Abstract
Soon for the security of phosphorus world supply, which comes primarily from non-renewable sources, the moderate carbonate phosphates will need further geochemical multidiscipline investigations to participate in the phosphorous supply chains necessary to increase human productivity. Dolomitic phosphates represented the main carbonate phosphate rocks of the Upper Member of Duwi Formation, at Um Queih Mine, South-Western Quseir, that phosphate can be classified as intermediate grade phosphate ore. It was enriched in V, Ni, Mo, U, Cu, Cr, Cd, Co as well as Zn and their ratios indicated that the deposition occurred in anoxic environment (reducing conditions). Mineralogical investigations indicated that the studied phosphorites are composed of two main mineral phases; fluorapatite and non-phosphatic minerals (dolomite, calcite, pyrite, gypsum, and quartz). The petrographic examination revealed that these phosphorites are composed of phosphatic lithoclasts, phosphatic bioclasts, opaques, and quartz grains embedded in a cryptocrystalline phosphatic matrix. The parent rocks of the studied phosphorites represented by basaltic mafic provenance were affected by low chemical weathering and deposited under marine anoxic environment. The mineralogical and geochemical characteristics indicated that the studied phosphorites deposited in marine anoxic condition. The weathering of these rocks can be harmful to the surrounding environment owing to its content of pyrite and potentially toxic elements (PTEs), the EF (enrichment factor) gives extremely high enriched with Mo, Cd, and Se.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phosphorite at Duwi Formation around the area of Quseir–Safaga in Eastern Desert, Egypt attracted the attention of previous authors, e.g., Youssef (1957); Baioumy and Tada (2005); Abou El-Anwar et al. (2017, 2019a), Abou El-Anwar et al. (2022). The model of quadratic regression theoretically was used in multiple practices (Narula and Wellington 2007). The phosphatic rocks contain a lot of million tons of U. It can be extracted as a product of manufactured fertilizers (Abou El-Anwar et al. 2019b). Generally, the occurrence of U in marine phosphorus illustrates either by replacement Ca in the constitution of apatite mineral or the incorporation under mixed set apatite and organic matter (Peter et al. 1986).
Phosphate is one of the most important strategic raw materials, since it is involved in many vital industries, the most important of which is the fertilizer industry. Phosphate is one of the raw materials rich in trace and rare earth elements that are used in many modern technological industries (Abou El-Anwar et al. 2022). Researchers in Egypt have been interested in evaluating phosphate deposits from an economic point of view for the production of phosphoric acid and its derivatives, without addressing the phosphate content of these elements and their environmental and economic importance (El-Kammar et al. 1990; Abou El-Anwar et al. 2017, 2022). Generally, apatite was accumulated in euxinic condition at calm environment where anaerobic microbes attack the organic matter and release PO4 3−. So, this acid medium dissolved the enclosing sediments and released some soluble cations such as Ca2+, Mg2+, etc. Thus, PO3− can react with Ca2+ and Mg2+ to form the transitional mineral “ Struvite (PO4 (Mg) NH4”. This mineral is unbalanced, can be re-precipitated in stable forms (Abou El-Anwar 2019a; Abou El-Anwar, et al. 2019c, 2020).
Potentially toxic elements (PTEs) (e.g., As, Cd, Cr, Pb, Hg, and Ni) are enriched in some natural geological materials such as black shales and phosphatic rocks (Nganje et al. 2020). Phosphatic rocks are the main source for the P-fertilizers production worldwide to improve the fertility and yield of agriculture soil. Salman et al. (2017) concluded that the P-fertilizers are the main source of some PTEs in the agriculture soil of Sohag, Egypt. In some cases, the concentrations of some PTEs are such that economic extraction is viable (Abou El-Anwar et al. 2021). REEs are subjected to enrichment during the authigenesis processes of phosphatic ores, and hence can be used to estimate the paleo-environment (McMurtry 2019).
Thus, the main target of the present article is to give detailed petrographical and geochemical remarks on the diagenesis of the dolomitic phosphates that represented the main phosphatic rocks of Queih Mine (Duwi Formation) at Quseir province, in a try to identify its environmental condition and the economic evolution. Also, to discuss some aspects regarding the environmental conditions through the deposition of the Upper Member of Duwi Formation in Quseir region in its type locality at the Queih Mine.
Materials and methods
Geology of the area
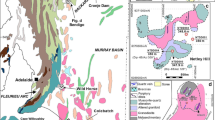
Late Cretaceous phosphorites in Egypt, which make up part of the Middle East to North Africa phosphogenic province with more than 3 billion tons of reserves, are widely distributed in the Eastern Desert, Western Desert, and Nile Valley (Baioumy and Tada 2005). The Duwi Formation, phosphorites bearing, is conformable overlaid by the Quseir variegated shales and underlaid by the Dakhla shales in the Quseir–Safaga zone (Fig. 1a). According to Baioumy and Tada (2005), the Duwi Formation is subdivided into four members—the lower member is composed of quartzose sandstone and siliceous shale; the middle one consists of soft laminated organic-rich black shale; the upper one is composed of phosphatic sandstone and oyster calcarenite in the Red Sea area; the uppermost member is composed of hard massive grayish brown to gray shale, that is mined (underground) in the study area. The thickness of the productive phosphorites bed is about 80 cm. The chosen area is represented by Queih Mine (Fig. 1a, b). It is located at longitudes 34° 05′ 00″ E and latitudes 26° 19′ 60″ N. It is located 5 km north of Al-Hamrawin area and at 25 km north of El-El-Quseir.
Sampling and analyses
The field trip was done to study the geological setting and collect the rock samples of the phosphorites from Queih Mine. The mine is ceased for technical problems, such as many mines in the study area. Eleven samples were collected from the carbonate phosphorites in the horizontal direction of Queih Mine. The petrographic investigations of the 11 dolomitic phosphates samples were done by advanced research microscope. The mineralogical composition of two bulk samples (No. 1 and 9) was studied by the X-ray diffraction (XRD) analyses ((Philips type’PanALytica’ equipment model’X-Pert-PRO’ with’Ni-filter), a Cu-radiation (ƛ = 1.542 Å) of 45 kV, 35 mA, and a normal scanning speed of 0.03◦/sec, the diffractograms were obtained using a range of 2–60◦) at the Egyptian Mineral Resources Authority (Dokki, Egypt).
The chemical composition of the collected 11 bulk samples was performed using XRF (X-ray fluorescence—Axios, Sequential WD- XRF, PANalytical 2005) with reference to the ASTM-E1621 standard. Microstructure characterization or surface morphology was performed using SEM/EDX (scan electron microscope/energy dispersive X-ray—Quanta FEG 250, 30 kV, 14x-350x). All these measurements have been carried out at the laboratories of the NRC (National Research Center).
The Index of Compositional Variability (ICV) values were determined using the concentrations of major oxides as wt. % following Cox et al. (1995) equation:
The Chemical Index of Alteration (CIA) and the Chemical Index of Weathering (CIW) that provide information about the intensity of sediments chemical were calculated using Nesbitt and Young (1982) equations:
Pollution indices were determined using the following equations:
The contamination factor (CF) (Hakanson 1980);
The enrichment factor (EF) (Buat-Menard and Chesselet 1979);
Index of Geoaccumulation (Igeo) (Muller 1979);
where Cm represents the content of the examined element in the phosphate samples, Bm represents the content of the inspected element in the upper continental crust (UCC) (Rudnick and Gao 2014), Rs represents the content of the reference element in the phosphatic ore, and Rc represents the content of the reference element in the UCC. In the current study, Zr had been used to represent the conventional traces that distinguish the natural from anthropogenic components. Generally, Zr is represented as mostly originating from natural origin and have no considerable anthropogenic source (Bam, et al. 2011).
Results and discussion
Petrographical and mineralogical study
Microscopically, the phosphorite at Um Queih area is mainly constituting of collophane, fluorapatite (phosphatic phase), pyrite, and quartz grains embedded in a cryptocrystalline phosphatic matrix according to their decreasing order of abundance (Fig. 2A–D). Dolomite, calcite, gypsum, pyrite, and quartz grains (non-phosphatic phase) occurred in the studied samples in association with the phosphatic phase as identified by the XRD (Fig. 3a). The occurrence of framboidal pyrite indicated the deposition of these sediments in dyoxic marine condition under the effect of microbial activity (Boulemia et al. 2021; Ciobota et al. 2014). The paragenetic sequence of the studied phosphorite is fluorapatite followed by collophane.
Microscopical investigations of Um Queih phosphorite (in PPL. X40): A fluorapatite (colorless subhedral rectangular crystals), collophane (brown grains, pellets, spheroids, ovoids), and dolomite as well as calcite (colorless anhedral grains and rhombohedral crystals) embedded in a cryptocrystalline phosphatic matrix. Collophane is replaced by the phosphatic matrix. B Fluorapatite, collophane, coprolite (brown cylindrical form), and dolomite as well as calcite (colorless anhedral grains and rhombohedral crystals) embedded in a cryptocrystalline phosphatic matrix. C Fluorapatite, collophane, and dolomite as well as calcite (colorless anhedral grains and rhombohedral crystals) embedded in a cryptocrystalline phosphatic matrix. Framboidal pyrite (opaque) scattered in the collophane. D Fluorapatite, collophane, and dolomite as well as calcite (colorless anhedral grains and rhombohedral crystals) embedded in a cryptocrystalline phosphatic matrix
a XRD for the phosphatic rocks of the studied mine. b BSE image and EDX analysis data viewing a domination of the bone remains, halite crystals, dolomite and phosphatic grains scattered in the matrix, EDX recorded P2O5 (10.52%), Ca (30.75%), and Na (3.11%). c BSE image and EDX analysis data viewing an elongated bone fragments and quartz grains scattered in the matrix, P2O5 (18.03%), SiO2 (46.7%). and SO3 (3.82%)
Based on the XRD, the analyzed samples are characterized by two dominant mineral phases; the prominence phosphatic phase, fluorapatite (Fig. 3a). In contrast, the non-phosphatic phase encloses carbonate minerals (mostly dolomite and calcite), evaporated minerals (gypsum), sulphide minerals (pyrite), as well as silicates, expressed as a quartz mineral which was approved by the petrographic study.
SEM and EDX-ray investigation of the dolomitic phosphates was exhibiting the prominence of bone fragmentations, crystalline halite, and quartz grains disassembled in the phosphatic matrix and within phosphatic grains (Fig. 3b and c). EDX recorded P (10.52%), Mg (6.22%), Ca (30.75%), and Na (3.11%), (Fig. 3b). Elongated bone fragment and quartz granules are sprinkled in the phosphatic matrix (Fig. 3c), EDX got P (18.03%), Si (48.71%), and Mg (9.38%).
Generally, Phosphorites, the most important sources of phosphate rock in the Middle East and North Africa, is mineralogically monotonous; composed of carbonate-fluorapatite (called collophane or francolite) (Dar et al. 2017; Howari et al. 2022). Usually, during authigenesis processes, admixtures of detrital impurities from hosting rocks are associated with the carbonate-fluorapatite. Also, this deposit usually contains 5% to 28% P2O5 with some element enrichment such as Sr and Y (McMurtry 2019).
Geochemical characterization
Major oxides features
The studied samples’ geochemical content of the major oxides, traces, REEs and their ratios are recorded in Table 1, and inter-correlation among them is present in Table 2.
Generally, CaO is the main constituent (30.24–38.93%) of the studied dolomitic phosphates followed by P2O5 in the studied phosphatic samples that vary from 16.7 to 20.85% and averaging 19.33%, (Table 1). The strong significant positive relation (r = 0.89) between CaO and P2O5 (Table 2) indicated the presence of apatite, while the high concentration of CaO indicated the presence of other Ca-bearing minerals, gypsum, calcite, and dolomite as confirmed by petrography, XRD, and EDX (Figs. 2 and 3). MgO is the third in the abundance, ranges from 7.01 to 10.01% with an average of 8.42%, and its positive correlation (r = 0.60) with CaO (Table 2) indicted their association in the dolomite mineral, which was verified by petrographic, XRD, SEM, and EDX techniques (Figs. 2 and 3). SiO2 ranges from 6.17 to 8.1% averaging 7.41, revealed the presence of quartz grains which detected with XRD (Fig. 3a). Its strong positive correlation with Fe2O3 (r = 0.8) and weak to moderate with other major elements (Table 2) indicated its presence as detrital grains stained with iron oxides.
The content of Fe2O3 is generally low, averaging 1.96% compared with the matching value for UCC, 4.5% (Taylor and McLennan 1985) and Post-Archean Australian Shale (PAAS), 6.5% (Rudnick and Gao 2014). The high content of Fe2O3 in the studied samples indicates the occurrence of free hydrate iron oxides and iron minerals (pyrite, Figs. 2 and 3a). Thus, iron oxides may be present as authigenic in origin. Al, Na, K, Ti, and F are documented in small percentage ratios. The weak and moderate positive linkage among Al2O3 with both SiO2 and NaO (r = 0.34 and 0.5; respectively), point out their association in aluminosilicate minerals. A positive relationship (r = 0.57) among Na and Cl revealed the existence of halite, which was confirmed with SEM and XRD investigations.
Trace and rare earth elements
The traces and rare earths enrichment in sediments perhaps are coming from seawater, hydrothermal, or diagenesis processing. The moderate traces and rare earths content in Queih phosphatic ores are excess enrichments rather than those in the UCC and PAAS countable (Table 3 and Fig. 4a). Commonly, the examined Queih phosphatic ores are enriched in all most traces, and Y (as REE) except Cr, Ba, Ce, La, and Ga more than those in the UCC and PAAS countable.
a Distribution of trace elements in the studied phosphorites in comparison with other locations. b 3D pyramids plotting showing the distribution of Um, Uc, F, and P2O5 in the studied phosphorites. c Relationship between U and P2O5. d Element ratio paleo-environment classes, star is the current results (Yan et al. 2018), e V/Ni vs. Co/Ni (Galarraga et al. 2008)
Accordingly, REEs conjoined with ore of phosphate can be ascribed to either exchange process through diagenesis or adsorption (McLennan 1989; Kohn and Moses 2013) and/or exposed to the demanding chemical weathering (El-Kammar et al. 1979; Abou El-Anwar et al. 2019b,c).
P2O5 has very intensive positive association with the trace elements; Cr, Cu, Co, Ni, V, Zn, Zr, and Cd, (r = 0.84, 0.43, 0.63, 0.62, 0.81, 0.51, 0.24, and 0.46, respectively, (Table 2), and SiO2 has positive association with some trace metal elements; Cr, Ni, Zr, and V (r = 0.34, 0.14, 0.29, and 0.41, respectively). Thus, traces are mostly associated with phosphatic rocks and/or detrital quartz. La, Ce, Nd, Sc, and Y are represented as the rare earth elements. La has recorded a positive correlation with Si, Mo, Cr, Zr, V, and Rb (r = 0.34, 0.49, 0.44, 0.49, 0.51, and 0.37; respectively) indicating the incorporation of these elements in silicates. Ce has moderate positive correlation with Na, K, and Ni (r = 0.26, 0.30, 0.36, respectively), Ce associated with clay minerals and/or heavy metals. Nd has weak to moderate positive correlation with P, Ca, K, Cr, Cu, Ni, Zn, Cd, and As (r = 0.38, 0.41, 0.34, 0.27, 0.31, 0.49, 0.36, 0.59, and 0.15, respectively, Table 2). Thus, Nd may be coupled with phosphate, clay minerals, and heavy metals. Sc positively correlated with Al, Ti, and Ni (r = 0.28, 0.69, and 0.34, respectively), so it associated with clay minerals and heavy metals. Finley, Y was positively correlated with Zr and Rb (r = 0.28 and 0.65, respectively), so it accumulated in heavy and trace metals.
Some heavy metal elements (such as Cr, Ni, V, As, and Cd) and rare earth elements, Sc are positively linked to Fe2O3, TiO2, and each other, which indicated that these components may be connected with TiO2, or the heavy mineral elements (Table 2), (Abou El-Anwar 2019a).
Dolomitization processes
The positive linkage (r = 0.51) among MgO and P2O5 indicated that the dolomite mineral was associated with the studied phosphatic ores. The dolomite rhombs occur in the thin sections (Fig. 2), SEM (Fig. 3b and c) and XRD indicated the studied phosphatic rocks subjected to the dolomitization effect. The dolomitization process must be attributed to the presence of magnesium (Abou El-Anwar 2019b). Thus, the studied samples represented the first occasion of the dolomite mineral was associated with phosphate rocks. The source of MgO is probable to be the presence of ultramafic rocks in the area. Hence, under the suitably conditions, the magnesium element replaces calcium in calcite and led to the precipitation of dolomite.
There are strong to medium positive relation (r = 0.25, 0.66, 0.48, 0.40, 0.32, and 0.28, respectively) for the heavy metals Co, Zn, Cu, Cr, V, and Ni, respectively, with MgO (Table 2). Also, there is positive correlation between MgO and some toxic and rare earth elements; Cd, Ga, Nd, As, Sc, and U (r = 0.42, 0.41, 0.13, 0.20, 0.20, and 0.15, respectively).
Thus, dolomite mineral can play role in scavenge and absorbing (Abou El-Anwar 2014 and 2019a) some heavy and rare elements and increasing their concentration in phosphate rocks. Consequently, this study is adding an important role for dolomite to increase the concentration of the heavy and rare elements in phosphates, and not only leached it from the black shales which spread in the region.
Mobilization of rare earth elements
Generally, concentrations of REEs logically present in sediments related to the parent rocks. They are mostly initiated from rock, chemical weathering, erosion, and soil.
Table 1 reveals LREEs (average 35.58 ppm) enrichment and HREEs (average 26.27 ppm) depletion. LREEs and HREEs are representing 57.5 and 42.5%, respectively, from the total content of the REEs which is a result of the weathering effect. The ratio LREE/HREE is 1.36 indicating the prevailing of LREEs, and hence the role of weathering processes on the fractionation of REEs in the studied area and also the metaluminous granites source rock of these sediments (Yusoff, et al. 2013). Low mobility, during weathering process, of LREEs compared with HREEs led to the LREEs enhancement (Yusoff et al. 2013 and Gao et al. 2016).
Concentration of U
The concentration of U in the studied phosphatic samples varies from 33 to 45 ppm with an average of 37.33 ppm (Table 1), which is higher than U content within the UCC and PAAS content (2.5 and 2.7 ppm, respectively).
U has strong positive relationship with P2O5 (r = 0.76) and CaO (r = 0.68), indicating the incorporation of U within the apatite minerals (Fig. 4b and Table 2). This relationship, also, pointed out the significant function of phosphate in deposition of U as secondary uranium mineral; phosphuranylite during weathering. So, phosphuranylite may be deposited in Queih phosphatic rocks as secondary mineral which agree with Abou El-Anwar et al. (2019b). In addition, U recorded strong to moderate positive correlation with Cr, Zr, Co, V, Zn, As, and La (r = 0.79, 0.60, 0.66, 0.53, 0.44, 0.49, and 0.45, respectively) indicating their geochemical association. Moderate positive correlation between U and SO3 (r = 0.55) may not be possibly present only in fluorapatite but also associated with other apatite group. Therefore, U may be out through the weathering and act as immobile and it may be transformed to a new phase (Venter and Boylett 2009). U was negatively correlated with HREE and weakly to moderately correlated with some LREE. The anoxic sediments are more enriched in U than oxic ones (Abou El-Anwar et al. 2019b, c).
Phosphatic rocks are considered as an important source of U worldwide (Gabriel, et al. 2013). The relationship between P2O5, F, and U can be deduced from the linear regression Eq. (1) based on the obtained results of the current study.
where P2O5 in %, U and F in ppm.
It was found that the calculated U (Uc) and measured U (Um) are nearly identical. Figure 4c shows the 3D pyramids plotting of Um and Uc. This model can be used to expect the U concentration in the phosphatic rocks.
Depositional environment
Deposition geochemical indices
The paleo-depositional environments can be identified using redox-sensitive elements; e.g., V, Ni, Mo, U, Cu, Cr, Re, Cd, Sb, and Tl (Adegoke, et al. 2015; Pattan and Pearce 2009; Pi, et al., 2014), as their concentration in the sediment increases in the anoxic environment.
The mean (in ppm), V = 1253.08, Ni = 208, Mo = 338.38, U = 39.74, Cu = 73.69, Cr = 27, Cd = 28.08, Co = 27.92, and Zn = 1062.85, in the studied phosphate rocks from Um Queih Mine is higher than the UCC (Rudnick and Gao 2014), Table 3 and Fig. 4a. Accordingly, those sediments are enriched with the oxidation-sensitive elements, indicating the deposition of phosphate rock in an anoxic environment (Adegoke et al. 2015 and Pi et al. 2014). It is also possible that the reason for the increase in the concentration of these elements in phosphates is due to their leaching from the above Dakhla Formation rocks in the study area. V is more stable and resistant to geochemical weathering than Ni, especially in the anoxic marine environments with bacterial activity, which led to an enrichment of V compared to Ni (Abou El-Anwar et al. 2019b; 2020).
The positive correlation between Cr and Ni (r = 0.47) have been used as a marker of mafic origin for the sedimentary source. The enrichment in Ni in the studied phosphatic rocks may indicate that they originated from mafic to ultramafic rocks (Graver and Royce 1993; Abou El-Anwar et al. 2020).
The average V/Mo for the studied phosphatic rocks is 3.9, which mentioned that the study phosphatic samples are formed in suboxic conditions (Gallego et al. 2010). V/Ni value ranges from 2.71 to 7.93 ratio and average is 6.29 (Table 1), which indicates that the study phosphatic rocks are deposited in marine reducing environments (Fig. 4d). This result was supported with the relationship between V/Ni and Co/Ni which indicated all the studied samples present in marine source organic matter field, except one sample (sample No. 3) in the field of mixed source organic matter due to the low content of V as shown in Fig. 4e (Galarraga et al. 2008).
The average (V/Cr) value was recorded as 47.63 (Table 1); thus, the study phosphatic samples are deposited in anoxic condition. The (Ni/Co) value varies from 5.13 to 12.11 and average is 7.77, which revealed the study phosphatic samples are deposited in anoxic condition (Jones and Manning 1994; Shi et al. 2015).
V/(V + Ni) and V/(V + Cr) ratios for the study phosphatic samples are recorded as 0.85 and 0.98, respectively; these indicate the study phosphatic rocks are deposited in strongly reducing conditions (Zho and Jiang 2009 and Pi et al. 2014).
The low U/Mo (average 0.10, Table 1) revealed that the studied phosphatic rocks in Queih Mine were deposited under sulfidic marine anoxic environment (Arning et al. 2009), within marine source organic matter (Abou El-Anwar et al. 2019b, c; 2021).
Weathering effects and paleosalinity
The Chemical Index of Alteration (CIA) is used to determine the degree of weathering of the parent rocks (Nesbitt and Young 1982). The average CIA (51.76) value for the studied phosphate samples, which is lower than the PAAS value “88.32”(Taylor and McLennan 1985), indicates that the studied samples have low chemical weathering at the source area (Fedo et al. 1995) (Table 1).
The Queih phosphatic rocks have ICV values in the range 7.12–10.13, with an average of 8.12% (Table 1). The ICV values are higher than the PAAS values (0.85) and those values of the UCC (1.2). Consequently, the Queih phosphatic rocks are geochemically more mature, and were derived from a low weathered source. On average, the CIA, CIW, and ICV average values of the studied samples are 51.76%, 56.41%, and 8.12%, respectively) (Table 1). These values suggest that the studied phosphatic samples were subjected to low weathering either of the novel source or during transportation before deposition.
The studied phosphate samples of Um Queih Mine recorded Rb/Sr ratio as average of 0.02 (Table 1). It is lower than that of the average UCC (0.33) and is comparatively equivalent to PAAS (0.08). It indicates that the rate of the chemical weathering of the study rocks was similar to the PAAS values (McLennan et al. 1993).
Consequently, the Duwi Formation can be significantly affected by the chemical weathering, which agrees with Abou El-Anwar et al. (2017; 2019b, c; 2022a).
The Sr/Ba ratio can used to conclude the dissimilarity in the salinity of water and climatic environment (Wang et al. 2005 and Shi et al. 2015). High Sr/Ba ratio is related to arid hot climate conditions and high salinity, whereas a low ratio is related to low salinity and warm climate (Wang et al. 2005). The Sr/Ba ratios for the studied phosphatic rocks are relatively high (13.89–32.42) with an average of 21.9, indicating a high salinity of water and a humid warm climate, which is in agreement with Abou El-Anwar and Abdel Rahim (2022); Boulemia et al. (2021); Mekky, et al. (2019). This is confirmed with the relation between Sr/Ba and V/Ni ratio plot (Adegoke et al. 2015), where the Um Queih samples plot in high salinity field (Fig. 5a).
a Sr/Ba vs. V/Ni (Adegoke et al. 2015). b Distribution of the Σtrace and ΣREE elements for the study area and others. c Distribution of the ΣLREE and ΣHREE elements for the study area and others
Rare earths infer to the parent rocks
REEs in sediments can be indicating the origin rocks. Ce/La (> 2) is indicated of the hydrogenic and (< 2) reveals a hydrothermal impact on REEs accumulation in the marine sediments (Khadijeh, et al. 2009). The average ratio of Ce/La in the studied phosphate samples of Queih Mine is 0.70 which is less than 2, thus indicating the hydrothermal origin also, it is lower to those of the average PAAS (2.13). Resulting of REEs has low mobility within near-surface environments. Thus, these minerals were reflecting near sources.
Comparison of the chemical composition with those of others localities
Table 3 shows the concentrations of the traces, rare earths, Σ trace, ΣREEs, P2O5, and CIA for the studied phosphatic rocks and other locations; Um El-Huwtat (Safaga), Yunis, and Gebel Duwi mines (Quseir) and El-Sabiya, in the Nile Valley.
The studied phosphatic rocks in Queih Mine are enriched in Mn, Zr, Sr, and Br than all the other localities (Table 3). Um El-Huwtat (Safaga) phosphatic rocks are enriched in Pb, Ba, Ni, Co, Rb, Sc, Ce, Ga, and U than all the other mines (Table 3). Yunis phosphatic rocks had high contents of Mo, Cr, V, Cd, Y, As, and Se than the studied phosphatic rocks. In addition, phosphatic rocks from Gebel Duwi and El-Sabiya are only enriched in La and Nd (32.2 and 16 ppm, receptivity) as shown in Table 3.
The studied phosphatic rocks in Queih Mine are represented with the highest content of the total trace elements (Σtrace = 5613.5ppm) than other mines, while El-Sabiya phosphatic rocks recorded the lowest content (Σtrace = 479.5 ppm) (Table 3 and Fig. 5b). Um El-Huwtat phosphatic rocks represented the highest content of the total rare earth elements and light rear earth elements (ΣREEs and LREEs = 92 and 47 ppm, respectively), and El-Sabiya phosphatic rocks represented the lowest content (Table 3 and Fig. 5b). In contrast, the phosphatic rocks for Yunis Mine recorded the highest values for HREEs (70.25 ppm), although has the lowest content of P2O5 12.46% (Table 3 and Figs. 5c and 6a).
The studied phosphatic samples in Queih Mine represented the highest content of the heavy metals Zn and Zr (average value 1062.85 and 160.23 ppm, respectively) as shown in Fig. 6b, c); thus, these elements are enriched in Quseir than those for Safaga and Nile Valley.
Thus, the rare earths are generally decreasing from Safaga to Quseir, toward Nile Valley, while the traces are increasing. This finding is in agreement with Abou El-Anwar et al. (2019b, c); Abou El-Anwar et al. (2022).
Pollution indices of PTEs
The enrichment of the studied phosphatic rocks with some PTEs can be deduced from the CF, EF, and Igeo (Fig. 7).
The CF is the simplest pollution indices used to express the extent to which the rock is enriched with the element. There is a large range in the recorded values of the CF, as the values ranged between 0.1 and 355.1 (Fig. 7a). The lowest values were recorded for Ba, Cr, Rb, Y, Ga, Ce, La, and Nd, and considered low contaminated with these elements. While, the highest values were recorded for Sr, Mo, V, Zn, Cd, As, Se, Br, and U, and classified as very high contaminated with these elements.
The EF gives a clear picture of the process of enriching the element in the rock, as it is compared with the CF of an element that is resistant to different weathering processes. The calculated EF values were ranged from 0.1 to 437.7 (Fig. 7b). The elements with EF < 2 indicating depletion or no enrichment in the studied rock are Ba, Cr, Co, Rb, Y, Ga, Ce, La, and Nd. The studied phosphate was extremely highly enriched with Mo, Cd, and Se.
Since it was introduced by Müller (1979), the Igeo was worldwide applied for determination of trace elements pollution evaluation (Mekky, et al. 2019). The Igeo calculated values varied from −3.1 to 7.8 (Fig. 7c). The studied phosphate was practically uncontaminated with Ba, Pb, Cr, Rb, Y, Sc, Ga, Ce, La, and Nd, while it was extremely contaminated with Mo, Cd, and Se.
It was observed that Ba showed the lowest CF, EF, and Igeo values; 0.1, 0.01, and −3.7, respectively, while the highest values were 355.1, 437.7, and 7.8, respectively, for Mo. The studied phosphatic rocks are extremely enriched with Mo, Cd, and Se, as well as relatively enriched with V, Zn, As, Br, and U. Although, Mo is essential for human, animal, and plant health and industrial applications, exposure to high doses of Mo can be detrimental to plant, animal, and human health (Smedley and Kinniburgh 2017). While Se is an essential micronutrient element considered also toxic to the living things, it is toxic for humans at > 400 μg /d (Gebreeyessus and Zewge 2019). Owing to the unique physical and chemical properties of Cd, it is widely used in many industrial processes. Among all the PTEs, it is known that Cd is harmful to almost all forms of life, as it has no benefit for the biofunctions processes in living organisms (Suhani, et al. 2021). The recorded REEs (Y, Sc, La, Ce, and Nd) are generally depleted in the studied samples with Cf < 1, EF < 2, and Igeo < 0. The study area at Queih is characterized by agricultural projects development. The weathering or excavation of phosphatic rocks can potentially be of environmental concern since this type of phosphate is enriched in PTEs.
Conclusion
The studied phosphorites from Queih Mine, Quseir Area, Duwi Formation are enriched in some trace and rare earth elements, which have an additional important role to the studied phosphatic rocks. Also, they are represented by an intermediate grade. They are deposited under anoxic reducing marine environment and are subjected to low chemical weathering in depositional environment. The petrographic studies show that the phosphorites at Queih Mine are composed of phosphatic lithoclasts, bioclasts, pyrite, and quartz grains enclosed in a cryptocrystalline phosphatic matrix. Mineralogically, the phosphorites are consisting of two main mineral phases. Phosphatic phase is represented by apatite (fluorapatite). Non-phosphatic phase is represented by calcite, dolomite, gypsum, pyrite, and quartz. The heavy and rare earth elements are associated with apatite, quartz grains, and titanium-iron oxides minerals; thus, there are many sources for these elements. The measurement U is relatively higher than calculated U values; this is credited to the post-depositional enrichment of U under the chemical weathering in Quseir area. Petrographic studies and geochemical analysis indicated that the studied phosphorites deposited in a high salinity marine anoxic condition. The pollution indices for the ore were considered low contaminated for Ba, Cr, Rb, Y, Ga, Ce, La, and Nd with low CF, while the EF indicates extremely highly enriched with Mo, Cd, and Se.
Availability of data and materials
All the data are provided within the manuscript.
References
Abou El-Anwar EA (2014) Composition and origin of the dolostones of Um Bogma formation, lower carboniferous, West Central Sinai, Egypt. Carbonates Evaporates 29:129–205. https://doi.org/10.1007/s13146-014-0188-3
Abou El-Anwar EA (2019a) Lithologic characterization of the phosphorite-bearing Duwi formation (Campanian), South Esna, West Nile Valley Egypt. Carbonates Evaporates 34:793–805. https://doi.org/10.1007/s13146-018-0442-1
Abou El-Anwar EA (2019b) Geochemical studies of carbonates of the Tayiba formation (Upper Eocene), Abu Zenima area, West Central Sinai. Bulletin Natl Res Centre 43:209. https://doi.org/10.1186/s42269-019-0240-5
Abou El-Anwar EA, Abd El Rahim SH (2022) Mineralogy, geochemistry and origin of the phosphorites at Um El-Huwtat mine, Quseir, Central Eastern Desert, Egypt. Carbonates Evaporites 37:16. https://doi.org/10.1007/s13146-022-00759-4
Abou El-Anwar EA, Mekky HS, Abd El Rahim SH, Aita SK (2017) Mineralogical, geochemical characteristics and origin of Late Cretaceous phosphorite in Duwi formation (GebleDuwi Mine) Red Sea region, Egypt. Egypt J Petrol 26:157–169
Abou El-Anwar EA, Abd El Rahim SH, Mekky HS (2019a) Spherulitic dahllite of Duwi formation phosphorite. Carbonates Evaporites 34:557–562. https://doi.org/10.1007/s13146-017-0377-y
Abou El-Anwar EA, Mekky H, AbdelWahab W (2019b) P2O5–F-U characterization and depositional environment of phosphatic rocks for the Duwi formation, Qussier-Safaga region, Red Sea coast. Egypt Egypt J Chem 62(12):2213–2228
Abou El-Anwar EA, Mekky HS, Abdel Wahab W (2019c) Geochemistry, mineralogy and depositional environment of black shales of the Duwi formation Quseir area, Red Sea coast, Egypt. Carbonates Evaporites 34:883. https://doi.org/10.1007/s13146-017-0417-7
Abou El-Anwar EA, Mekky HS, Abd El Rahim SH (2020) Discovery of spherulitic dahllite associated with carbonates at Hamadat phosphorite mine, Qusseir, Central Eastern Desert, Egypt. Carbonates Evaporites 35:106. https://doi.org/10.1007/s13146-020-00637-x
Abou El-Anwar EA, Salman AS, Mousa DA, Aita SK, Makled W, Gentzis T (2021) Organic petrographic and geochemical evaluation of the black shale of the Duwi formation, El Sebaiya, Nile valley, Egypt. Minerals 11:1416. https://doi.org/10.3390/min11121416
Abou El-Anwar EA, Abdelhafiz MA, Salman AS (2022) Rare earth and trace elements enrichment and implications in black shales of Safaga-Qussier sector, Egypt. J Afr Earth Sci 188:104482
Adegoke AK, Abdullah WH, Hakimi MH, Yandoka BMS (2015) Geochemical characterisation and organic matter enrichment of Upper Cretaceous Gongila shales from Chad (Bornu) Basin, northeastern Nigeria: bioproductivity versus anoxia conditions. J Petrol Sci Eng 135:73–87. https://doi.org/10.1016/j.petrol.2015.08.012
Arning ET, Lückge A, Breuer C, Gussone N, Birgel D, Peckmann J (2009) Genesis of phosphorite crusts of Peru. Mar Geol 262(1–4):68–81. https://doi.org/10.1016/j.margeo.2009.03.006
ASTM E1621-13 (2021). Standard guide for elemental analysis by wavelength dispersive X-ray fluorescence spectrometry. ASTM book of standards V. 03.05. https://doi.org/10.1520/E1621-13
Baioumy HM, Tada R (2005) Origin of upper cretaceous phosphorites in Egypt. Cretac Res 26:261–275
Bam EKP, Akiti TT, Osea SD, Ganyaglo SY, Gibrilla A (2011) Multivariate cluster analysis of some major and trace elements distribution in an un-saturated zone profile, Densu river Basin. Ghana Afr J Environ Sci Technol 5:155–167
Boulemia S, Hadji R, Hamimed M (2021) Depositional environment of phosphorites in a semiarid climate region, case of El Kouif area (Algerian–Tunisian border). Carbonates Evaporites 53(3):1–15. https://doi.org/10.1007/s13146-021-00719-4
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:399–411
Ciobota V, Salama W, Jentzsch PV, Tarcea N, Rosch P, El Kammar A, Morsy RS, Popp J (2014) Raman investigations of upper cretaceous phosphorite and black shale from Safaga district, Red Sea Egypt. Spectrochim Acta Part a Mol Biomol Spectrosc 118:42–47. https://doi.org/10.1016/j.saa.2013.08.059
Conoco, 1987. Geologic map of Egypt. Egyptian General Authority for Petroleum (UNESCO Joint Map Project). Scale (1:500,000), NG 36 SNE Quseir sheet
Cox R, Lowe DR, Cullers RL (1995) The influence of sediment recycling and basement composition on evolution of mud rock chemistry in the south-western United States. Geochem Cosmochim Acta 59:2919–2940
Dar SA, Khan KF, Birch WD (2017). Sedimentary: Phosphates. Reference Module in Earth Systems and Environmental Sciences. Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.10509-3
El-Kammar AM, Zayed M, Amer SA (1979) Rare earths of the Nile Valley phosphorites, Upper Egypt. Chem Geol 24:69–81
El-Kammar AM, Darwish M, Philip G, El-Kammar MM (1990) Composition and origin of black shales from Quseir area, Red Sea. Egypt Journal of the University of Kuwait-Science 17:177–1989
Fedo CM, Nesbitt HW, Young GM (1995) Unraveling the effects of K metasomatism in sedimentary rocks and paleosols with implications for palaeoweathering conditions and provenance. Geology 23:921–924
Gabriel S, Baschwitz A, Mathonnière G, Eleouet T, Fizaine F (2013) A critical assessment of global uranium resources, including uranium in phosphate rocks, and the possible impact of uranium shortages on nuclear power fleets. Ann Nucl Energy 58:213–220
Galarraga F, Llamas JF, Martinez A, Martinez M, Marquez G, Reategui K (2008) V/Ni ratio as a parameter in palaeoenvironmental characterization of non-mature medium-crude oils from several Latin American basins. J Petrol Sci Eng 61:9
Gallego-Torres D, Martinez-Ruiz F, De Lange GJ, Jimenez-Espejo FJ, Ortega-Huertas M (2010) Trace-elemental derived paleoceanographic and paleoclimatic conditions for Pleistocene Eastern Mediterranean sapropels. Palaeogeogr Palaeoclimatol Palaeoecol 293(1–2):76–89
Gao P, Zheng YF, Zhao ZF (2016) Distinction between S-type and peraluminous I-type granites: zircon versus whole-rock geochemistry. Lithos 258–259:77–91
Gebreeyessus GD, Zewge F (2019) A review on environmental selenium issues. SN Appl Sci 1:55. https://doi.org/10.1007/s42452-018-0032-9
Graver JI, Royce PR (1993). Chromium and nickel in shale of the foreland deposits of the Ordovician Tectonic Orogeny: using shale as a provenance indicator of ultra- mafic rocks. Geol. Soc. Amer., Abstract, 25, 17
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. Water Res 14:975–1001
Howari F, Salman A, Goodell P (2022) Uranium resources in the Middle East and North Africa. In: Howari F, Salman A, Goodell P (eds) Uranium geology of the Middle East and North Africa. Elsevier, Amsterdam, pp 135–264. https://doi.org/10.1016/B978-0-323-90992-1.00005-1
Jones B, Manning DAC (1994) Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem Geol 111:111–129
Khadijeh RK, Saion B, Elias WA, Reza A (2009) Rare earth elements distribution in marine sediments of Malaysia coasts. J Rare Earths 27(6):1066
Kohn MJ, Moses RJ (2013) Trace element diffusivities in bone rule out simple diffusive uptake during fossilization but explain in vivo uptake and release. Proc Natl Acad Sci 110:419–424. https://doi.org/10.1073/pnas.1209513110
Mahfouz KH, Obaidalla NA, Hewaidy AA, Mostafa A, El-Sheikh I (2021) Evolution of the Maastrichtian-Paleocene sedimentary basin in the Safaga-Quseir region, Red Sea Coast. Egypt. Marine Micropaleontol 169:102039. https://doi.org/10.1016/j.marmicro.2021.102039
McLennan SM (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. Rev Mineral Geochem 21(1):169–200
McLennan SM, Hemming S, McDaniel DK, Hanson GN (1993). Geochemical approaches to sedimentation, provenance and tectonics. In: M.J. Johnson, A., Basu (Eds), Processes Controlling the Composition of Clastic Sediments. Geological Society of American, Special Paper, USA, 21- 40
McMurtry GM (2019) Deep-Sea sediment authigenic deposits. In: Cochran et al (eds) Encyclopedia of ocean sciences, 3rd edn. Academic Press, Amsterdam, pp 121–132. https://doi.org/10.1016/B978-0-12-409548-9.11645-8
Mekky HS, Abou El-Anwar EA, Salman SA, Elnazer AA, Abdel Wahab W, Asmoay AS (2019) Evaluation of heavy metals pollution by using pollution indices in the soil of Assiut district Egypt. Egypt J Chem 62(9):1673–1683
Muller G (1979) Schwermetalle in den sedimenten des Rheins, VeranderungemSeit 1971. Umschau 79:778–783
Narula SC, Wellington JF (2007) Multiple criteria linear regression. European Journal of Operational Research : EJOR 181(2):767–772
Nesbitt HW, Young GM (1982) Early proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 299:715–717
Nganje TN, Edet A, Cuthbert S, Adamu CI, Hursthouse AS (2020) The concentration, distribution and health risk from potentially toxic elements in the soil—plant—water system developed on black shales in SE Nigeria. J Afr Earth Sc 165:103806
Pattan JN, Pearce NJG (2009) Bottom water oxygenation history in Southeastern Arabian Sea during the past 140 ka: results from redox-sensitive elements. Palaeogeogr Palaeoclimatol Palaeoecol 280:396–405. https://doi.org/10.1016/j.palaeo.2009.06.027
Peter LM, Glen BM, Paul WW (1986) Uranium in early proterozoic phosphate-rich metasedimentary rocks of east-central Minnesota. Econ Geol 81(1):173–183
Pi DH, Jiang SY, Luo L, Yang JH, Hong-Fei LHF (2014) Depositional environments for stratiformwitherite deposits in the lower Cambrian black shale sequence of the Yangtze platform, southern Qinling region, SW China: evidence from redox-sensitive trace element geochemistry. Palaeogeogr Palaeoclimatol Palaeoecol 398:125
Rudnick RL, Gao S (2014) Composition of the continental crust. Treatise Geochem 3:1
Salman SA, Elnazer AA, El Nazer HA (2017) Integrated mass balance of some heavy metals fluxes in Yaakob village, south Sohag Egypt. Int J Environ Sci Technol 14:1011–1018
Shi D, Adinolfi V, Comin R, Yuan M, Alarousu E, Buin A, Chen Y, Hoogland S, Rothenberger A, Katsiev K, Losovyj Y, Zhang X, Dowben PA, Mohammed OF, Sargent EH, Bakr OM (2015) Low trap-state density and long carrier diffusion in organo-lead-trihalide-perovskite single crystals. Science 347(6221):519–522. https://doi.org/10.1126/science.aaa2725
Smedley PL, Kinniburgh DG (2017) Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl Geochem 84:387–432
Suhani I, Sahab S, Srivastava V, Singh RP (2021) Impact of cadmium pollution on food safety and human health. Curr Opin Toxicol 27:1–7. https://doi.org/10.1016/j.cotox.2021.04.004
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell, Oxford, p 312p
Venter R, Boylett M (2009). The evaluation of various oxidants used in acid leaching of uranium. Hydrometallurgy Conference, The Southern African Institute of Mining and Metallurgy, 445–456
Wang M-F, Jiao Y-Q, Wang Z-H, Yang Q, Yang S-k (2005) Recovery paleosalinity in sedimentary environment-an example of mudsone in Shuixigou group, southwestern margin of Turpan-Hami basin. Xinjiang Petroleum Geology 12(6):719–722
Yan C, Jin Z, Zhao J, Du W, Liu Q (2018) Influence of sedimentary environment on organic matter enrichment in shale: a case study of the Wufeng and Longmaxi formations of the Sichuan Basin, China. Mar Pet Geol 92:880–894. https://doi.org/10.1016/j.marpetgeo.2018.01.024
Youssef MI (1957) Upper Cretaceous rocks in Quseir area. Bull. Inst. Desert Egypt. 7:35–547
Yusoff ZM, Ngwenya BT, Parsons I (2013) Mobility and fractionation of REEs during deep weathering of geochemically contrasting granites in a tropical setting. Malaysia Chem Geol 349:71
Zhou CM, Jiang SY (2009) Palaeoceanographic redox environments for the lower Cambrian Hetang formation in South China: evidence from pyrite framboids, redox sensitive trace elements, and sponge biota occurrence. Palaeogeogr Palaeoclimatol Palaeoecol 271:279–286
Acknowledgements
Many thanks for the National Research Centre for facilities and lab work during sampling and analyses.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Abou El-Anwar: Conceptualization, supervision, chemical analyses, project management and revising the manuscript. Lotfy: Collecting the samples, Visualization, interpretation, writing the main draft, revising and editing the manuscript Salman: Conceptualization, collecting the data and samples, supervision, chemical analyses, and revising the manuscript. Abd El Rahim: Collecting the samples, chemical analysis of samples, petrographic studies, and revising the manuscript. Abdelwahab:Conceptualization, supervision, visualization, revising and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abou El‑Anwar, E.A., Belal, Z.L., Salman, S.A. et al. Geochemical and mineralogical analysis of the Upper Cretaceous dolomitic phosphates at Queih Mine, Quseir, Central Eastern Desert, Egypt: depositional environment implications and pollution indices. Carbonates Evaporites 38, 70 (2023). https://doi.org/10.1007/s13146-023-00894-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s13146-023-00894-6