Abstract
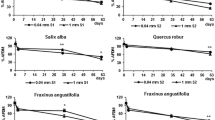
The invasion by emerald ash borer (Agrilus planipennis) of extensive black ash wetlands in the upper Great Lakes region of North America is expected to alter plant community structure and composition and, therefore, abiotic factors like temperature and hydrology. We conducted two experiments to examine how changes in leaf litter could alter ecosystem function via 1) changes in litter breakdown and 2) aquatic invertebrate feeding. For the first experiment, we placed litter bags containing black ash (Fraxinus nigra), swamp white oak (Quercus bicolor), and lake sedge (Carex lacustris), in either control or clear-cut black ash plots. We found that black ash litter broke down 2-3 times faster than other species and broke down faster in control plots than in clear-cuts. There was no effect of clear cutting on swamp white oak or lake sedge breakdown rates. For the second experiment, we fed shredding caddisfly larvae (Limnephilus indivisus) one of six species: black ash, swamp white oak, lake sedge, balsam poplar (Populus balsamifera), American elm (Ulmus americana), or speckled alder (Alnus incana) for 14 days. Caddisflies had the highest survival and greater resource use when given black ash or speckled alder, which are abundant in black ash wetlands. These results suggest that loss of black ash will alter ecosystem processes via changes in the physical environment, changes in leaf litter properties, and changes in shredder processing rates of leaf litter.





Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry, and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Alonso A, González-Muñoz N, Castro-Díez P (2010) Comparison of leaf decomposition and macroinvertebrate colonization between exotic and native trees in a freshwater ecosystem. Ecological Research 25:647–653
Baker TT, Lockaby BG, Conner WH et al (2001) Leaf litter decomposition and nutrient dynamics in four southern forested floodplain communities. Soil Science Society of America Journal 65:1334
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48
Battle JM, Golladay SW (2001) Hydroperiod influence on breakdown of leaf litter in cypress-gum wetlands. The American Midland Naturalist 146:128–145
Battle JM, Golladay SW (2007) How hydrology, habitat type, and litter quality affect leaf breakdown in wetlands on the Gulf coastal plain of Georgia. Wetlands 27:251–260
Batzer PB, Palik BJ, Buech R (2004) Relationships between envrionmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. Journal of the North American Benthological Society 23:50–68
Batzer DP, Dietz-Brantley SE, Taylor BE, DeBiase AE (2005) Evaluating regional differences in macroinvertebrate communities from forested depressional wetlands across eastern and Central North America. Journal of the North American Benthological Society 24:403–414
Batzer DP, Palik BJ (2007) Variable response by aquatic invertebrates to experimental manipulations of leaf litter input into seasonal woodland ponds. Fundamental and Applied Limnology 168:155–162
Batzer DP, Cooper R, Wissinger SA (2014) Wetland animal ecology. In: Batzer DP, Sharitz RR (eds) Ecology of freshwater and estuarine wetlands. University of California Press, Berkeley, pp 242–284
Benfield EF, Webster JR, Golladay SW, Peters GT, Stout BM (1991) Effects of forest disturbance on leaf breakdown in southern Appalachian streams. Verhandlungen des Internationalen Verein Limnologie 24:1687–1690
Bird GA, Kaushik NK (1985) Processing of elm and maple leaf discs by collectors and shredders in laboratory feeding studies. Hydrobiologia 126:109–120
Boulton AJ, Boon PI (1991) A review of methodology used to measure leaf litter decomposition in lotic environments: time to turn over an old leaf? Australian Journal of Marine and Freshwater Research 42:1–43
Boyero L, Barmuta LA, Ratnarajah L et al (2012) Effects of exotic riparian vegetation on leaf breakdown by shredders: a tropical–temperate comparison. Freshwater Science 31:296–303
Britson A, Wardrop D, Drohan P (2016) Plant community composition as a driver of decomposition dynamics in riparian wetlands. Wetlands Ecology and Management 24:335–346
Colburn EA (2004) Vernal pools: natural history and conservation. The McDonald and Woodward Publishing Company, Blacksburg
Covich A, Palmer M, Crowl T (1999) The role of benthic invertebrate species in freshwater ecosystems - Zoobenthic species influence energy flows and nutrient cycling. BioScience 49:119–127
Cox DR (1972) Regression models and life tables. Journal of the Royal Statistical Society, Series B (Methodology 34:187–220
Cross WF, Benstead JP, Frost PC, Thomas SA (2005) Ecological stoichiometry in freshwater benthic systems: recent progress and perspectives. Freshwater Biology 50:1895–1912. https://doi.org/10.1111/j.1365-2427.2005.01458.x
Cummins KW (1974) Structure and function of stream ecosystems. BioScience 24:631–641
D’Amato AW, Palik BJ, Slesak RA et al (2018) Evaluating adaptive management options for black ash forests in the face of emerald ash borer invasion. Forests 9:348
Diamond JS, McLaughlin D, Slesak RA et al (2018) Forested vs. herbaceous wetlands: can management mitigate ecohydrologic regime shifts from invasive emerald ash borer? Journal of Environmental Management 222:436–446
Earl JE, Castello PO, Cohagen KE, Semlitsch RD (2014) Effects of subsidy quality on reciprocal subsidies: how leaf litter species changes frog biomass export. Oecologia 175:209–218
Eggert SL, Wallace JB (2003) Litter breakdown and invertebrate detritivores in a resource-depleted Appalachian stream. Archives of Hydrobiology 156:315–388
Eggert SL, Wallace JB (2007) Wood biofilm as a food resource for stream detrivores. Limnology and Oceanography 52:1239–1245
Ellison AM, Bank MS, Clinton BD et al (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment 3:479–486
Flower CE, Gonzalez-Meler MA (2015) Responses of temperate forest productivity to insect and pathogen disturbances. Annual Review of Plant Biology 66:547–569
Fox J, Weisberg S (2011) An {R} companion to applied regression, 2nd edn. Sage, Thousand Oaks URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion
Gandhi KJK, Herms DA (2010) North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biological Invasions 12:1839–1846
Golladay SW, Webster JR, Benfield EF (1983) Factors affecting food utilization by a leaf shredding aquatic insect: leaf species and conditioning time. Holarctic Ecology 6:157–162
González JM, Graça MAS (2003) Conversion of leaf litter to secondary production by a shredding caddis-fly. Freshwater Biology 48:1578–1592
Graça MAS, Canhoto C (2006) Leaf litter processing in low order streams. Limnetica 25:1–10
Graça MAS, Cressa C, Gessner MO et al (2001) Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshwater Biology 46:947–957
Herbst GN (1982) Effects of leaf type on the consumption rates of aquatic detritivores. Hydrobiologia 89:77–87
Hooker KL, Marzolf GR (1987) Differential decomposition of leaves in grassland and gallery forest reaches of kings creek. Transactions of the Kansas Academy of Science 90:17–24
Hooper DU, Adair EC, Cardinale BJ et al (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50:346–363
Hutchens JJ, Benfield EF, Webster JR (1997) Diet and growth of a leaf-shredding caddisfly in southern Appalachian streams of contrasting disturbance history. Hydrobiologia 346:193–201
Jonsson M, Malmqvist B (2005) Species richness and composition effects in a detrital processing chain. Journal of the North American Benthological Society 24:798–806
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Klemmer AJ, Wissinger SA, Greig HS, Ostrofsky ML (2012) Nonlinear effects of consumer density on multiple ecosystem processes. Journal of Animal Ecology 81:770–780
Klooster WS, Herms DA, Knight KS et al (2014) Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biological Invasions 16:859–873
Kominoski JS, Pringle CM, Ball BA, Bradford MA, Coleman DC, Hall DB, Hunter MD (2007) Nonadditive eff ects of leaf litter species diversity on breakdown dynamics in a detritus-based stream. Ecology 88:1167–1176
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. Journal of Statistical Software 82:1–26
LeRoy CJ, Marks JC (2006) Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshwater Biology 51:605–617
Looney CE, D’Amato AW, Palik BJ et al (2017) The response of Fraxinus nigra forest ground-layer vegetation to emulated emerald ash borer mortality and management strategies in northern Minnesota, USA. Forest Ecology and Management 389:352–363
Looney CE, D’Amato AW, Palik BJ, Slesak RA (2015) Overstory treatment and planting season affect survival of replacement tree species in emerald ash borer threatened Fraxinus nigra forests in Minnesota, USA. Canadian Journal of Research 45:1728–1738
Lousier JD, Parkinson D (1978) Chemical element dynamics in decomposing leaf litter. Canadian Journal of Botany 56:2795–2812
Lovett GM, Weiss M, Liebhold AM et al (2016) Nonnative forest insects and pathogens in the United States: impacts and policy options. Ecological Applications 26:1437–1455
Marcarelli AM, Baxter CV, Mineau MM, Hall RO (2011) Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–1225
Martins RT, Melo AS, Gonçalves JF, Hamada N (2014) Estimation of dry mass of caddisflies Phylloicus elektoros (Trichoptera: Calamoceratidae) in a Central Amazon stream. Zoologia 31:337–342
Mehring AS, Maret TJ (2011) Red maple dominance enhances fungal and shredder growth and litter processing in temporary ponds. Limnology and Oceanography 56:1106–1114
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
MNDNR (2003) Field guide to the native plant communities of Minnesota: the Laurentian mixed Forest Province. Minnesota Department of Natural Resources, St. Paul
Nisbet D, Kreutzweiser D, Sibley P, Scarr T (2015) Ecological risks posed by emerald ash borer to riparian forest habitats: a review and problem formulation with management implications. Forest Ecology and Management 358:165–173
Ostrofsky ML (1997) Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. Journal of the North American Benthological Society 16:750–759
Palik B, Batzer DP, Kern C (2006) Upland forest linkages to seasonal wetlands: litter flux, processing, and food quality. Ecosystems 9:142–151
Palik BJ, Ostry ME, Venette RC, Abdela E (2012) Tree regeneration in black ash (Fraxinus nigra) stands exhibiting crown dieback in Minnesota. Forest Ecology and Management 269:26–30
Peterson RC, Cummins (1974) Leaf processing in a woodland stream. Freshwater Biology 4:343–368
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. Journal of Forestry 104:118–124
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
PRISM Climate Group (2018) PRISM climate data: Recent years (Jan 1981–Jan 2018). Northwest Alliance Computational Science and Engineering. <http://www.prism.oregonstate.edu/recent/>
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. <URL https://www.R-project.org/.>
Reinhart KO, VandeVoort R (2006) Effect of native and exotic leaf litter on macroinvertebrate communities and decomposition in a western Montana stream. Diversity and Distributions 12:776–781
Richardson JS, Mackay RJ (1984) A comparison of the life history and growth of Limnephilus indivisus (Trichoptera:Limnephilidae) in three temporary pools. Archiv für Hydrobiologie 99:515–528
Rodriguez P, Martinez-Madrid M, Arrate JA, Navarro E (2001) Selective feeding by the aquatic oligochaete Tubifex tubifex (Tubificidae, Clitellata). Hydrobiologia 463:133–140
Rubbo MJ, Kiesécker JM (2004) Leaf litter composition and community structure: translating regional species changes into local dynamics. Ecology 85:2519–2525
Santonja M, Pellan L, Piscart C (2018) Macroinvertebrate identity mediates the effects of litter quality and microbial conditioning on leaf litter recycling in temperate streams. Ecology and Evolution 8:2542–2553
Slesak RA, Lenhart CF, Brooks KN et al (2014) Water table response to harvesting and simulated emerald ash borer mortality in black ash wetlands in Minnesota, USA. Canadian Journal of Forest Research 44:961–968
Stephens JP, Berven KA, Tiegs SD (2013) Anthropogenic changes to leaf litter input affect the fitness of a larval amphibian. Freshwater Biology 58:1631–1646
Stoler AB, Burke DJ, Relyea RA (2016) Litter chemistry and chemical diversity drive ecosystem processes in forest ponds. Ecology 97:1783–1795
Stoler AB, Relyea RA (2011) Living in the litter: the influence of tree leaf litter on wetland communities. Oikos 120:862–872
Telander AC, Slesak RA, D’Amato AW et al (2015) Sap flow of black ash in wetland forests of northern Minnesota, USA: hydrologic implications of tree mortality due to emerald ash borer. Agricultural and Forest Meteorology 206:4–11
Therneau T (2015) A package for survival analysis in S version 2.38, <URL: https://CRAN.R-project.org/package=survival>
Webster JR, Waide JB (1982) Effects of forest clearcutting on leaf breakdown in a southern Appalachian stream. Freshwater Biology 12:331–344
Whiles MR, Wallace JB (1997) Leaf litter decomposition and macroinvertebrate communities in headwater streams draining pine and hardwood catchments. Hydrobiologia 353:107–119
Wiggins GB (1973) A contribution to the biology of caddisflies (Trichoptera) in temporary pools. Royal Ontario Museum, Life Sciences Contributions 88:1–28
Wiggins GB (1996) Larvae of the North American caddisfly genera (Trichoptera). 2nd Ed. Univ of Toronto Press
Williams BK, Rittenhouse TAG, Semlitsch RD (2008) Leaf litter input mediates tadpole performance across forest canopy treatments. Oecologia 155:377–384
Williams DD (2005) Temporary forest pools: can we see the water for the trees? Wetlands: Ecology and Management 13:213–233
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wissinger SA, Bohonak AJ, Whiteman HH, Brown WS (1999) Subalpine wetlands in Colorado: habtat permanence, salamander predation, and invertebrate communities. Invertebrates in Freshwater Wetlands of North America: Ecology and Management 757–790
Wissinger SA, Perchik ME, Klemmer AJ (2018) Role of animal detritivores in the breakdown of emergent plant detritus in temporary ponds. Freshwater Science 37:826–835
Youngquist MB, Eggert SL, D’Amato AW et al (2017) Potential effects of foundation species loss on wetland communities: a case study of black ash wetlands threatened by emerald ash borer. Wetlands 37:787–799
Acknowledgements
Funding was provided by Minnesota Environment and Natural Resources Trust Fund to the Legislative Citizens Committee for Minnesota Resources, The Upper Midwest and Great Lakes Landscape Conservation Cooperative, USDA Forest Service Northern Research Station, and The Department of Interior Northeast Climate Adaptation Science Center. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Youngquist, M.B., Wiley, C., Eggert, S.L. et al. Foundation Species Loss Affects Leaf Breakdown and Aquatic Invertebrate Resource Use in Black Ash Wetlands. Wetlands 40, 839–852 (2020). https://doi.org/10.1007/s13157-019-01221-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01221-3