Abstract
Due to their relatively small sizes, temperate forest vernal pools are less studied than other wetlands, despite being potential biogeochemical hotspots in landscapes. We investigated spatial and temporal factors driving N2O and CH4 emission rates from vernal pools in a temperate forest. We determined higher N2O (3.66 ± 0.53 × 10−6, μg N2O/m2/h) and CH4 (2.10 ± 0.7 × 10−3, μg N2O/m2/h) rates in spring relative to fall (~50% and 77% lower for N2O and CH4 rates, respectively) and winter (~70% and 94% lower for N2O and CH4 rates, respectively). Soil organic matter, nitrate content and bacterial 16S rDNA, nirS, and norB gene abundances emerged as significant drivers of N2O rates, whereas, soil pH, organic matter content and mcrA abundance were significant drivers of CH4 rates. Denitrification gene abundances were negatively correlated with N2O rates, whereas mcrA abundance correlated positively with CH4 rates. Results suggest that CH4 rates may be directly coupled to methanogen abundance, whereas N2O rates may be directly impacted by a variety of abiotic variables and indirectly coupled to the abundance of potential denitrifier assemblages. Overall, additional studies examining these dynamics over extended periods are needed to provide more insights into their control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vernal pools are ephemeral wetlands formed in relatively small permanent basins/depressions (less than 2 m deep) that experience periodic inundation and drying (Zedler 2003). Vernal pools are variable in size, depth, and volume (Brooks and Hayashi 2002). The relatively shallow depths, small to medium sizes, and ephemeral nature of vernal pools results in considerable mixing between upper (water column) and lower (soil and organic matter) layers during periods of inundation that differ from larger wetland systems. This mixing changes biotic and abiotic variables at the oxic and anoxic interface (Zedler 2003; Carrino-Kyker and Swanson 2007; Holgerson and Raymond 2016) and impacts biogeochemical processes.
Several abiotic factors impact biogeochemical processes in terrestrial wetland systems. Elevated soil moisture (and reduced soil aeration) (Liu et al. 2011; Signor and Cerri 2013; Butterbach-Bahl et al. 2013; Holgerson and Raymond 2016), and increasing soil temperature (Le Mer and Roger 2001; Butterbach-Bahl et al. 2013) create suitable conditions for enhancing denitrification and methanogenesis in anaerobic microsites within soils. Other soil abiotic factors, such as soil type, pH, nitrate, and organic matter content, also impact these processes (Knowles 1982; Brentrup et al. 2000; Le Mer and Roger 2001; Chapuis-Lardy et al. 2006; Cameron et al. 2013; Signor and Cerri 2013; Butterbach-Bahl et al. 2013; Holgerson 2015). Additionally, biogeochemical processes in wetlands also depend on the presence, composition, and activity of microbial populations that are capable of responding to seasonal changes in abiotic factors (Butterbach-Bahl et al. 2013; Capps et al. 2014). These microbial assemblages are crucial for the maintenance of ecosystem processes under fluctuating conditions (Allison and Martiny 2008). However, the impacts of varying environmental conditions on functional groups within assemblages are not straightforward. The uncertainty around this is because microbial diversity and abundances of functional populations vary spatially and seasonally, and the effects of changing environmental variables on functional groups within these assemblages are complex and not well-understood (Braker et al. 2010; Dandie et al. 2011; Hallin et al. 2012; Chen et al. 2015; Saarenheimo et al. 2015).
Few studies have studied the spatial and temporal variations of methanogenesis and denitrification rates in temperate forest vernal pools within landscapes (but see Carrino-Kyker and Swanson 2008; Capps et al. 2014). Temporally, inundated vernal pools are characterized by low dissolved oxygen, high soil moisture, high organic matter content, low pH, low redox, and high enzyme activities (Carrino-Kyker and Swanson 2007; Capps et al. 2014). These conditions result in rates of denitrification (Capps et al. 2014) and methanogenesis rates (Kutzbach et al. 2004) that considerably higher in inundated vernal pool soils than in adjacent terra firma sites. Spatially, vernal pools may also be considered biogeochemical “hot spots” where denitrification and methane emission occur at disproportionately higher rates during periods of inundation, compared to the surrounding landscape (Butterbach-Bahl et al. 2013; Capps et al. 2014). This is because vernal pools are linked spatially along hydrologic flow paths within landscapes, sharing precursors that are potentially converted into gases (N2, N2O, CH4) when microbial assemblages with the potential for transformation are present and responsive (Mayorga et al. 2003). However, the specific location of vernal pools within the surrounding landscape may also be a contributing factor. For example, in general, upland sites across various landscapes are characterized by lower soil moisture content and water holding capacity (Bayabil et al. 2016; Arias-Navarro et al. 2017), which can negatively impact biogeochemical processes in vernal pools located in these regions. In contrast, bottomland regions in floodplains tended to have higher soil moisture content and reduced soil aeration, which can positively enhance anaerobic biogeochemical processes in these vernal pools (Bayabil et al. 2016; Arias-Navarro et al. 2017).
Given the nature of vernal pools, the biogeochemical processes, and abiotic and microbial factors that regulate biogeochemical processes may differ in vernal pools from other wetland types. In this study, we measured denitrification and methanogenesis rates from vernal pools along a topographic gradient (bottomland and upland) across seasons in a temperate forest ecosystem to investigate the nature of the relationships among abiotic and microbial variables and measured rates. We anticipated higher denitrification and methanogenesis rates 1) during periods of inundation, and 2) in bottomland pools relative to upland pools. Abiotic variables (soil moisture, % soil organic matter, soil nitrate concentration, and soil pH) and microbial community characteristics (16S rRNA, nirS, norB, nosZ, and mcrA gene abundances) were quantified and utilized in determining the best predictors of these processes across seasons.
Methods
Study Site
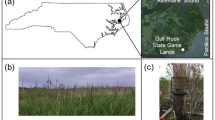
Ten vernal pools at Kent State University’s research forest, Jennings Woods, were sampled. Jennings is a 30 ha hardwood temperate forest with two distinct zones along a topographical gradient (Blackwood et al. 2013). The upland zone is 15–20 m above a riparian border on a plateau above an incline and is characterized by well-drained Chili loam and Geeburg-Glenford silt loam complex soils (fine-loamy Alfisols), with poorly drained vernal pools. The bottomland zone is located at the bottom of the incline within 80 m of the West Branch of the Mahoning River and is characterized by the Geeburg-Glenford silt loam complex and Holly silt loam soil series (a fine-loamy Inceptisol) (Blackwood et al. 2013; Valverde-Barrantes et al. 2015). These distinct spatial zones with different edaphic factors (bulk density, water holding capacity, total nitrogen, and carbon content) were selected to examine the impact of existing vernal pool soil conditions on biogeochemical process rates. Five soils cores from five vernal pools were sampled in the upland (UL) and bottomland (BL) zones. Samples were taken in spring (mid-June, entirely inundated with water) and fall (late September to early October, dry with little surface water) of 2016, and winter (late February-early March, covered with ice) of 2017. We were unable to sample in summer due to extenuating circumstances.
Sample Collection and Processing
Five soil core samples (~7.62 cm long, with litter layer) were randomly collected from each of the ten vernal pools with a soil corer and placed into polyvinyl chloride (PVC) tubes (diameter = 2.5 cm, length = 15.24 cm, volume = 74.80 cm3) and sealed at both ends with rubber septa (N = 25 tubes per zone). Soil cores were transferred to PVC tubes in spring, without any concerted effort to drain water. In winter, the frozen surface layer of water from the upper portion of soil cores was removed, before the transfer of soil cores to PVC tubes without any effort to drain water. N2O emission rates was measured immediately in the field using the acetylene inhibition method (Groffman and Turner 1995). Because soil temperatures in wetlands vary and are dependent on factors, such as the presence of surface water (Kurylyk et al. 2019) and depth, we incubated samples at prevailing atmospheric temperatures during sampling periods. Briefly, 5 ml of acetylene gas was injected into each tube and point 0 (t0) (5 ml) headspace samples collected and injected into evacuated 25 ml glass vials for storage. Three additional 5 ml headspace samples were collected at 20-min intervals for 1 h in the field. At each sampling point, 5 ml of headspace removal was followed by re-injection of 5 ml of acetylene into each tube to make up headspace volume and pressure. Samples were incubated in the field at atmospheric temperatures in between sampling periods. To minimize additional disturbances following the initial removal of soil core samples, they were immediately transferred into the PVC tubes. PVC tubes were incubated on adjacent dry sites to avoid variations associated with differences in depth of surface water and sizes of vernal pools within and across seasons. N2O and CH4 concentrations were quantified via gas chromatography using a Shimadzu GC-2014 Gas Chromatograph equipped with an electron capture detector (ECD) for N2O (detector temperature: 350 °C) and a flame ionization detector (FID) for CH4 (detector temperature: 250 °C). Denitrification rates and methanogenesis rates were calculated from the linear increases (accumulation) in both N2O and CH4 gas concentrations (in parts per million, ppm or μg/g) over time (slope), standardized per gram of dry soil (dm), and multiplied by headspace volume within PVC tubes, then divided by area of PVC tubes used as μg N2O m−2 h−1 (Collier et al. 2014).
After gas sample collection, soil core samples were transported to the laboratory on ice. In the laboratory, total fresh weights of soil core samples were determined. Soil samples were then mixed thoroughly without sieving and divided into four subsamples. One subsample was used right away in the laboratory for determination of soil moisture content by drying for 48 h at 60 °C, and percent soil organic matter by combusting at 500 °C for 6 h. The second subsample (stored at −20 °C) was used to determine soil nitrate concentrations after incubation in 2 M KCl with shaking for 90 min to extract soil nitrate followed by spectrophotometric quantification of nitrate concentrations via Vanadium (III) reduction (Miranda et al. 2001). The third subsample (~3–5 g) (stored at −20 °C) used to determine soil pH after incubation in 1 M KCL with shaking for 60 min, using a Delta 320 pH meter (Mettler, Toledo, OH, USA). The final subsample (stored −80 °C) was used for DNA extraction.
DNA Extraction and Molecular Analyses
The fourth subsample (~0.2–0.3 g) was used for DNA extraction. Samples were homogenized via vortexing for 15 min before DNA extraction with Qiagen DNeasy PowerSoil DNA extraction kit (Qiagen Inc., Germantown, MD, USA). Bacterial 16S rDNA gene was confirmed in samples using the universal forward (357F) and reverse (1391R) bacterial primer pair (10 mM each) (Turner et al. 2007). The abundances of five genes were quantified: bacterial 16S rRNA gene, nitrite reductase gene nirS, [responsible for reducing nitrite (NO−2) to nitric oxide (NO)], nitric oxide reductase gene, norB [responsible for reducing nitric oxide (NO) to nitrous oxide (N2O)], nitrous oxide reductase gene nosZ (responsible for the final reduction of N2O to N2 gas) (Moreno-Vivián et al. 1999), and methyl co-enzyme M reductase, subunit A gene (mcrA), which catalyzes the synthesis of methane from methyl-coenzyme M (Friedrich 2005). Gene abundances were quantified via quantitative polymerase chain reaction (qPCR) in a subset of vernal pool soil core DNA samples (five vernal pools per location and three replicates per vernal pool across three sampling periods). Primer pairs obtained from the literature were used to quantify the abundances of a 180 bp 16S rDNA fragment (Fierer et al. 2005), a 256 bp nirS fragment (Graham et al. 2010), a 389 bp cnorB fragment (Braker and Tiedje 2003), a 259 bp nosZ fragment from the nosZ clade I (Henry et al. 2006; Jones et al. 2013), and a 438 bp mcrA fragment (Luton et al. 2002). Each 20 μl qPCR reaction mixture contained template DNA, PerfecTa SYBR Green FastMix (Quanta bio, Beverly, MA, USA), water, and primers (0.2 μM each). qPCR reactions were carried out with a Stratagene MX3005P Real-time PCR System (Agilent Technologies, Santa Clara, CA, USA). Primer information and qPCR conditions for these genes are shown in Table 1. All runs were followed by a melt curve step comprised of 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s, in order to assess PCR efficiency (i.e., presence of a single distinct peak for each gene). Standard curves for runs were generated using serial dilutions of plasmids acquired following cloning using the TOPO TA Cloning kit (Thermo Fisher Scientific, Waltham, MA, USA). Gene abundances were calculated as copy number/g dry weight. Standards were plasmids containing 16S rRNA, nirS, norB, and nosZ genes from Pseudomonas aeruginosa (ATCC number BAA-47; GenBank accession number AE004091), and a plasmid containing the mcrA gene from Methanosarcinia acetivorans.
Statistical Analyses
All variables were log-transformed for normality before statistical analysis. Analyses were carried out in JMP Pro 13 (SAS Inc. NC, USA). A mixed model analysis (equivalent to a repeated-measures ANOVA) with a residual covariance structure was used to examine the impact of season, vernal pool location, and their interaction on measured variables and rates. Season, location (BL and UL), and season x location were fixed effects, and individual vernal pools were treated as random effects to account for lack of independence among samples from individual sites within and across seasons, followed by post hoc student’s t test for pairwise comparisons. Data used in the mixed model analyses are provided in Table S1. Subsequently, stepwise multiple regression analyses were carried out to determine the abiotic (nitrate concentration, % organic matter, soil moisture, and pH) and microbial (16S rDNA, nirS, norB, nosZ, and mcrA) variables that were the best predictors of N2O and CH4 emission rates rates. Analyses were carried out using an all possible combination fit model option with selection of the most parsimonious model according to the lowest Akaike information criterion (AIC). The all possible combination fit model option performs variable selection, parameter estimation, as well as minimizes/eliminates collinearity among variables. Best models in each instance were selected based on a combination of lowest AIC, highest R2, and the Mallows statistic value, Cp, which best approximates the number of predictors used in generating the regression model (Mallows 1973). Finally, least-squares linear regression analyses were used to examine individual relationships among identified predictors and N2O and CH4 emission rates rates. Data used in the stepwise regression analyses are provided in Table S2.
Results
Measured N2O emission rates ranged from 0 to 17.4 μg N2O/m2/h across all seasons and sites. There was a significant season by site interaction (P = 0.01), indicating that the impact of location on rates was different across seasons. Within seasons, N2O emission rates in bottomland (BL) vernal pools was ~35% higher relative to upland (UL) vernal pools in fall and winter; rates were higher in BL vernal pools (~ 46%) than in UL vernal pools in winter (P = 0.01), but not in fall (Fig. 1a). In contrast, N2O emission rates rates were higher in UL vernal pools (~ 12%) relative to BL vernal pools in spring, but not statistically significant (Fig. 1a). Furthermore, by site, rates in UL vernal pool sites differed significantly among seasons, whereas denitrification rates in BL vernal pool sites did not (Fig. 1a). Across seasons, average N2O emission rates in bottomland (BL) vernal pools (23.3 ± 3.14 × 10−7, mean ± S.E.M, standard error of the mean) was slightly higher than in upland (UL) pools (21.4 ± 3.20 × 10−7), although this was not statistically significant. Finally, there was an overall seasonal effect on N2O emission rates (P < 0.0001). Rates were highest in spring (3.66 ± 0.53 × 10−6, mean ± S.E, standard error), intermediate in fall (1.83 ± 0.21 × 10−6) and lowest in winter (1.10 ± 0.13 × 10−6) (Fig. 1a).
N2O and CH4 emission rates across seasons. Season, location, and season x location for a N2O emission rates (μg N2O m−2 h−1 × 10−6) and b CH4 emission rates (μg CH4 m−2 h−1 × 10−6) from bottomland (BL) and upland (UL) vernal pools soil. In the figure, *, **, and *** represents significant differences among seasons, and different letters represent significant season by location differences at P = 0.05. N2O and CH4 emission rates are non-transformed in the figure. Bars represent standard error of means
Measured CH4 emission rates ranged from 1.56 × 10−6 to 30.06 × 10−4 μg CH4/m2/h across all sites and dates. Season had a significant impact (P < 0.0001), with rates highest in spring (21.03 ± 7.05 × 10−5, mean ± S.E), followed by fall (4.80 ± 1.22 × 10−5), and lowest in winter (1.22 ± 0.27 × 10−5) (Fig. 1b). There was no season by site interaction (P = 0.50) nor significant site effect (P = 0.22), CH4 emission rates were on average higher in BL vernal pools (12.0 ± 4.4 × 10−5) relative to UL pools (6.3 ± 2.42 × 10−5) throughout the study.
Vernal pool soil abiotic variables are summarized in Table 2. Season also significantly affected soil moisture (P < 0.0001), soil % organic matter content (P < 0.0001), and pH (P < 0.001). On average, soil moisture (g H2O/g dry soil) was highest in spring (1.87 ± 0.20) and winter (1.90 ± 0.14), and lowest in fall (0.86 ± 0.07). Soil % organic matter was highest in fall (10.61 ± 0.62%) and significantly lower in winter (5.98 ± 0.47) and spring (6.28 ± 0.28). Finally, soil pH was high in fall (4.90 ± 0.07), intermediate in spring (4.45 ± 0.04), and least in winter (4.20 ± 0.21). There were no significant effects of site or season by site interactions for these variables. There was, however, was a significant season (P = 0.003) and site (P = 0.0005) effect on soil nitrate concentrations. On average, vernal pool soil nitrate concentrations were highest in fall (0.78 ± 0.22, μg NO3/g dm), intermediate in winter (0.29 ± 0.04), and lowest in spring (0.16 ± 0.04). Average nitrate concentration was significantly higher in bottomland vernal pools (0.70 ± 0.09) relative to upland vernal pools (0.13 ± 0.12) across seasons. There was no significant season by site interaction on vernal pool soil nitrate concentrations (P = 0.07).
Season significantly impacted abundances of microbial 16S rDNA (P < 0.0001) and norB (P < 0.0001) gene abundances, with no significant site effect or season by site interactions (Fig. 2). On average, 16S rRNA gene abundance was highest in winter (125.3 ± 9.0 × 105, copy number/g dry mass), followed by fall (71.7 ± 8.7 × 105), and spring (23.4 ± 2.5 × 105). norB gene abundance was highest in fall (14.9 ± 1.5 × 105), followed by winter (5.6 ± 0.50 × 105) and spring (1.6 ± 0.18 × 105). In contrast, season (P = 0.07), site (P = 0.20), and their interaction (P = 0.62) did not significantly impact nosZ gene abundances, whereas the interaction between site and season significantly impacted nirS gene abundances (P = 0.04). On average, nirS gene abundance was highest in fall (1.2 ± 0.20 × 105), and not statistically different (i.e. comparable) in spring (0.53 ± 0.11 × 105) and winter (0.78 ± 0.13 × 105). The abundances of the nirS gene were higher in BL pools than UL pools across seasons (Fig. 2). Finally, the abundance of the methanogenesis-related mcrA gene was only significantly impacted by season (P < 0.0001) (Fig. 2). mcrA gene abundances were highest in the spring (1.1 ± 0.35 × 105, copy number/g dry mass), reduced in fall (0.16 ± 0.04 × 105), and lowest in winter (0.025 ± 0.0053 × 105) following the methane emission rate pattern.
Changes in gene abundances across seasons. Raw gene abundances (copy number g −1 dry mass × 106) of bacterial, denitrification-related, and methanogenesis-related genes within and across seasons for bottomland and upland vernal pool soil core samples across seasons. *, **, and *** represents significant differences among seasons, and different letters represent significant season by location differences at P = 0.05. Bacterial abundances are non-transformed in the figure. Bars represent standard error of means
From the stepwise multiple regression analyses, a combination of the abiotic (% organic matter and nitrate concentration) and microbial (16S rDNA, nirS, and norB gene abundances) variables emerged as the best set of predictors of N2O emission rates (AICc = 38.70, R2 = 0.46, Cp = 5.87; number of predictors = 5) (Table 3). The coefficients of the abiotic variables in the regression model were positively related to N2O emission rates, as expected. However, the coefficients of the microbial variables were negatively related (Table 3). Soil organic matter, soil pH, and mcrA gene abundances were selected as the best set of predictors of CH4 emission rates (AICc = 133.6, R2 = 0.36, Cp = 2.13; number of predictors = 4). All three coefficients were positively related (Table 3). Data used in the stepwise regression analyses are provided in Table S2.
Discussion
Soil moisture and atmospheric temperature are recognized major drivers of landscape-level biogeochemical process rates due to their respective impacts on soil oxygen availability (increasing soil anaerobiosis) and increased microbial enzymatic activities in well-studied wetland systems (Butterbach-Bahl et al. 2013; Holgerson 2015). However, the impacts of other drivers and more importantly, their interactions, on these landscape-level biogeochemical process rates and the ability to predict landscape-level responses in less-studied systems remains a challenge (Butterbach-Bahl et al. 2013). Capps et al. (2014) in a seminal study, demonstrated that vernal pools within a temperate forest landscape were a significant nitrogen removal “hotspots” relative to broader adjacent terra firma sites, and suggested the need for further spatial and temporal examinations of biogeochemical process rates in these systems. We uncovered significant season- and site-specific differences in N2O emission rates and significant seasonal differences in CH4 emission rates, as well as season- and site-specific differences among measured soil abiotic and microbial variables in vernal pool soil samples in this study. The seasonal patterns of N2O and CH4 emission rates from vernal pools in this study are consistent with reported seasonal patterns from other wetlands, such as vernal pools (Kutzbach et al. 2004; Capps et al. 2014), ditches (Hansen et al. 2016), and estuaries and mangrove systems (Allen et al. 2007). Overall, our examination of drivers of both emission rates in vernal pools suggests that the impacts of biotic and abiotic variables on these rates are context-dependent and complex, underscoring the influence of spatial and temporal heterogeneity within landscapes on biogeochemical processes.
Drivers of Denitrification Rates
Organic matter and nitrate concentration were significant and positive predictors of denitrification rates in this study, as expected because this process utilizes organic matter as an electron donor and nitrate as an electron acceptor in the first step of denitrification (i.e., nitrate reduction) (Hansen et al. 2016; Tomasek et al. 2017; Sabba et al. 2017). The positive impacts of soil nitrate and organic matter content are consistent with other studies that demonstrate positive influences of nitrate concentrations on denitrification rates in sediments (Kjellin et al. 2007; Groffman et al. 2009; Song et al. 2012) from comparatively larger wetland types, agricultural soils (Signor and Cerri 2013), and one singular study of vernal pools (Carrino-Kyker et al. 2012), as well as positive impacts of organic matter on denitrification rates across a variety of systems (Brentrup et al. 2000; Cameron et al. 2013; Capps et al. 2014). The importance of soil nitrate and organic matter content in predicting denitrification rates in wetlands, in general, might be particularly crucial in vernal pools in temperate forests. This is because these vernal pools receive significant seasonal organic matter input from external sources (leaf fall from surrounding tree stands, and insect remains), as well as experience considerable changes in temperature due to their shallow depths (Williams 2006; Kurylyk et al. 2019). These seasonal inputs vary among vernal pools in different locations in an ecosystem, and can potentially impact physicochemical properties of vernal pools under rapidly changing season-associated environmental conditions (pH, nitrate, and temperature) leading to different seasonal and site rates (Brentrup et al. 2000; Cameron et al. 2013). Differences organic matter content and overall N2O emission rates between BL and UL sites in this study may be related to differences in dominant forest tree species (and consequently, leaf input), the decomposition rates of these leaf inputs, and underlying soil properties (bulk density, water holding capacity, etc.,), and at the specific study sites as previously reported (Blackwood et al. 2013). The UL site is dominated by red maple (Acer rubrum) and sugar maple (Acer saccharum) followed by American beech (Fagus grandifolia), and black cherry (Prunus serotina), whereas the BL site is dominated by Acer rubrum, Spanish oak (Quercus palustris), black ash (Fraxinus nigra), American sycamore (Palanus occidentalis), and bitternut hickory (Carya cordiformis) (Blackwood et al. 2013). The presence of beech in the UL site and its complete absence in the BL site may impact decomposition rates (and consequently higher organic matter and lower nitrate content in UL sites), as beech litter tends to have slower decomposition rates compared to other temperate forest tree species (Gosz et al. 1973; Berger et al. 2015). This results in overall slower decomposition rates in mixed tree litter input studies (Jacob et al. 2010). The high soil nitrate content in fall may be due to high microbial and macroinvertebrate decomposing activities following leaf litter input), whereas the intermediate nitrate content by winter may be due to slowed down microbial and macroinvertebrate decomposing activities, and lowest nitrate content in spring may be attributed to thawing and increased microbial utilization and possibly leaching into deeper layers of vernal pool soils.
Interestingly, soil moisture was not a significant predictor of N2O emission rates in this study. High soil moisture, and consequently low dissolved oxygen, are essential variables that often drive soil denitrification rates in terrestrial systems, but are not as crucial in wetland ecosystems (Vargas et al. 2018). Major wetland systems experience consistently low dissolved oxygen and high moisture content, leaving other variables, such as organic matter and nitrate content as the limiting variables (Taylor and Townsend 2010; Hansen et al. 2016). Unlike larger and deeper wetlands, the shallow and ephemeral nature of vernal pools dictates that, at some point, there is water limitation. In this study, there was a 54% decrease in soil moisture content in vernal pools in fall relative to spring. Although considerable, this 54% decrease in soil moisture did not appear to be a significant driver of N2O emission rates. A possible explanation of this might be that there was reduced evaporative water loss (desiccation) from vernal pools as a result of increased forest vegetation cover during the fall. Thus, perhaps, forested vernal pools that are shaded by dense vegetation during periods of high temperature and low rainfall, may not be completely desiccated even if there is no actual surface water. It remains to be determined if this is solely a climatic effect of the study period, or if this relationship between moisture content and denitrification rates in vernal pools changes with varying annual temperature and precipitation regimes.
There were three significant microbial predictors of N2O emission rates from vernal pools (16S rRNA, nirS, and norB); however, abundances of these genes were all unexpectedly negatively related to N2O emission rates. This trend did not change following normalizing functional gene copy numbers to 16S rRNA gene copy numbers (data not shown). These denitrification-related microbial gene abundances have been used as indicators or predictors of denitrification in several studies, with mixed results. For example, studies have reported positive correlations between denitrification rates and nirS gene abundances from a variety of systems (Morales et al. 2010; Braker et al. 2010; Graham et al. 2010; Saarenheimo et al. 2015; Yang et al. 2017; Tomasek et al. 2017), suggestive of linkages between gene abundances and process rates since all denitrifiers have the nir genes (Zumft 1997). The detection of nirS is crucial because it is a definitive marker for denitrifying bacteria and is not found in nitrifying bacteria, unlike nirK (Casciotti and Ward 2006, 2001). Additionally, most nirS-containing (nitric oxide, NO-producing) denitrifiers also contain the norB (nitrous oxide, N2O- producing) gene (Philippot 2002; Zumft 1997), and have similar gene expression patterns (Yoshida et al. 2012). This co-occurrence is significant because NO is toxic to bacteria and requires a rapid and efficient conversion of NO to N2O (Sabba et al. 2017). However, similar to the data for vernal pools reported here, Chen et al. (2015) found a negative correlation between denitrification rates and denitrification-related gene abundances in grassland soils, while other studies have reported no significant correlations (Ducey et al. 2010; Dandie et al. 2011).
The observed negative relationships between gene abundances and N2O emission rates in vernal pools were unexpected. A common explanation for lack of expected positive relationships between gene abundances and microbe-mediated rates is that these gene abundances do not necessarily translate directly to actual gene expression. While this would be adequate to explain a casual relationship, it does not entirely explain the negative relationships observed here. The impacts of environmental variables on specific genotypes or functional groups in driving biogeochemical processes are not straightforward, particularly for denitrification. Denitrification is biochemically similar to aerobic respiration, with denitrification-related genes widespread among phylogenetically diverse facultatively anaerobic bacteria (Martiny et al. 2015). This makes it particularly challenging to assign nitrate reducing or denitrifying functions to the organisms carrying denitrification related genes. For example, fluctuating environmental variables (temperature, salinity) were shown to change the abundances and community compositions of denitrifying bacterial taxa in soils, resulting in incongruent patterns between selected gene abundances and denitrification rates (Braker et al. 2010; Hallin et al. 2012). Thus, in this study, the lack of congruence between gene abundances and denitrification activity may be attributed to specific small populations of denitrifying bacteria selected for by environmental conditions within vernal pools that are poised to take advantage of prevailing conditions but were undetected in this study. The shallow, and organic matter-rich nature of forested vernal pools relative to larger and deeper wetland types and non-forested vernal pools suggest perhaps that factors regulating microbial diversity, abundance, and activity are still largely unknown. Overall, the presence and ubiquity of denitrification genes across several bacterial phyla may have masked the detection of any positive associations between measured N2O emission rates and denitrification gene abundances in vernal pools in this study.
Drivers of Methanogenesis Rates
Vernal pools and small temporary ponds tend to be rich in organic matter and relatively acidic to neutral in pH, emitting disproportionately more methane than surrounding terra firma sites (Kutzbach et al. 2004; Holgerson 2015; Holgerson and Raymond 2016). Much like denitrification, the seasonal relationship between CH4 emission rates and inundation observed in this study is consistent with reported methane emission patterns in vernal pools (Capps et al. 2014) and other types of wetlands (Le Mer and Roger 2001; Allen et al. 2007; Hansen et al. 2016). Positive predictors of methanogenesis in this study were soil pH and organic matter content. The dominant sources of biogenic methane in wetlands are hydrogenotrophic methanogenesis, which is more pronounced in comparatively acidic soils and is limited by hydrogen (H2) availability, and acetotrophic/acetoclastic methanogenesis, which becomes more dominant and relevant when hydrogen has been consumed, as in comparatively alkaline soils (Conrad 1999). Both sources of methane are connected to the availability of organic matter since both substrates are by-products of organic matter fermentation. The relatively high soil organic matter content in the studied vernal pools in this study may explain the positively correlated, but not statistically significant relationship between CH4 emission rates and soil organic matter. Whereas, the positive correlation between CH4 emission rates and the slightly acidic soil pH in this study is perhaps suggestive of hydrogenotrophic methanogenesis underscored by the relatively low pH of the studied vernal pools following fermentation of organic matter (Conrad 1999; Le Mer and Roger 2001; Holgerson 2015; Holgerson and Raymond 2016).
Unlike denitrification, the abundance of the methanogenesis-related mcrA gene was positively and significantly correlated with measured CH4 emission rates. This positive correlation between mcrA gene abundance is consistent with reports from studies in anaerobic reactors (Morris et al. 2014), rice paddies (Ma et al. 2012; Bao et al. 2014), streams (Crawford et al. 2017), and bogs (Milferstedt et al. 2010). This suggests a more direct linkage between mcrA gene abundance and methanogenesis. This linkage may be attributed to the fact that methanogenesis (both hydrogenotrophic and acetotrophic) is an extremely conserved microbial functional trait involving several gene subsystems present in a diverse but phylogenetically unique microbial group (Friedrich 2005; Martiny et al. 2015). Furthermore, unlike denitrifiers, methanogens are obligate anaerobes incapable of surviving using alternate respiratory mechanisms. This complex methanogenesis gene system is not readily amenable to horizontal gene transfer, and thus, the presence of the most conserved methanogenesis gene, mcrA, tends to be a good indicator of methanogenesis rates (Martiny et al. 2015). Thus, in this study, the observed pattern of CH4 emission rates from vernal pools can be primarily attributed to the abundance of methanogens which vary between dry and wet periods in soils (He et al. 2015) and wetlands (Fu et al. 2015), the tightly-regulated methanogenesis gene system, the obligately anaerobic nature of methanogens in contrast to the derived nature of denitrification-related genes, and the heterotrophic nature of denitrifiers and nitrate reducers. This study is the first to our knowledge to report this positive relationship between CH4 emission rates and methanogen abundance in forested vernal pools.
Conclusion
Although soil moisture and atmospheric temperature are conventionally recognized predictors of biogeochemical process rates in wetland systems, we demonstrate in this study that other soil abiotic variables, such as nitrate, pH, and organic matter content may be relevant drivers of biogeochemical processes, such as denitrification and methanogenesis in ephemeral, shallow forested vernal pool wetland systems across seasons. Denitrification and methanogenesis rates in vernal pools in temperate northeastern forests were context-dependent and differentially impacted by microbial and abiotic variables. The spatial and temporal variations of these proximal soil and microbial variables within the studied vernal pools in the forested landscape, and how these impacted measured biogeochemical process rates (i.e., C and N cycling), underscore the “hot spot” nature of these wetland systems within landscapes. Vernal pools may be potential sites of significant N and C removal processes during “hot moments” when factors besides soil moisture and elevated temperature become relevant for vernal pool microbial assemblages. Long-term studies (≤ 2 years of seasonal data) examining the relationships among environmental, and microbial (composition and functional gene abundance) parameters, and biogeochemical process rates in these systems will provide more insights into the controls of these dynamics. Overall, studies of this nature provide insights into other factors that drive biogeochemical process rates in wetlands. They also improve the accuracy of emission factor assignments to wetland ecosystems (varying in size and nature) in greenhouse gas emission models within landscapes for improved predictive capabilities in response to changing climate (Sherlock et al. 2002; Li et al. 2002; Davidson and Kanter 2016; Gaillard et al. 2017).
References
Allen DE, Dalal RC, Rennenberg H et al (2007) Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biology and Biochemistry 39:622–631. https://doi.org/10.1016/j.soilbio.2006.09.013
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proceedings of the National Academy of Sciences 105:11512–11519. https://doi.org/10.1073/pnas.0801925105
Arias-Navarro C, Díaz-Pinés E, Klatt S et al (2017) Spatial variability of soil N2O and CO2 fluxes in different topographic positions in a tropical montane forest in Kenya. Journal of Geophysical Research: Biogeosciences 122:514–527. https://doi.org/10.1002/2016JG003667
Bao Q-L, Xiao K-Q, Chen Z et al (2014) Methane production and methanogenic archaeal communities in two types of paddy soil amended with different amounts of rice straw. FEMS Microbiology Ecology 88:372–385
Bayabil KH, Stoof RC, Mason C et al (2016) Nitrous oxide and methane fluxes from smallholder farms: a scoping study in the Anjeni watershed. Climate. https://doi.org/10.3390/cli4040062
Berger TW, Duboc O, Djukic I et al (2015) Decomposition of beech (Fagus sylvatica) and pine (Pinus nigra) litter along an alpine elevation gradient: decay and nutrient release. Geoderma 251–252:92–104. https://doi.org/10.1016/j.geoderma.2015.03.024
Blackwood CB, Smemo KA, Kershner MW et al (2013) Decay of ecosystem differences and decoupling of tree community–soil environment relationships at ecotones. Ecological Monographs 83:403–417. https://doi.org/10.1890/12-1513.1
Braker G, Tiedje JM (2003) Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Applied and Environmental Microbiology 69:3476–3483. https://doi.org/10.1128/AEM.69.6.3476-3483.2003
Braker G, Schwarz J, Conrad R (2010) Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiology Ecology 73:134–148. https://doi.org/10.1111/j.1574-6941.2010.00884.x
Brentrup F, Küsters J, Lammel J, Kuhlmann H (2000) Methods to estimate on-field nitrogen emissions from crop production as an input to LCA studies in the agricultural sector. The International Journal of Life Cycle Assessment 5:349. https://doi.org/10.1007/BF02978670
Brooks RT, Hayashi M (2002) Depth-area-volume and hydroperiod relationships of ephemeral (vernal) forest pools in southern New England. Wetlands. https://doi.org/10.1672/0277-5212(2002)022[0247:DAVAHR]2.0.CO;2
Butterbach-Bahl K, Baggs EM, Dannenmann M et al (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society B: Biological Sciences 368:20130122. https://doi.org/10.1098/rstb.2013.0122
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Annals of Applied Biology 162:145–173. https://doi.org/10.1111/aab.12014
Capps KA, Rancatti R, Tomczyk N et al (2014) Biogeochemical hotspots in forested landscapes: the role of vernal pools in denitrification and organic matter processing. Ecosystems 17(8):1455–1466
Carrino-Kyker SR, Swanson AK (2007) Seasonal physicochemical characteristics of thirty northern Ohio temporary pools along gradients of GIS-delineated human land-use. Wetlands: The journal of the Society of the Wetland Scientists 27:749–760
Carrino-Kyker SR, Swanson AK (2008) Temporal and spatial patterns of eukaryotic and bacterial communities found in vernal pools. Applied and Environmental Microbiology 74:2554–2557. https://doi.org/10.1128/AEM.01482-07
Carrino-Kyker S, Smemo K, Burke D (2012) The effects of pH change and NO−3 pulse on microbial community structure and function: a vernal pool microcosm study. FEMS Microbiology Ecology 81:660–672
Chapuis-Lardy L, Bernoux M, Chotte JL et al (2006) Soils, a sink for N2O? A review. Global Change Biology 13:1–17. https://doi.org/10.1111/j.1365-2486.2006.01280.x
Chen Z, Wang C, Gschwendtner S et al (2015) Relationships between denitrification gene expression, dissimilatory nitrate reduction to ammonium and nitrous oxide and dinitrogen production in montane grassland soils. Soil Biology and Biochemistry 87:67–77. https://doi.org/10.1016/j.soilbio.2015.03.030
Collier SM, Ruark MD, Oates LG et al (2014) Measurement of greenhouse gas flux from agricultural soils using static chambers. Journal of Visualized Experiments: JoVE:52110. https://doi.org/10.3791/52110
Conrad R (1999) Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiology Ecology 28:193–202
Crawford JT, Loken LC, West WE et al (2017) Spatial heterogeneity of within-stream methane concentrations. Journal of Geophysical Research: Biogeosciences 122:1036–1048. https://doi.org/10.1002/2016JG003698
Dandie CE, Wertz S, Leclair CL et al (2011) Abundance, diversity and functional gene expression of denitrifier communities in adjacent riparian and agricultural zones. FEMS Microbiology Ecology 77:69–82
Davidson E, Kanter D (2016) Inventories and scenarios of nitrous oxide emissions. https://doi.org/10.1201/b20720-11
Ducey TF, Shriner AD, Hunt PG (2010) Nitrification and denitrification gene abundances in swine wastewater anaerobic lagoons. Journal of Environmental Quality - Waste Management 40:610–619
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Applied and Environmental Microbiology 71:4117–4120. https://doi.org/10.1128/AEM.71.7.4117-4120.2005
Friedrich MW (2005) Methyl-coenzyme M reductase genes: unique functional markers for methanogenic and anaerobic methane-oxidizing archaea. Methods in enzymology. Academic Press, pp 428–442
Fu L, Song T, Lu Y (2015) Snapshot of methanogen sensitivity to temperature in Zoige wetland from Tibetan plateau. Frontiers in Microbiology 6:131. https://doi.org/10.3389/fmicb.2015.00131
Gaillard RK, Jones CD, Ingraham P et al (2017) Underestimation of N2O emissions in a comparison of the DayCent, DNDC, and EPIC models. Ecological Applications 28:694–708. https://doi.org/10.1002/eap.1674
Gosz JR, Likens GE, Bormann FH (1973) Nutrient release from decomposing leaf and branch litter in the Hubbard brook forest, New Hampshire. Ecological Monographs 43:173–191. https://doi.org/10.2307/1942193
Graham DW, Trippett C, Dodds WK et al (2010) Correlations between in situ denitrification activity and nir-gene abundances in pristine and impacted prairie streams. Environmental pollution (Barking, Essex: 1987) 158:3225–3229. https://doi.org/10.1016/j.envpol.2010.07.010
Groffman PM, Turner CL (1995) Plant productivity and nitrogen gas fluxes in a tallgrass prairie landscape. Landscape Ecology 10:255–266. https://doi.org/10.1007/BF00128993
Groffman PM, Butterbach-Bahl K, Fulweiler RW et al (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93:49–77. https://doi.org/10.1007/s10533-008-9277-5
Hallin S, Welsh A, Stenström J et al (2012) Soil functional operating range linked to microbial biodiversity and community composition using denitrifiers as model guild. PLoS One 7:e51962
Hansen A, Dolph C, Finlay A (2016) Do wetlands enhance downstream denitrification in agricultural landscapes? Ecosphere 7:e01516. https://doi.org/10.1002/ecs2.1516
He S, Malfatti SA, McFarland JW et al (2015) Patterns in wetland microbial community composition and functional gene repertoire associated with methane emissions. mBio 6:e00066-15
Henry S, Bru D, Stres B et al (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Applied and Environmental Microbiology 72:5181–5189. https://doi.org/10.1128/aem.00231-06
Holgerson MA (2015) Drivers of carbon dioxide and methane supersaturation in small, temporary ponds. Biogeochemistry 124:305–318. https://doi.org/10.1007/s10533-015-0099-y
Holgerson MA, Raymond PA (2016) Drivers of carbon dioxide and methane supersaturation in small, temporary ponds. Nature Geoscience 9:222
Jacob M, Viedenz K, Polle A, Thomas FM (2010) Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 164:1083–1094. https://doi.org/10.1007/s00442-010-1699-9
Jones CM, Graf DRH, Bru D et al (2013) The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. The ISME Journal 7:417–426. https://doi.org/10.1038/ismej.2012.125
Kjellin J, Hallin S, Wörman A (2007) Spatial variations in denitrification activity in wetland sediments explained by hydrology and denitrifying community structure. Water Research 41:4710–4720. https://doi.org/10.1016/j.watres.2007.06.053
Knowles R (1982) Denitrification. Microbiological Reviews 46:43–70
Kurylyk BL, Irvine DJ, Bense VF (2019) Theory, tools, and multidisciplinary applications for tracing groundwater fluxes from temperature profiles. Wiley Interdisciplinary Reviews: Water 6:e1329. https://doi.org/10.1002/wat2.1329
Kutzbach L, Wagner D, Pfeiffer E-M (2004) Effect of microrelief and vegetation on methane emission from wet polygonal tundra, Lena Delta, northern Siberia. Biogeochemistry 69:341–362. https://doi.org/10.1023/B:BIOG.0000031053.81520.db
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. European Journal of Soil Biology 37:25–50. https://doi.org/10.1016/S1164-5563(01)01067-6
Li C, Stange F, Zechmeister-Boltenstern S et al (2002) A process-oriented model of N2O and NO emissions from forest soils: 2. Sensitivity analysis and validation. Journal of Geophysical Research: Atmospheres 105:4385–4398. https://doi.org/10.1029/1999jd900948
Liu C, Wang K, Meng S et al (2011) Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China. Agriculture, Ecosystems & Environment 140:226–233. https://doi.org/10.1016/j.agee.2010.12.009
Luton PE, Wayne JM, Sharp RJ, Riley PW (2002) The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 148:3521–3530. https://doi.org/10.1099/00221287-148-11-3521
Ma K, Conrad R, Lu Y (2012) Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Applied and Environmental Microbiology 78:445–454. https://doi.org/10.1128/AEM.06934-11
Mallows CL (1973) Some comments on Cp. Technometrics 15:661–675. https://doi.org/10.1080/00401706.1973.10489103
Martiny JBH, Jones SE, Lennon JT, Martiny AC (2015) Microbiomes in light of traits: a phylogenetic perspective. Science 350:aac9323. https://doi.org/10.1126/science.aac9323
Mayorga E, Dent CL, McClain ME et al (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312. https://doi.org/10.1007/s10021-003-0161-9
Milferstedt K, Youngblut ND, Whitaker RJ (2010) Spatial structure and persistence of methanogen populations in humic bog lakes. The ISME Journal 4:764–776
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71. https://doi.org/10.1006/niox.2000.0319
Morales SE, Cosart T, Holben WE (2010) Bacterial gene abundances as indicators of greenhouse gas emission in soils. The ISME Journal 4:799–808
Moreno-Vivián C, Cabello P, Martínez-Luque M et al (1999) Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. Journal of Bacteriology 181:6573–6584
Morris R, Schauer-Gimenez A, Bhattad U et al (2014) Methyl coenzyme M reductase (mcrA) gene abundance correlates with activity measurements of methanogenic H2/CO2-enriched anaerobic biomass. Microbial Biotechnology 7:77–84. https://doi.org/10.1111/1751-7915.12094
Saarenheimo J, Tiirola MA, Rissanen AJ (2015) Functional gene pyrosequencing reveals core proteobacterial denitrifiers in boreal lakes. Frontiers in Microbiology 6:674. https://doi.org/10.3389/fmicb.2015.00674
Sabba F, Picioreanu C, Nerenberg R (2017) Mechanisms of nitrous oxide (N2O) formation and reduction in denitrifying biofilms. Biotechnology and Bioengineering 114:2753–2761. https://doi.org/10.1002/bit.26399
Sherlock RR, Sommer SG, Khan RZ et al (2002) Ammonia, methane, and nitrous oxide emission from pig slurry applied to a pasture in New Zealand. Journal of Environmental Quality 31:1491–1501. https://doi.org/10.2134/jeq2002.1491
Signor D, Cerri CEP (2013) Nitrous oxide emissions in agricultural soils: a review. Pesquisa Agropecuaria Tropical 43:322–338
Song K, Kang H, Zhang L, Mitsch WJ (2012) Seasonal and spatial variations of denitrification and denitrifying bacterial community structure in created riverine wetlands. Ecological Engineering 38:130–134. https://doi.org/10.1016/j.ecoleng.2011.09.008
Taylor PG, Townsend AR (2010) Supplementary information: stoichiometric control of organic carbon–nitrate relationships from soils to the sea. Nature 464:1178–1181. https://doi.org/10.1038/nature08985
Tomasek A, Kozarek JL, Hondzo M et al (2017) Environmental drivers of denitrification rates and denitrifying gene abundances in channels and riparian areas. Water Resources Research 53:6523–6538. https://doi.org/10.1002/2016WR019566
Turner S, Pryer MK, Miao VP, Palmer J (2007) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. Journal of Eukaryotic Microbiology 46:327–338. https://doi.org/10.1111/j.1550-7408.1999.tb04612.x
Valverde-Barrantes OJ, Smemo KA, Feinstein LM et al (2015) Aggregated and complementary: symmetric proliferation, overyielding, and mass effects explain fine-root biomass in soil patches in a diverse temperate deciduous forest landscape. New Phytologist 205:731–742. https://doi.org/10.1111/nph.13179
Vargas R, Sánchez-Cañete PE, Serrano-Ortiz P et al (2018) Hot-moments of soil CO2 efflux in a water-limited grassland. Soil Systems. https://doi.org/10.3390/soilsystems2030047
Williams DD (2006) The biology of temporary waters. Oxford University Press (OUP), Oxford
Yang L, Zhang X, Ju X (2017) Linkage between N2O emission and functional gene abundance in an intensively managed calcareous fluvo-aquic soil. Scientific Reports 7:43283. https://doi.org/10.1038/srep43283
Yoshida M, Ishii S, Fujii D et al (2012) Identification of active denitrifiers in rice paddy soil by DNA- and RNA-based analyses. Microbes and Environments 27:456–461. https://doi.org/10.1264/jsme2.ME12076
Zedler PH (2003) Vernal pools and the concept of “ isolated wetlands.”. Wetlands 23:597–607. https://doi.org/10.1672/0277-5212(2003)023
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61:533–616
Acknowledgements
We would also like to acknowledge Jonathan von Gray, Allison Beckwith, and Sohini Bhattacharyya for assisting with sample collection and processing. We want to also acknowledge Professor Gregory Ferry in the Department of Biochemistry and Molecular Biology at the Pennsylvania State University for providing the methanogen used for mcrA plasmid generation. The Department of Biological Sciences and Office of Research and Sponsored Programs at Kent State University provided funding for this study.
Funding
Funding for this study was provided by the Department of Biological Sciences and Office of Research and Sponsored Programs at Kent State University.
Author information
Authors and Affiliations
Contributions
PAA, LGL, and CB conceived and designed the study. PAA, JT, and AR executed the study. PAA, LGL, AB, JT, TEA, and CB wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts Interests
The authors declare no conflict of interest.
Ethical Statement
No animal rights were violated in the execution of this study. Guidelines of the Kent State University’s Office of Research Compliance and Kent State Institutional Animal Care and Use Committee (IACUC) did not apply to the use of insects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ayayee, P.A., Taura, J., Roberto, A.A. et al. Patterns of Denitrification and Methanogenesis Rates from Vernal Pools in a Temperate Forest Driven by Seasonal, Microbial Functional Gene Abundances, and Soil Chemistry. Wetlands 40, 721–731 (2020). https://doi.org/10.1007/s13157-019-01225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01225-z