiology study. Circ J. 2012;76:1904–10.
Moreno LM, Vergara J, Alarcón R. Predictive risk model for the diagnosis of diabetes mellitus type 2 in a follow-up study 15 years on: PRODI2 Study. Eur J Public Health. 2019;29:178–82.
Wang Y, Qu X, Kam NW, Wang K, Shen H, et al. An inflammation-related nomogram for predicting the survival of patients with non-small cell lung cancer after pulmonary lobectomy. BMC Cancer. 2018;18:692.
Wu J, Zhang H, Li L, Hu M, Chen L, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond). 2020;40:301–12.
Duarte AA, Mohsin S, Golubnitschaja O. Diabetes care in figures: current pitfalls and future scenario. EPMA J. 2018;9:125–31.
Wang Y, Zhang Y, Wang K, Su Y, Zhuge J, et al. Nomogram model for screening the risk of type II diabetes in Western Xinjiang. China Diabetes Metab Syndr Obes. 2021;14:3541–53.
Wang K, Gong M, Xie S, Zhang M, Zheng H, et al. Nomogram prediction for the 3-year risk of type 2 diabetes in healthy mainland China residents. EPMA J. 2019;10:227–37.
Xue M, Su Y, Feng Z, Wang S, Zhang M, et al. A nomogram model for screening the risk of diabetes in a large-scale Chinese population: an observational study from 345,718 participants. Sci Rep. 2020;10:11600.
Cho NH, Jang HC, Choi SH, Kim HR, Lee HK, et al. Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care. 2007;30:2566–8.
Xu W, Lu Z, Wang X, Cheung MH, Lin M, et al. Gynura divaricata exerts hypoglycemic effects by regulating the PI3K/AKT signaling pathway and fatty acid metabolism signaling pathway. Nutr Diabetes. 2020;10:31.
Park JM, Lee HS, Park JY, Jung DH, Lee JW. White blood cell count as a predictor of incident type 2 diabetes mellitus among non-obese adults: a longitudinal 10-year analysis of the Korean genome and epidemiology study. J Inflamm Res. 2021;14:1235–42.
Kachekouche Y, Dali-Sahi M, Benmansour D, Dennouni-Medjati N. Hematological profile associated with type 2 diabetes mellitus. Diabetes Metab Syndr. 2018;12:309–12.
Wang W, Yan Y. Suboptimal health: a new health dimension for translational medicine. Clin Transl Med. 2012;1:28.
Multhaup ML, Seldin MM, Jaffe AE, Lei X, Kirchner H, et al. Mouse-human experimental epigenetic analysis unmasks dietary targets and genetic liability for diabetic phenotypes. Cell Metab. 2015;21:138–49.
Kowluru RA, Mohammad G. Epigenetic modifications in diabetes. Metabolism. 2022;126:154920.
Bollati V, Baccarelli A. Environmental epigenetics. Hered (Edinb). 2010;105:105–12.
Wang W. Glycomedicine: the current state of the art. Engineering 2022; in press.
Fournet M, Bonté F, Desmoulière A. Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis. 2018;9:880–900.
Özdemir V, Arga KY, Aziz RK, Bayram M, Conley SN, et al. Digging deeper into precision/personalized medicine: cracking the sugar code, the third alphabet of life, and sociomateriality of the cell. OMICS. 2020;24:62–80.
Russell A, Wang W. The rapidly expanding nexus of immunoglobulin G N-glycomics, suboptimal health status, and precision medicine. Exp Suppl. 2021;112:545–64.
Štambuk J, Nakić N, Vučković F, Pučić-Baković M, Razdorov G, et al. Global variability of the human IgG glycome. Aging (Albany NY). 2020;12:15222–59.
Acknowledgements
The authors thank the participants and researchers who contributed or collected data. The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
Funding
This work was supported by the Jiangsu Innovative and Entrepreneurial Talent Program (grant number 202131530).
Author information
Authors and Affiliations
Contributions
TCX participated in the study design and data analysis. DCY contributed to the study design. WHZ contributed to data collection. LY participated in the study design and drafting of the manuscript and acquired the funding for this study. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This is a regression observational study and this research protocol was approved by the Nanjing Drum Tower Hospital Institutional Review Board.
Consent to participate
Participants have given their written informed consent to participate in this study.
Consent for publication
Participants have given their written informed consent for publishing the data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The author name ‘Tiancheng Xu’ was incorrectly written as ‘Tianchen Xu’.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, T., Yu, D., Zhou, W. et al. A nomogram model for the risk prediction of type 2 diabetes in healthy eastern China residents: a 14-year retrospective cohort study from 15,166 participants. EPMA Journal 13, 397–405 (2022). https://doi.org/10.1007/s13167-022-00295-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-022-00295-0