Abstract
Seed banks play an important role in determining the spatial and temporal distribution of halophytes in salt marshes. We tested the ability of native Spartina maritima and invasive S. densiflora spikelets to disperse by flotation on water with different salinity concentrations, and the longevity of spikelet viability relative to environmental conditions including dry or wet storage at low or moderate temperatures, and aqueous salinity concentrations from fresh to sea water. We quantified pre-dispersal seed production and pre- and post-dispersal focal Spartina spp. presence in seed banks along intertidal gradients within salt marshes at the Gulf of Cádiz (Southwest Iberian Peninsula). Spartina densiflora spikelets, especially from middle and high elevation marshes, showed greater ability than S. maritima to remain afloat, which suggests this species could be dispersed over longer distances. Wet-stored Spartina seeds were able to maintain viability for months, while seeds under dry storage rapidly lost viability. This decline was most significant for S. maritima, and for seeds stored at moderate temperatures. Storage of spikelets under wet and cold conditions optimized spikelet viability. Native S. maritima did not establish transient or persistent seed banks, while invasive S. densiflora established transient seed banks mainly at higher marsh elevations. Our results on the dynamics of seed dispersal and seed banks and seedling recruitment provide fundamental knowledge that can be applied for conservation of native S. maritima, management of invasive S. densiflora, and ecological restoration of tidal salt marshes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Soil seed banks play an important role in the establishment, maintenance, regeneration and restoration of vegetation in many plant communities (Bao et al. 2021; An et al. 2022). In salt marshes, seed dispersal and soil seed banks play an important role in determining the spatial and temporal distribution of halophytes (Egan and Ungar 2000; Rand 2000; Crain et al. 2008), and the successional assembly of halophytic communities following disturbance or restoration actions (Dausse et al. 2007; Kottler and Gedan 2019).
The presence and abundance of a species in the soil seed bank depends on its seed production, seed dispersal and its longevity in the soil. Regarding longevity in the soil, there are two general types of seed banks: transient and persistent (Fenner and Thompson 2005; Thompson and Grime 1979). This temporal classification of seed banks is useful in the context of exotic plant invasions, because it provides a predictive model for how long a species may persist in soil as a latent source of invasive propagules (Gioria and Pyšek 2016). In the transient seed bank, seeds are renewed annually, with accumulation of viable seeds for only up to one year. Persistent seed banks include live dormant seeds that are one or more years old. One way for species to survive in stressful environments with high mortality risk is to establish persistent seed banks (Baskin and Baskin 2001). Most perennial halophyte species form predominantly transient soil seed banks (Hutchings and Russell 1989; Ungar 2001; Wolters and Bakker 2002; Polo-Ávila et al. 2019).
Species with a transient seed bank depend on seed dispersal to maintain diversity and colonize new areas (van den Broek et al. 2005; Polo-Ávila et al. 2019). Since long-term persistent seed banks usually do not exist for perennials in salt marshes, seed dispersal by seawater (hydrochory) also plays an important role in the preservation and ecological restoration of salt-marsh communities (Wolters and Bakker 2002). In this sense, seed buoyancy enhances seed dispersal in aquatic environments, as salt marshes (van den Broek et al. 2005; Elsey-Quirk et al. 2009). Thus, sexual propagules of many salt marsh species are able to float in seawater (Huiskes et al. 1995; Wolters and Bakker 2002). Seeds of some halophytes can remain afloat for hours, while others may float during months, increasing the chance to disperse further away from their sources (Huiskes et al. 1995). In addition, seeds of halophytes may be influenced by salinity during their dispersal phase, affecting the seed flotation time (Elsey-Quirk et al. 2009). In salt marshes, tidal flooding can disperse and redistribute the seeds locally, affecting to the distributions of local species, or can disperse the seeds over long distances, favouring the colonization of new habitats (Huiskes et al. 1995; Xiao et al. 2016). In addition, seed dispersal seems to be an important factor that affects the spatial distribution of seed banks in salt marshes (Wang et al. 2009).
The spread of exotic species is an important threat to salt marsh conservation (Adam 2002). In this context, the study of soil seed banks and seed dispersal of invasive species is a crucial aspect since our knowledge on seed dynamics in the soil plays an important role in the management of salt marshes, and also may help to improve conservation and restoration actions (Wang et al. 2009; Xiao et al. 2009; Hazelton et al. 2014). Evaluation of soil seed banks can also improve our understanding of factors contributing to the invasiveness of species (Gioria et al. 2012).
The genus Spartina (cordgrasses, a monophyletic clade of perennial grasses) is abundant in salt marshes and is distributed worldwide in every continent except Antartica (Bortolus et al. 2019). Frequently, exotic Spartina species become invasive when introduced to new geographical areas (Ainouche and Gray 2016). In this context, Spartina maritima (Curtis) Fernald is a primary colonizer in salt marshes and the only European native cordgrass (Marchant and Goodman 1969). In Southwest Iberian Peninsula, this native cordgrass co-occurs with exotic and invasive Spartina densiflora Brongn. introduced from South America, which colonizes very contracted habitats along the intertidal gradient (Nieva et al. 2001). In general, Spartina species disperse spikelets that are well adapted to float in sea water, allowing them to be transported over long distances (Morgan and Sytsma 2013; McDonald 2014; Xiao et al. 2016). Moreover, Spartina species can establish transient seed banks that may vary in seed density and characteristics among different salt marsh habitats (Ungar 2001; Wang et al. 2009; Xiao et al. 2009, 2016).
Until very recently, seed production in S. maritima had been described as very low or non-existent (Marchant and Goodman 1969; Castellanos et al. 1994; Castillo et al. 2010), but Infante-Izquierdo et al. (2019a) observed in the Southwest Iberian Peninsula that this species produces a moderate number of caryopses with high seed viability. For this reason, to our knowledge, this is the first work that evaluates seed bank dynamics in S. maritima. Only one work has studied the seed bank of S. densiflora showing that this invasive species forms transient or persistent seed banks depending on environmental conditions (Abbas et al. 2021). In this work, we tested the ability of S. maritima and S. densiflora spikelets to disperse in water, the spikelet longevity relative to different environmental conditions, and we quantified the pre-dispersal seed production and the seed banks of both cordgrasses along the intertidal gradient. Differences in the ecology behaviour of these species, S. maritima as a pioneer species of mudflat soils, and S. densiflora as an invader of all habitats along the intertidal gradient, could be affected to a great extend by their seed dispersal and soil seed bank dynamics. In this sense, we hypothesized that both Spartina species would form transient seed banks in salt marsh habitats. We also postulated that daily submersion of inflorescences in tidal waters would decrease seed buoyancy, especially in more frequently inundated low elevation marshes. Our aim was to evaluate the dynamics of seed dispersal and seed banks to provide fundamental knowledge critical for science-based conservation of native S. maritima, and for restoration and management of invasive S. densiflora-invaded tidal marshes. With this aim, we studied seed viability, spikelet dispersal, seed banks and seed storage requirements for both native S. maritima and alien S. densiflora in three estuaries located in Southwest Iberian Peninsula.
Methods
Study Sites
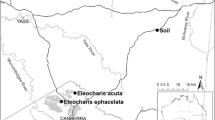
This work was carried out in tidal salt marshes in the estuaries of Odiel, Tinto and Piedras Rivers located along the Gulf of Cádiz (Southwest Iberian Peninsula) (Fig. 1). This area is under a Mediterranean climate with Atlantic influence. The coast of the Gulf of Cádiz is mesotidal and the mean sea level in this area is + 1.85 m relative to Spanish Hydrographic Zero (SHZ). The tides are semidiurnal and have a mean range of 2.10 m and a mean spring tidal range of 2.97 m, representing 0.40–3.37 m above SHZ (Castellanos et al. 1994). Native vegetation in salt marshes along the Gulf of Cádiz has been described in previous works (Castellanos et al. 1994; Fernández-Illescas et al. 2010). Different vegetation zones can be distinguished based on tidal influence and soil characteristics (Contreras-Cruzado et al. 2017). Low elevation intertidal marshes (hereafter LM) occur between Mean High Water Neap and Mean High Water, and they are dominated by Sarcocornia perennis (Mill.) A.J. Scott and Spartina maritima; middle marshes (MM) are located between Mean High Water to Mean High Water Spring, and are dominated by Sarcocornia fruticosa (L.) A.J. Scott, Sarcocornia hybrids (Figueroa et al. 2003) and Halimione portulacoides Aellen; high marshes (HM) are at Mean High Water Spring to Highest Astronomical Tide, and they are colonized by halophytes such as Arthrocnemum macrostachyum (Moric.) K. Koch, Suaeda vera Forssk. ex J. F. Gmel. and Limoniastrum monopetalum (L.) Boiss. (Long and Mason 1983; Fernández-Illescas et al. 2010). Native S. maritima inhabits LM, and is considered a primary colonizer of bare intertidal mudflats (Castellanos et al. 1994; Infante-Izquierdo et al. 2019a), while exotic and invasive S. densiflora invades LM, MM and HM, and with invasive spread, displaces native vegetation (Nieva et al. 2001).
Sampled points for native Spartina maritima (Sm) and invasive S. densiflora (Sd) in the Gulf of Cádiz (Southwest Iberian Peninsula). The assays for which locations were sampled (B: buoyancy; S: storage; SB: seed bank) are indicated after the species. The three beaches sampled (B) to study the spikelet dispersion are also indicated (source: Google Maps, data from ©2019 Instituto Geográfico Nacional Spain)
Spikelet Buoyancy
Mature spikelets were randomly collected from one S. maritima population at LM in August 2017, and from one S. densiflora population at LM, MM and HM in November 2017 (Fig. 1). Then, spikelets containing caryopses were randomly selected and stored in paper bags in dark and dry conditions at + 5 ºC. Four sets of 25 spikelets from each sampled location and habitat were placed in 500 ml beakers (8 cm diameter), each containing 100 ml of one of three different salt solutions (0.0, 0.3 and 0.6 M NaCl) at + 20–25 ºC and a 12 h light/dark photoperiod. Radiation was provided by fluorescent lamps that produced a photosynthetic photon flux density of 60 µmol m− 2 s− 1. Floating and sunken spikelets were counted seven times on the first day and twice on the following days during 5 days until practically no spikelets were floating. Beakers were agitated before counting floating and sunken spikelets to simulate wave action that can affect buoyancy (Van den Broek et al. 2005). The percentage of spikelets that remained afloat was calculated over time for each beaker. Sinking rate was calculated as the number of sinking spikelets per hour.
Spikelet Storage
Mature spikelets were randomly collected from one S. maritima population at LM in August 2017 and from one S. densiflora population at HM in November 2016 (Fig. 1). Immediately following collection, spikelets containing caryopses were randomly selected and sowed to establish a control germination before storage. Spikelets were surface-sterilized before sowing in 5% (v/v) sodium hypochlorite for 10 min to prevent fungal contamination, and then rinsed with distilled water (Muñoz-Rodríguez et al. 2012; Infante-Izquierdo et al. 2019a). Four replicates, each with 25 spikelets, were sown in Petri dishes (9 cm diameter) on two layers of autoclaved filter paper, dampened with distilled water, and sealed with adhesive tape (Parafilm™) to avoid desiccation. Germination was carried at + 20–25 ºC and a 12 h light (60 µmol m− 2 s− 1)/dark photoperiod. Germination was recorded every 2 or 3 days for 2 months. Spikelets were considered germinated when the coleoptile emerged. The rest of spikelets were stored for 1, 3, 6 and 12 months in one of five storage treatment conditions: (1) dry at + 20 °C); (2) dry at + 5 ºC; (3) immersed in distilled water (0 M NaCl) at + 5 ºC; (4) immersed in 0.3 M NaCl solution at + 5 ºC; and (5) immersed in 0.6 M NaCl at + 5ºC. Then, these pre-treated spikelets were sown as reported above. Viability of the caryopses from spikelets that did not germinate was tested using the tetrazolium test (Mackay 1972). For this purpose, the embryo was incised with a scalpel and submerged in a 1% aqueous solution of 2,3,5 triphenyl tetrazolium chloride at + 25 ºC in darkness for 24 h. Then, red stained viable embryos were counted through a magnifying glass. The percentage of spikelets with viable caryopses (germinated plus dormant), the germination percentage (based on spikelets with viable caryopses) and the days necessary to reach 50% of the final germination percentage (T50) were calculated for each Petri dish (Muñoz-Rodríguez et al. 2012; Infante-Izquierdo et al. 2019a).
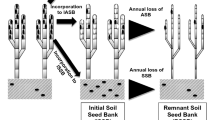
Spikelet Production and Soil Seed Banks
Both cordgrass species produce spikelets which break off from the mother plant and become a dispersal unit, in which glumes, palea and lemma cover the caryopsis. In our study we quantified the production and the density in the soil of total spikelets, including empty ones, spikelets containing caryopses and spikelets containing viable caryopses. In the soil we have also quantified the density of spikelets with germinating caryopses. We have used the term ‘seed bank’ as a generic term applied to the densities of all these types of spikelets in the soil, in concordance with seed bank literature. To study the dynamics of the seed banks of S. maritima and S. densiflora, the different phases in spikelet production and dispersal were followed and compared (Fig. 2). Annual spikelet production (ASP) and soil seed bank were studied at LM in four locations for S. maritima (Sm1, Sm2, Sm3 and Sm4; Fig. 1) and at LM, MM and HM, and adjacent bare mudflats (BM) in three locations (Sd1, Sd2 and Sd3; Fig. 1) for S. densiflora.
Annual Spikelet Production
The production of total spikelets (ASP) and of spikelets with caryopses per m2 of habitat were calculated for four populations of S. maritima in low marshes, and the production of spikelets with viable caryopses per m2 was calculated for two of those populations, using data obtained from Infante-Izquierdo et al. (2019a). For S. densiflora, spikelet production was calculated by counting the number of inflorescences on live plants prior to the onset of primary dispersal. Counts were made in 10 randomly distributed squared plots (50 × 50 cm) in patches of S. densiflora formed by coalescent tussocks in MM and HM, while in LM, in which S. densiflora grows in discrete tussocks, density of inflorescences was recorded by counting the number of inflorescences in 10 tussocks and measuring each tussock diameter to calculate its area. We randomly collected 20 inflorescences from each S. densiflora population and counted the number of total spikelets per inflorescence and the number of spikelets with caryopses, calculating then the density of spikelets with caryopses for each population. Density of spikelets with viable caryopses was calculated as the product of the mean percentage of viability by the density of spikelets with caryopses per population. These data were converted in production per m2 of habitat by using S. densiflora coverage proportion for each habitat and location for the same year, recorded along three 50 m transects in each habitat and location (LM: 0.10 ± 0.02; MM: 0.56 ± 0.09; HM: 0.51 ± 0.08).
Soil Seed Bank Sampling and Analysis
Spartina maritima seed bank was studied in LM at the four studied locations (Fig. 1) in October 2017, just after spikelet dispersal (initial seed bank). Spartina densiflora seed bank was sampled in LM, MM, HM and BM and at three locations (Fig. 1) in July 2017 (remnant seed bank) and in January 2018 (initial seed bank). To evaluate the presence of Spartina spp. in soil seed banks, we randomly collected ten sediment samples at each designated study location and habitat using stainless steel cores (50 mm diameter, 50 mm height) during low tides. We used this model of stainless steel core since previous seed bank studies in salt marshes have reported that most of the seeds accumulates in the first 50 mm of sediments (Coteff and Van Auken 2006; Zepeda et al. 2014).
To analyze spikelet dispersal away from source populations, we have studied the soil seed banks at three sandy beaches located more than 500 m from the nearest Spartina population (B1, B2 and B3 in Fig. 1). These beaches were sampled in February 2018, when both species had finished their spikelet dispersal periods. Three zones (bare mudflats, sand beach and high tide line) were sampled in each beach (n = 10–20 sediment samples per zone and beach).
Sediment samples were analysed in the laboratory just after sampling. Spikelets were directly extracted from sediment samples, since methods based on germination are less precise because many seeds may remain dormant and, therefore, the seed bank could be underestimated (Gross 1990; Brown 1992). Thus, each soil sample was washed to reduce the amount of sediment (Wolters and Bakker 2002) over a 1-mm sieve that retained all Spartina spikelets (Infante-Izquierdo et al. 2019b). The material that remained in the sieve (sand, gravel, shells, plant wrack and propagules) was placed on a filter paper and, spikelets were counted and extracted under a magnifying glass. Then, glumes, palea and lemma were removed from each spikelet in order to examine the presence or absence of caryopsis. Viability of caryopses was tested using the tetrazolium test as described above. Density of the total spikelets (total spikelets m− 2), density of spikelets with caryopses (caryopses m− 2), density of spikelets with viable caryopses (viable caryopses m− 2) and density of spikelets in which caryopses were germinating in the field at sampling moment (germinated caryopses m− 2) was calculated for each habitat for initial and remnant seed banks.
Statistical Analysis
Statistical analyses were carried out with STATISTICA 8.0 (StatSoft Inc., USA). Deviation from the mean was calculated as Standard Error (SE) for all variables. Test results were considered significant when p ≤ 0.05. Kolmogorov-Smirnov test and Levene test were used to evaluate the normality and homogeneity of all data series, respectively. Data series were transformed using √x, 1/(x + 1), ln(x + 1) or arcsine(x) functions trying to achieve normality and homogeneity of variance normality and homogeneity of variance, but they were not achieved in any case. Seed viability and germinability responses of S. maritima and S. densiflora to storage period treatments (0, 1, 3, 6, 12 months), spikelet production, and seed bank characteristics were all analysed using one-way ANOVAs with Tukey’s Honest Significant Difference (HSD) as post hoc tests. In cases where data transformations did not support use of the parametric ANOVA, non-parametric Kruskal-Wallis H test with Mann-Whitney U as the post-hoc test were used. Spearman’s correlation coefficient (ρ) was used to correlate the percentage of spikelets of S. maritima and S. densiflora that remained afloat over time.
Results
Spikelet Buoyancy
In S. maritima, there was a significant and linear reduction of floating spikelets over time in the three saline solutions, from 100 ± 0% to 9 ± 1% during the first 24 h, reaching 2 ± 1% at 48 h (Spearman correlations coefficient, 0 M NaCl: ρ = -0.9591; 0.3 M NaCl: ρ = -0.9359; 0.6 M NaCl: ρ = -0.9518; p < 0.05) (Fig. 3a). Sinking rate was 2.50 seeds h− 1 in 0.0 M and 0.6 M NaCl, and 2.44 seeds h− 1 in 0.3 M NaCl.
Percentage of floating spikelets in each beaker over time in different salt solutions of native S. maritima (a) and invasive S. densiflora (b) (M: molar NaCl), and in the different S. densiflora habitats (c) (LM: low marsh, MM: middle marsh and HM: high marsh). Regression lines and equations are presented in each case (Spearman correlation coefficient showed in Results)
The percentage of floating S. densiflora spikelets after the first 24 h was 70 ± 3%. After 70 h, S. densiflora maintained 8 ± 2% of floating spikelets, with 1 ± 0% reduction in buoyancy recorded on the fourth day (94 h). A linear and significant decrease of floating S. densiflora spikelets over time was observed under the three saline solution treatments (Spearman correlations coefficient, 0 M NaCl: ρ = -0.9022; 0.3 M NaCl: ρ = -0.9145; 0.6 M NaCl: ρ = -0.8911; p < 0.05) (Fig. 3b), with a sinking rate of 1.25 seeds h− 1 in 0.0 M and 0.3 M NaCl, and 1.17 seeds h− 1 in 0.6 M NaCl. Spartina densiflora spikelets from LM, MM and HM showed a significant decrease of floating spikelets over time (Fig. 3c) (Spearman correlations coefficient, LM: ρ = -0.9748; MM: ρ = -0.9206; HM: ρ = -0.9281; p < 0.05) with similar sinking rates: 1.27 seeds h− 1 at HM, 1.22 seeds h− 1 at MM and 1.18 seeds h− 1 at LM. However, while the first sunken spikelets from MM and HM were observed at 8.5 h, those from LM started to sink immediately. No S. maritima or S. densiflora spikelets germinated during the flotation experiment.
Spikelet Storage
In S. maritima, spikelet viability was 80 ± 4% for the control treatment. Spikelets stored dry at + 20–25 ºC showed a drastic reduction in viability after 1 month (12 ± 2%), with all spikelets dead after 3, 6 and 12 months in these conditions (Kruskal-Wallis test: H4,20 = 18.65, p < 0.001) (Fig. 4a). Spikelets dry-stored at + 5 ºC maintained 44 ± 4% of viable seeds up to 3 months, although with a significant reduction compared to the control (One-way ANOVA test: F = 110.87, df = 4, p < 0.0001) (Fig. 4b). Seeds stored wet at + 5 ºC maintained viability for at least 6 months, decreasing after 12 months (Fig. 4c, d, e). This reduction of seed viability over time was more pronounced in distilled water (3 ± 1%) (One-way ANOVA test: F = 56.40, df = 4, p < 0.0001), than in 0.3 M and 0.6 M NaCl, in which viability always remained above 40% (One-way ANOVA test: F = 24.34, df = 4, p < 0.0001; F = 12.33, df = 4, p < 0.001, respectively).
Germination (white bars) and viability percentages (black bars) for seeds of Spartina maritima and S. densiflora stored under different conditions: dry at + 20–25 ºC (a), dry at + 5 ºC (b), wet (0.0 M NaCl) at + 5 ºC (c), wet (0.3 M NaCl) at + 5 ºC (d) and wet (0.6 M NaCl) at + 5 ºC (e); during 0 (control), 1, 3, 6 and 12 months
S. maritima viable seed germination was ca. 98% after all storage periods in all conditions (One-way ANOVA or Kruskal-Wallis test, p > 0.05) (Fig. 4a, b, c, d, e). S. maritima spikelets showed T50 values of 23.3 ± 1.6 days for the control treatment. Germination was significantly delayed (higher T50) when spikelets were stored dry at + 20–25 ºC for 1 month. However, spikelets stored dry at + 5 ºC did not delay their germination (Fig. 5). In contrast, in the three wet and cold storage conditions, germination was significant and progressively accelerated (lower T50) when storage time increased (Fig. 5).
Days necessary to reach 50% of the final germination percentage (T50) for native Spartina maritima and invasive S. densiflora spikelets stored under different conditions during 0, 1, 3, 6 and 12 months. Data are means ± SE. Different letters indicate significant differences among storage periods within each storage condition (Mann-Whitney U-test or Tukey’s HSD test, p < 0.05)
Spartina densiflora seed viability was 92 ± 2% for the control treatment. Seeds dry-stored at + 20–25 ºC maintained initial viability for 1 month, followed by a 71 ± 1% reduction in viability after 3 months, with senescence of nearly all seeds after 6 months (Kruskal-Wallis test: H4,20 = 17.41, p < 0.01) (Fig. 4a). Spikelets stored dry at + 5 ºC maintained initial viability until 6 months, with a reduction in viability to 83% after 12 months (One-way ANOVA test: F = 7.52, df = 4, p < 0.01) (Fig. 4b). Spikelets stored wet at + 5 ºC maintained initial viability for 6 months (Fig. 4c, d, e). Seed viability was reduced significantly in distilled water and 0.3 M NaCl solution after 12 months (One-way ANOVA test: F = 22.44, df = 4, p < 0.0001; F = 19.35, df = 4, p < 0.0001, respectively) (Fig. 4c, d), and viability remained constant in 0.6 M NaCl solution (One-way ANOVA test: F = 2.01, df = 4, p > 0.05) (Fig. 4e). Viable seed germination was 52 ± 5% for the control sowing, with an initial dormancy level of 48%. Germination of spikelets storage dry at + 20–25 ºC for 1 month increased to 81%, being maintained this high for 3 and 6 months (One-way ANOVA test: F = 10.65, df = 3, p < 0.01) (Fig. 4a). Germination percentage remained constant during the entire storage period for dry-stored seeds at + 5 ºC (One-way ANOVA test: F = 1.85, df = 4, p > 0.05) (Fig. 4b). In the three wet and cold storage conditions, initial seed germinability was maintained until 3 months, increasing significantly after 6 or 12 months (Kruskal-Wallis test, 0.0 M: H4,20 =14.32, p < 0.01; One-way ANOVA test, 0.3 M: F = 18.71, df = 4, p < 0.0001; 0.6 M: F = 21.32, df = 4, p < 0.0001) (Fig. 4c, d, e). At the time of spikelet collection (control), T50 was 22.9 ± 0.6 days. At dry and + 20–25 ºC, germination was significantly delayed (higher T50) after 1 month or more, whereas T50 was not significantly affected in dry at + 5 ºC (Fig. 5). In contrast, germination was significantly accelerated (lower T50) in the three wet and cold storage conditions in storage periods longer than 3 months (Fig. 5). No S. maritima and S. densiflora spikelet germinated during the storage experiment.
Annual Spikelet Production (ASP)
ASP of S. maritima from field sampling was 7538 ± 896 total spikelets m− 2 in Sm1, 8069 ± 1454 total spikelets m− 2 in Sm2, 1699 ± 760 total spikelets m− 2 in Sm3, and 6565 ± 1441 total spikelets m− 2 in Sm4. Production of spikelets with caryopses per m2 was 889 ± 106 in Sm1, 991 ± 178 in Sm2 562 ± 252 in Sm3, and 2177 ± 478 in Sm4; and the production of spikelets with viable caryopses per m2 was 450 ± 201 in Sm3 and 2111 ± 463 in Sm4.
ASP of S. densiflora showed no significant differences among the three studied S. densiflora habitats in any production trait. Production of total spikelets per m2 ranged from 1623 ± 304 to 6652 ± 1893, production of spikelets with caryopses per m2 ranged from 533 ± 80 to 1722 ± 476 and production of spikelets with viable caryopses per m2 oscillated between 304 ± 52 and 1390 ± 380 (Table 1).
Soil Seed Bank
Regarding S. maritima seed bank, we only found 153 ± 109 spikelets without caryopses m− 2 in Sm2 and 51 ± 51 spikelets without caryopses m− 2 in Sm3 in the initial seed bank. No spikelets with caryopses were recorded in any of the studied S. maritima populations neither in any studied S. densiflora habitat or beaches.
In the initial seed bank, density of total spikelets of S. densiflora was significantly higher in MM and HM than in LM and BM. Density of spikelets with caryopses was significantly higher in soils from LM, MM and HM than in BM. Density of spikelets with viable caryopses increased from BM to HM along the intertidal gradient. Finally, density of spikelets with caryopses germinating in the field at the moment of sampling was significantly higher in HM than in BM, LM and MM (Table 1).
In the remnant seed bank, density of total spikelets of S. densiflora was significantly higher in MM and HM than in BM and LM. We only found spikelets with caryopses at similar densities in MM and HM. Spikelets with viable caryopses were recorded only in HM. In addition, we did not find any germinated caryopsis in the remnant seed bank (Table 1).
In the studied beaches, total density of S. densiflora spikelets was higher at the high tide lines than at lower bare mudflats or sand beaches (Kruskal-Wallis test: H2,110 = 21.72, p < 0.0001). Density of spikelets with caryopses, with viable caryopses and with germinated caryopses also showed the highest values at high tide level, but without significant differences among zones (Kruskal-Wallis test, p > 0.05) (Table 2).
Discussion
Our results about seed dispersal potential and soil seed bank dynamics are useful to explain the distribution of S. maritima and S. densiflora along the intertidal gradient in salt marshes. An important result of this work is that no seed bank was found for S. maritima in either its low marsh populations nor in upper areas in the intertidal gradient. However, in accordance with our hypothesis, S. densiflora established a viable transient seed bank in all elevation zones of the marsh. This finding reveals a seed bank life stage of the invader that is present through the salt marsh, and can contribute to its invasiveness. Results of our work show that daily submersion of inflorescences decreases the buoyancy of spikelets as predicted in our hypothesis.
In buoyancy trials, most of the native Spartina maritima spikelets sank within the first 24 h in water with different salinities, whereas only c. 30% of S. densiflora spikelets sank after 24 h and practically all S. densiflora spikelets were submerged after 94 h in water with different salinities. This poor buoyancy ability of S. maritima likely contributes to why we did not detect any S. maritima seeds in soil seed banks at locations away from their population sites, including sampled S. densiflora locations and isolated beaches. These results do provide support for the better dispersal ability of S. densiflora which was present in soils seed banks at isolated beaches some distance away from its populations. Moreover, the density of S. densiflora spikelets in the seed bank before the germination period (initial seed bank) in HM exceeded the sum of the density of remnant spikelets and the in situ annual production, meaning that the HM was a sink habitat for propagules, whereas LM and MM were propagule production areas from which propagules were exported. Our results coincide with studies on Spartina alterniflora Loisel. in the Yangtze Estuary (China) that have reported a higher density of spikelets in MM and HM than in LM and bare mudflats (Wang et al. 2009; Xiao et al. 2009). Upper salt marsh zones are less exposed to tides at the same time that halophytes canopies reduce the speed of tidal currents, favouring the deposition of seeds that have been dragged by the tide (Lambrinos and Bando 2008). In contrast, tidal currents favour the exportation of propagules in low marshes (Wolters and Bakker 2002).
The recorded flotation period for S. densiflora spikelets was similar to that found by McDonald (2014). Other cordgrasses showed longer buoyancy periods than S. densiflora. For example, S. alterniflora and Spartina patens (Aiton) Muhl. from the Atlantic and the Gulf Coasts of North America presented flotation times ca. 25 days (Elsey-Quirk et al. 2009) and 50% of spikelets remained afloat after 9 days and 8% after 40 days for South American Spartina ciliata Brongn. (Cordazzo and Davy 1994). Moreover, S. densiflora spikelets from LM started to sink before than those from MM and HM. This behaviour was also observed by Xiao et al. (2016) in S. alterniflora. Waterlogging of Spartina spp. propagules can cause loss of buoyancy (Morgan and Sytsma 2013). In this sense, Elsey-Quirk et al. (2009) found that S. alterniflora spikelets pre-treated during 30 days with wet stratification sank before than those not pre-treated. The ability of S. densiflora spikelets, especially from MM and HM, to remain afloat may causes that this species could be dispersed over long distances favouring the colonization of new areas (McDonald 2014). This could explain the extension of S. densiflora invasion from San Francisco Bay to Vancouver Island along the Pacific Coast of North America (Castillo et al. 2014) as well as its invasion from South Portugal to the Strait of Gibraltar along the Gulf of Cadiz (Nieva et al. 2001). In contrast, European native S. maritima spikelets capacity to colonize new habitats located at medium and long distances would be more restricted. This limitation in spikelet dispersion may favour population isolation that could explain why S. maritima shows a low genetic diversity, especially in North European Marshes (Raybould et al. 1991; Yannic et al. 2004). In agreement with our results, Polo-Ávila et al. (2019) found only one S. maritima spikelet in all 420 soil samples analyzed along the whole intertidal gradient. Thus, due to their low buoyancy, most of S. maritima spikelets may be deposited on the bottom of salt marsh channels and mudflats, where they could maintain viability for longer due to humidity conditions according to our storage experiments, and where if the elevation is adequate, they could potentially germinate and establish as primary colonizers (Castellanos et al. 1994; Castillo et al. 2000).
In this context and in view of our storage results, wet Spartina seeds transported by currents and tides would be able to keep their viability during months in contrast with dry seeds that rapidly lost their viability, especially at moderate temperature and in the case of S. maritima, like many aquatic plants with recalcitrant seeds (Probert and Longley 1989; Biber and Caldwell 2008). This behaviour was also observed for S. alterniflora seeds that cannot withstand drying at moderate temperature, losing their viability within 40 days (Mooring et al. 1971). Cold mitigated the deleterious effects of dry storage since refrigeration prevents desiccation, being S. maritima able to keep some viable seeds until 3 months and S. densiflora until 12 months. These results are in accordance with Kittelson and Boyd (1997), who reported that S. densiflora caryopses did not lose viability in dry storage at 0 ºC, and with Mooring et al. (1971) who stated that S. alterniflora viability was preserved in cold storage, but after 8 months viability is lost.
There are numerous endogenous and exogenous types of seed dormancy. Spartina spp. have physiological dormancy that inhibits germination (Baskin and Baskin 2001). This dormancy can prevent germination of seeds in unfavourable conditions for seedling growth (Ungar 1978). Dormancy is an important process for establishment of persistent soil seed banks in salt marshes (Ungar 2001), and seeds of many halophytes that disperse during the fall have dormancy mechanisms (Baskin and Baskin 2001). Spartina maritima seeds without spikelet bracts showed dormancy levels that ranged from 9 to 19% (Infante-Izquierdo et al. 2019a), whereas, in the present study, S. maritima did not show dormancy sowing the entire spikelet as occurred in our study about germination at different salinities (Infante-Izquierdo et al. 2019c). This means that its seeds were ready to germinate just after being dispersed from the mother plant. In the case of S. maritima, its lack of dormancy and its inability to maintain viable seeds covered with bracts for long periods at moderate temperatures supports the characterization of this species as a short distance dispersal, as discussed above, and its inability to establish even transient seed banks as recorded in this study. The lack of seedling emergence of S. patens and Spartina foliosa Trin. from seed bank assays also suggests these congeners do not maintain persistent soil seed banks (Hopkins and Parker 1984; Baldwin et al. 1996). Spartina foliosa can be an abundant seed producer and quickly colonizes marsh restoration sites, but a low frequency of detection in seed rain and seed banks at San Francisco Estuary is thought to be due to low seed viability, particularly at elevated salinity (Diggory and Parker 2011).
In contrast, S. densiflora exhibited dormancy after being dispersed from the mother plant of 47%, which would allow to disperse to long distances and establish seed banks as recorded in our study. This dormancy was broken after 1 month in dry and moderate temperature conditions, accompanied by a decrease in seed viability, and was also broken by storage over 6 months in wet and cold conditions. However, S. densiflora dormancy at dispersal moment was retained when spikelets were stored in dry and cold conditions. These results are similar than those recorded previously for S. patens (Plyler and Proseus 1996) and S. alterniflora (Wijte and Gallagher 1996; Biber and Caldwell 2008; Xiao et al. 2009). Thus, wet and cold conditions were the best for spikelets storage in both cordgrasses, as occurs in S. alterniflora (Mooring et al. 1971; Wijte and Gallagher 1996), maintaining seed viability longer, mainly in 0.6 M NaCl solutions, in which high numbers of seeds remained viable during 12 months. Moreover, germination was progressively accelerated when storage time increased in these conditions for both species. In this sense, Xiao et al. (2009) observed that a wet and chilling treatment shorten the time of onset seed germination for S. alterniflora. Due to its dormancy, S. densiflora was able to establish transient seed banks mainly at higher elevations along the intertidal gradient as recorded in this study. Abbas et al. (2021) reported that S. densiflora formed short-term persistent seed banks at Humboldt Bay (North California) probably related to a cooler climate than in the Odiel Marshes. Nevertheless, S. densiflora was unable to establish permanent seed bank in the Odiel Marshes since only 1 spikelet with viable caryopsis remained in a HM soil sample after the germination period. Transient seed bank has been reported for other Spartina species such as S. alterniflora (Wang et al. 2009; Xiao et al. 2009, 2016), Spartina argentinensis Parodi (Feldman et al. 2007) and allopolyploid Spartina anglica C.E. Hubb. (Ungar and Woodell 1993). In S. alterniflora, Xiao et al. (2009) found that its transient seed bank lasted less than 9 months. We recorded high germination percentages (14–56% of viable caryopses) for dispersed S. densiflora spikelets before the beginning of the germination period in the field (initial seed bank). Thus, all these seeds were being subtracted from the transient seed bank during and just after winter rainfalls, that reduce salinity promoting germination as occurs in most halophytes (Keiffer and Ungar 1997; Muñoz-Rodríguez et al. 2017; Infante-Izquierdo et al. 2019c). This first germination window is followed by other germination periods later in the year (J.M. Castillo, personal observation), as S. densiflora seeds would lose their dormancy after 6 months in cold and wet conditions. On the other hand, the decay of non-germinated caryopses in the soil bank may be determined by the desiccation that occurs in summer, as reported by Chang et al. (2001) and as we observed. Other factors that could explain the depletion of the soil seed bank is seed transport by tides (Wang et al. 2009; Xiao et al. 2009) and seed mortality caused by microbial and fungi activity (Wagner and Mitschunas 2008; Xiao et al. 2009), or by seed predation (Espinar et al. 2004; Xiao et al. 2009).
Our results on the dynamics of seed dispersal and seed banks provide fundamental information for the conservation of native S. maritima and the management of invasive S. densiflora. The limited spikelet dispersal and the absence of soil seed banks in native S. maritima habitat reveals the need to maintain high levels of genetic variability in populations, which must be consider in marsh restoration works using this species. The limited dispersal ability and the absence of S. maritima in soil seed banks, together with the results from other studies that indicate invasive S. densiflora is better adapted to salinity changes expected with climate change than native S. maritima (Infante-Izquierdo et al. 2019c), suggest that native S. maritima is highly vulnerable to future changes in the littoral environment such as sea level rise. Existing natural S. maritima populations should be carefully preserved and used as source sites for seeds to actively create new populations. Thus, the limited dispersal and soil seed bank ecology of S. maritima suggests revegetation at restored salt marshes will likely require augmentative introductions since passive restoration efforts would not be enough. Ex situ collections and storage of plant species propagules to bank and preserve plant genetic resources for marsh restoration can provide an important safety net against extinction of native species in the face of climate change, invasive species, and habitat loss (Maunder et al. 2004; Millennium Ecosystem Assessment 2005). The results of storage experiments provide important information about the best way to preserve the seeds of native S. maritima. For invasive S. densiflora, attempts to manage this invasive species should focus on eliminating adult plants before annual seed release to reduce augmentation of seed banks. Also, regional weed management strategies are needed to reduce the entry of propagules from other nearby populations that will drive secondary invasions. In general, our results add useful knowledge to preserve European salt marshes using S. maritima as biotool and to fight exotic S. densiflora invasion in North American, European and African marshes.
Data Availability
The datasets used and analysed during the current study are available from the authors upon reasonable request.
Code Availability
Not applicable.
References
Abbas AM, Pickart AJ, Goldsmith LM, Davenport DN, Newby B, Muñoz-Rodríguez AF, Grewell BJ, Castillo JM (2021) Seed bank persistence of a south american cordgrass in invaded northern Atlantic and Pacific Coast estuaries. AoB Plants 13:plab014. https://doi.org/10.1093/aobpla/plab014
Adam P (2002) Saltmarshes in a time of change. Environmental Conservation 29:39–61. https://doi.org/10.1017/S0376892902000048
Ainouche M, Gray A (2016) Invasive Spartina: lessons and challenges. Biological Invasions 18:2119–2122. https://doi.org/10.1007/s10530-016-1201-7
An H, Baskin CC, Ma M (2022) Nonlinear response of the soil seed bank and its role in plant community regeneration with increased grazing disturbance. Journal of Applied Ecology 59:2593–2603. https://doi.org/10.1111/1365-2664.14259
Baldwin AH, McKee KL, Mendelssohn IA (1996) The influence of vegetation, salinity, and inundation on seed banks of oligohaline coastal marshes. American Journal of Botany 83:470–479. https://doi.org/10.1002/j.1537-2197.1996.tb12728.x
Bao F, de Assis MA, Pott A (2021) Maintenance of wetland plant communities: the role of the seed bank in regeneration of native plants. Acta Botnica Braslica 35:70–78. https://doi.org/10.1590/0102-33062020abb0112
Baskin CC, Baskin JM (2001) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic, San Diego
Biber PD, Caldwell JD (2008) Seed germination and seedling survival of Spartina alterniflora Loisel. American Journal of Agricultural and Biological Sciences 3:633–638
Bortolus A, Adam P, Adams JB et al (2019) Supporting Spartina: interdisciplinary perspective shows Spartina as a distinct solid genus. Ecology 100:e02863. https://doi.org/10.1002/ecy.2863
Brown D (1992) Estimating the composition of a forest seed bank: a comparison of the seed extraction and seedling emergence methods. Canadian Journal of Botany 70:1603–1612. https://doi.org/10.1139/b92-202
Castellanos EM, Figueroa ME, Davy AJ (1994) Nucleation and facilitation in saltmarsh succession: interactions between Spartina maritima and Arthrocnemum perenne Journal of Ecology 82:239–248. https://doi.org/10.2307/2261292
Castillo JM, Fernández-Baco L, Castellanos EM, Luque CJ, Figueroa ME, Davy AJ (2000) Lower limits of Spartina densiflora and S. maritima in a Mediterranean salt marsh determined by different ecophysiological tolerances. Journal of Ecology 88:801–812. https://doi.org/10.1046/j.1365-2745.2000.00492.x
Castillo JM, Ayres DR, Leira-Doce P, Bailey J, Blum M, Strong DR, Luque T, Figueroa E (2010) The production of hybrids with high ecological amplitude between exotic Spartina densiflora and native S. maritima in the Iberian Peninsula. Diversity and Distributions 16:547–558. https://doi.org/10.1111/j.1472-4642.2010.00673.x
Castillo JM, Grewell BJ, Pickart A, Bortolus A, Peña C, Figueroa E, Sytsma M (2014) Phenotypic plasticity of invasive Spartina densiflora (Poaceae) along a broad latitudinal gradient on the Pacific Coast of North America. American Journal of Botany 101:448–458. https://doi.org/10.3732/ajb.1400014
Chang ER, Jefferies RL, Carleton TJ (2001) Relationship between vegetation and soil seed banks in an arctic coastal marsh. Journal of Ecology 89:367–384. https://doi.org/10.1046/j.1365-2745.2001.00549.x
Contreras-Cruzado I, Infante-Izquierdo MD, Márquez-García B, Hermoso-López V, Polo A, Nieva FJJ, Cartes-Barroso JB, Castillo JM, Muñoz-Rodríguez A (2017) Relationships between spatio-temporal changes in the sedimentary environment and halophytes zonation in salt marshes. Geoderma 305:173–187. https://doi.org/10.1016/j.geoderma.2017.05.037
Cordazzo CV, Davy AJ (1994) Seed production, seed size, and dispersal of Spartina ciliata Brongniart (Gramineae) in southern brazilian coastal dunes. Atlantica 16:143–154
Coteff C, Van Auken OW (2006) Sampling requirements for estimation of the soil seed bank of a west Texas salt marsh. Texas Journal of Science 58:349–370
Crain CM, Albertson LK, Bertness MD (2008) Secondary succession dynamics in estuarine marshes across landscape-scale salinity. Ecology 89:2889–2899
Dausse A, Bonis A, Bouzille IB, Lefeuvre JC (2007) Seed dispersal in a polder after partial tidal restoration: implications for salt marsh restoration. Applied Vegetation Science 11:3–12. https://doi.org/10.1111/j.1654-109X.2008.tb00199.x
Diggory ZE, Parker VT (2011) Seed supply and revegetation dynamics at restored tidal marshes, Napa River, California. Restoration Ecology 19:121–130. https://doi.org/10.1111/j.1526-100X.2009.00636.x
Egan TP, Ungar IA (2000) Similarity between seed banks and above ground vegetation along a salinity gradient. Journal of Vegetation Science 11:189–194. https://doi.org/10.2307/3236798
Elsey-Quirk T, Middleton BA, Proffitt CE (2009) Seed flotation and germination of salt marsh plants: the effects of stratification, salinity, and/or inundation regime. Aquatic Botany 91:40–46. https://doi.org/10.1016/j.aquabot.2009.02.001
Espinar JL, García LV, Figuerola J, Green AJ, Clemente L (2004) Helophyte germination in a Mediterranean salt marsh: gut passage by ducks changes seed response to salinity. Journal of Vegetation Science 15:315–322. https://doi.org/10.1111/j.1654-1103.2004.tb02267.x
Feldman SR, Alzugaray C, Lewis JP (2007) Relación entre la vegetación y el banco de semillas de un espartillar de Spartina argentinensis Ciencia e Investigación Agraria 34:41–48
Fenner M, Thompson K (2005) The ecology of seeds. Cambridge University Press, New York
Fernández-Illescas F, Nieva FJJ, Silva I, Tormo R, Muñoz AF (2010) Pollen production of Chenopodiaceae species at habitat and landscape scale in Mediterranean salt marshes: an ecological and phenological study. Review of Palaeobotany and Palynology 161:127–136. https://doi.org/10.1016/j.revpalbo.2010.03.006
Figueroa ME, Castillo JM, Redondo S, Luque T, Castellanos EM, Nieva FJJ, Luque CJ, Rubio-Casal AE, Davy AJ (2003) Facilitated invasion by hybridization of Sarcocornia species in a salt-marsh succession. Journal of Ecology 91:616–626. https://doi.org/10.1046/j.1365-2745.2003.00794.x
Gioria M, Pyšek P (2016) The legacy of plant invasions: changes in the soil seed bank of invaded plant communities. BioScience 66:40–53. https://doi.org/10.1093/biosci/biv165
Gioria M, Pyšek P, Moravcová L (2012) Soil seed banks in plant invasions: promoting species invasiveness and long-term impact on plant community dynamics. Preslia 84:327–350
Gross KL (1990) A comparison of methods for estimating seed numbers in the soil. Journal of Ecology 78:1079–1093. https://doi.org/10.2307/2260953
Hazelton EL, Mozdzer TJ, Burdick DM, Kettenring KM, Whigham DF (2014) Phragmites australis management in the United States: 40 years of methods and outcomes. AoB Plants 6. https://doi.org/10.1093/aobpla/plu001
Hopkins DR, Parker VT (1984) A study of the seed bank of a salt marsh in northern San Francisco Bay. American Journal of Botany 71:348–355. https://doi.org/10.1002/j.1537-2197.1984.tb12522.x
Huiskes AHL, Koutstaal BP, Herman PMJ, Beeftink WG, Markusse MM, De Munck W (1995) Seed dispersal of halophytes in tidal salt marshes. Journal of Ecology 83:559–567. https://doi.org/10.2307/2261624
Hutchings MJ, Russell PJ (1989) The seed regeneration dynamics of an emergent salt marsh. Journal of Ecology 77:615–637
Infante-Izquierdo MD, Castillo JM, Nieva FJJ, Rotundu ID, David FT, Grewell BJ, Muñoz-Rodríguez AF (2019a) Fruit set, seed viability and germination of the european native Spartina maritima in Southwest Iberian Peninsula. Wetlands 40:421–432. https://doi.org/10.1007/s13157-019-01188-1
Infante-Izquierdo MD, Gallego-Tévar B, Sánchez-Gullón E, Nieva FJJ, Grewell BJ, Castillo JM, Muñoz-Rodríguez AF (2019b) Morphological and anatomical evidence supports differentiation of new interspecific hybrids from native Spartina maritima and invasive S. densiflora (Poaceae, subfamily Chloridoideae). Plant Systematics and Evolution 305:531–547. https://doi.org/10.1007/s00606-019-01591-5
Infante-Izquierdo MD, Castillo JM, Grewell BJ, Nieva FJJ, Muñoz-Rodríguez AF (2019c) Differential effects of increasing salinity on germination and seedling growth of native and exotic invasive cordgrasses. Plants 8:372. https://doi.org/10.3390/plants8100372
Keiffer CH, Ungar IA (1997) The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. American Journal of Botany 84:104–111. https://doi.org/10.2307/2445887
Kittelson PM, Boyd MJ (1997) Mechanisms of expansion for an introduced species of cordgrass, Spartina densiflora, in Humboldt Bay, California. Estuaries 20:770–778. https://doi.org/10.2307/1352250
Kottler EJ, Gedan K (2019) Seeds of change: characterizing the soil seed bank of a migrating salt marsh. Annals of Botany. https://doi.org/10.1093/aob/mcz133
Lambrinos JG, Bando KJ (2008) Habitat modification inhibits conspecific seedling recruitment in populations of an invasive ecosystem engineer. Biological Invasions 10:729–741. https://doi.org/10.1007/s10530-007-9165-2
Long SP, Mason CF (1983) Saltmarsh ecology. Cambridge University Press, Cambridge
Mackay DB (1972) The measurement of viability. In: Roberts EH (ed) Viability of seeds. Chapman and Hall, London, pp 172–208
Marchant CJ, Goodman PJ (1969) Spartina maritima (Curtis) Fernald. Journal of Ecology 57:287–291. https://doi.org/10.2307/2258222
Maunder M, Edward O, Guerrant K Jr, Dixon KW (2004) Realizing the full potential of ex situ contributions to global plant conservation. In: Edward O, Guerrant K Jr, Maunder M (eds) Ex situ plant conservation: supporting species survival in the wild. Island Press, Washington, pp 389–418
McDonald K (2014) Tidal seed dispersal potential of Spartina densiflora in Humboldt Bay (Humboldt County, California). Doctoral dissertation, Humboldt State University, California
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: synthesis. Island Press, Washington DC
Morgan VH, Sytsma MD (2013) Potential ocean dispersal of cordgrass (Spartina spp.) from core infestations. Invasive Plant Sci Manag 6:250–259. https://doi.org/10.1614/IPSM-D-12-00042.1
Mooring MT, Cooper AW, Seneca ED (1971) Seed germination response and evidence for height ecophenes in Spartina alterniflora from North Carolina. American Journal of Botany 58:48–55. https://doi.org/10.1002/j.1537-2197.1971.tb09944.x
Muñoz-Rodríguez AF, Rodríguez-Rubio P, Nieva FJJ, Fernández-Illescas F, Sánchez-Gullón E, Soto JM, Hermoso-López V, Márquez-García B (2012) The importance of bracteoles in ensuring Atriplex halimus germination under optimal conditions. Fresenius Environmental Bulletin 21:3521–3526
Muñoz-Rodríguez AF, Sanjosé I, Márquez-García B, Infante-Izquierdo MD, Polo-Ávila A, Nieva FJJ, Castillo JM (2017) Germination syndromes in response to salinity of Chenopodiaceae halophytes along the intertidal gradient. Aquatic Botany 139:48–56. https://doi.org/10.1016/j.aquabot.2017.02.003
Nieva FJJ, Díaz-Espejo A, Castellanos EM, Figueroa ME (2001) Field variability of invading populations of Spartina densiflora Brong. In different habitats of the Odiel Marshes (SW Spain). Estuarine, Coastal and Shelf Science 52:515–527. https://doi.org/10.1006/ecss.2000.0750
Plyler DB, Proseus TE (1996) A comparison of the seed dormancy characteristics of Spartina patens and Spartina alterniflora (Poaceae). American Journal of Botany 83:11–14. https://doi.org/10.1002/j.1537-2197.1996.tb13868.x
Polo-Ávila A, Infante-Izquierdo MD, Soto JM, Hermoso-López V, Nieva FJJ, Castillo JM, Muñoz-Rodríguez AF (2019) Contrasting propagule dispersal and halophyte seed banks along the intertidal gradient. Marine Ecology Progress Series 616:51–65. https://doi.org/10.3354/meps12943
Probert RJ, Longley PL (1989) Recalcitrant seed storage physiology in three aquatic grasses (Zizania palustris, Spartina anglica and Porteresia coarctata). Annals of Botany 63:53–64. https://doi.org/10.1093/oxfordjournals.aob.a087728
Rand T (2000) Seed dispersal, habitat suitability and the distribution of halophytes across a salt marsh tidal gradient. Journal of Ecology 88:608–621. https://doi.org/10.1046/j.1365-2745.2000.00484.x
Raybould AF, Gray AJ, Lawrence MJ, Marshall DF (1991) The evolution of Spartina anglica C.E. Hubbard (Gramineae): genetic variation and status of the parental species in Britain. Biological Journal of the Linnean Society 44:369–380. https://doi.org/10.1111/j.1095-8312.1991.tb00626.x
Thompson K, Grime JP (1979) Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology 67:893–921. https://doi.org/10.2307/2259220
Ungar IA (1978) Halophyte seed germination. The Botanical Review 44:233–264. https://doi.org/10.1007/BF02919080
Ungar IA (2001) Seed banks and seed population dynamics of halophytes. Wetlands Ecology and Management 9:499–510. https://doi.org/10.1023/A:1012236829474
Ungar IA, Woodell SRJ (1993) The relationship between the seed bank and species composition of plant communities in two british salt marshes. Journal of Vegetation Science 4:531–536. https://doi.org/10.2307/3236080
van den Broek T, van Diggelen R, Bobbink R (2005) Variation in seed buoyancy of species in wetland ecosystems with different flooding dynamics. Journal of Vegetation Science 16:579–586. https://doi.org/10.1111/j.1654-1103.2005.tb02399.x
Wagner M, Mitschunas N (2008) Fungal effects on seed bank persistence and potential applications in weed biocontrol: a review. Basic and Applied Ecology 9:191–203. https://doi.org/10.1016/j.baae.2007.02.003
Wang CH, Tang L, Fei SF, Wang JQ, Gao Y, Wang Q, Chen JK, Li B (2009) Determinants of seed bank dynamics of two dominant helophytes in a tidal salt marsh. Ecological Engineering 35:800–809. https://doi.org/10.1016/j.ecoleng.2008.12.004
Wijte AH, Gallagher JL (1996) Effect of oxygen availability and salinity on early life history stages of salt marsh plants. I. different germination strategies of Spartina alterniflora and Phragmites australis (Poaceae). American Journal of Botany 83:1337–1342. https://doi.org/10.1002/j.1537-2197.1996.tb13919.x
Wolters M, Bakker JP (2002) Soil seed bank and driftline composition along a successional gradient on a temperate salt marsh. Applied Vegetation Science 5:55–62. https://doi.org/10.1111/j.1654-109X.2002.tb00535.x
Xiao D, Zhang L, Zhu Z (2009) A study on seed characteristics and seed bank of Spartina alterniflora at saltmarshes in the Yangtze Estuary, China. Estuarine, Coastal and Shelf Science 83:105–110. https://doi.org/10.1016/j.ecss.2009.03.024
Xiao D, Zhang C, Zhang L, Zhu Z, Tian K, Gao W (2016) Seed dispersal capacity and post-dispersal fate of the invasive Spartina alterniflora in saltmarshes of the Yangtze Estuary. Estuarine, Coastal and Shelf Science 169:158–163. https://doi.org/10.1016/j.ecss.2015.11.032
Yannic G, Baumel A, Ainouche M (2004) Uniformity of the nuclear and chloroplast genomes of Spartina maritima (Poaceae), a salt-marsh species in decline along the western european coast. Heredity 93:182–188. https://doi.org/10.1038/sj.hdy.6800491
Zepeda G, Lot A, Nemiga XA, Manjarrez J (2014) Seed bank and established vegetation in the last remnants of the Mexican Central Plateau wetlands: the Lerma marshes. Revista de Biologia Tropical 62:455–472
Acknowledgements
We thank to the management and staff of the Odiel Marshes Natural Park (Andalusia, Spain) for their collaboration. M.D. Infante-Izquierdo acknowledges to Ministerio de Educación, Cultura y Deporte of Spanish Government for the FPU Grant (FPU14/06556).
Funding
Funding for open access publishing: Universidad de Sevilla/CBUA Open Access funding enabled and organized by Universidad de Sevilla. M.D. Infante-Izquierdo acknowledges to Ministerio de Educación, Cultura y Deporte of Spanish Government for the FPU Grant (FPU14/06556).
Author information
Authors and Affiliations
Contributions
Research and methodology were conceived by MDII, AFMR, JMC, supervised by AFMR. Manuscript was written and edited by MDII, AFMR, JMC and BJG. Data collection and analysis was performed by MDII, RRM, JJS and FJJN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No ethics approvals were required for this research.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Infante-Izquierdo, M.D., Romero-Martín, R., Castillo, J.M. et al. Seed Viability, Spikelet Dispersal, Seed Banks and Seed Storage Requirements for Native and Invasive Cordgrasses (Genus Spartina) in Southwest Iberian Peninsula. Wetlands 43, 8 (2023). https://doi.org/10.1007/s13157-022-01655-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01655-2