Abstract
Debris-cloaking, a method of concealment with material collected from the environment, has evolved repeatedly with the purpose of avoiding detection in adults or immatures of various invertebrates including beetles. Fungus beetles in the family Anamorphidae (Coccinelloidea) are small-sized spore feeders of predominantly surface-dwelling habits. Debris-cloaking has been reported only for two genera in the family and nowhere else in Coccinelloidea. Here we report debris-cloaking behaviour in larvae of the Neotropical beetle genus Catapotia, describe its larvae, and compare it to confamilials. We summarise the knowledge on the defensive mechanisms and the natural history traits for members of the coccinellid group of Coccinelloidea. Possible evolutionary origins for the defensive mechanism are reconstructed on the basis of available phylogenetic hypotheses. Groups containing species with a larger body size also have a higher number of defensive strategies in larvae and adults; these include members of Endomychidae and Coccinellidae that exhibit reflex bleeding, aposematic colouration, and gregarious behaviour. Debris-cloaking has likely evolved only once, in the comparatively small-sized Anamorphidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camouflage, crypsis and especially concealment with objects collected by animals from their environment (Endler, 1981), has evolved repeatedly in adults or immatures of various organisms including insects (Pérez-de la Fuente et al., 2012; Ruxton & Stevens, 2015) that construct a larval case or fasten debris, cast-skins, faecal material, and even attach other live organisms to their body (Gressit et al., 1968). Different strategies of camouflage are employed to avoid detection by predators as well as prey (Pembury Smith & Ruxton, 2020). These strategies are either passive or active (Berke et al., 2006), and attachment of foreign material may be facilitated by glues (Bamuel, 1989) but is most often achieved with modified cuticular structures (Hultgren & Stachowicz, 2008; Rider & Hostetler, 2022). Active concealment with debris particles involves behavioural adaptations aimed at detecting, selecting, or accommodating debris particles in the desired location on the body. Though several terms exist in the literature for this behaviour, such as trash-carrying (Eisner et al., 1978) or decorating (Ruxton & Stevens, 2015), debris-cloaking was coined for anamorphid beetle larvae (Polyphaga: Coccinelloidea), the group we report on here, that may actively or passively load material onto their bodies (Leschen & Carlton, 1993).
Debris-cloaking may be mostly expressed in species that are active on open, exposed substrates where material may be passively attached to the body as they move through the environment, like assassin bugs (Brandt & Mahsberg, 2002), bark-lice (Kiesmüller et al., 2021), or slime mould beetles (McHugh & Kiselyova, 2003). Rarer are groups like decorator crabs (Guinot & Wicksten, 2015; Hultgren & Stachowicz, 2008), which load material with dexterity, using appendages and body movements. Yet, data suggest that debris-cloaking is not taxonomically ubiquitous and might be constrained by the energetic cost to the organism (Berke et al., 2006). Predatory lacewing larvae use their mandibles for debris loading and have modified setae to hold debris in place by frictional forces (Eisner et al., 1978; Tauber et al., 2014). Larvae of some species of the fungus-feeding Anamorphidae have evolved a unique loading mechanism that involves arching their bodies and making use of thoracic lobes to position debris onto their topsides (Leschen & Carlton, 1993).
Beetles feeding on the external surfaces of fungi have developed a series of adaptations akin to external plant-feeders to avoid predation (Leschen, 1994; Shockley et al., 2009b). Among the most common strategies used by larvae and adults to evade predators are aposematism (Robertson et al., 2004), reflex bleeding (Drilling & Dettner, 2010), gregarism (Roubik & Skelley, 2001), and stridulation (Navarrete-Heredia et al., 2021). Furthermore, females may conceal their eggs on the surface of the fungus (Leschen & Carlton, 1988) or insert them into substrates. Parental care is rare (Leschen, 1994; Hanley & Goodrich, 1995; Chaboo & McHugh, 2010). Due in part to the short-term availability of fungi (Hanski, 1989), some of the oddest tactics of protection are used by fungus beetle larvae (Leschen, 1994) and include autotomy of thoracic appendages in endomychids (McHugh & Pakaluk, 1997; Tomaszewska, 2002), the retention of exuvial skins in erotylids (Hayashi, 1984; Yoshida & Leschen, 2020), and debris-cloaking in Anamorphidae.
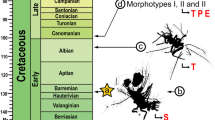
Anamorphidae form a moderately diverse group of small subhemisphaerical beetles, with nearly 175 described species (Shockley et al., 2009a, b). Until recently, Anamorphidae was considered a subfamily in Endomychidae. Following a comprehensive molecular phylogenetic hypothesis (Robertson et al., 2015), this group is now classified as a separate, monophyletic family within Coccinelloidea, as the sister or near relative of Corylophidae (Arriaga-Varela et al., 2023), both of which were recovered in the coccinellid group (sensu Robertson et al., 2015). Based on direct observation, gut analyses, and mouthpart structures, it is suspected that most species feed predominantly on fungal spores; however, very little is known about the natural history of most members of this family (Shockley et al., 2009a, b).
Debris-cloaking has been reported in two anamorphid genera. Hayashi (1984) mentioned that the larvae of Idiophyes niponensis (Gorham, 1874), from Japan, holds the exuvia of the previous instar as well as other debris from their environment. In a paper documenting a new species of Bystus Guérin-Méneville, 1857, from Peru, Leschen and Carlton (1993) described its larval morphology and debris-cloaking behaviour based on field observations and lab work. Specialised setae arising on the lobes of the body are used to position material into place. In the same paper, the debris-cloaking behaviour of two unidentified species of Anamorphidae from Mexico was observed. In one species, the placement of debris particles was passive while the other species gauged the size of the particle with its mandibles before loading. These species remained unidentified because they were not associated with adults. With newly available material, we revisited these Mexican specimens and can now assign them to the genus Catapotia Thomson, 1860.
Catapotia is a small genus, consisting of five species ranging from Mexico to Brazil: additional undescribed species exist, so the number will increase. Catapotia laevissima Thomson, 1860, is the most widespread species, known from Mexico, Guatemala, Nicaragua, Costa Rica, Panama, Ecuador, and Peru. One of us (EAV) discovered adults and larvae of this species in a cloud forest in south-central Mexico, the larvae of which were debris-cloaked and prompted further study. Here we describe the larval morphology of C. laevissima and confirm that the undetermined larvae studied by Leschen and Carlton (1993) belong to Catapotia.
New information about Catapotia provides an opportunity to compare and determine the ecological context in which these defensive strategies occurring in Anamorphidae and its relatives have evolved. Debris-cloaking and other defensive strategies (reflex bleeding, gregarism, aposematism, egg protection, autonomy) and natural history traits (larval and adult diet, feeding location, and body size) are mapped onto the most-comprehensive phylogenetic tree available for the group (Robertson et al., 2015). Though natural history and phylogenetic information remains incomplete for the coccinellid group, we propose that larval debris-cloaking has evolved from a spore-feeding, small-bodied ancestor. Other defensive traits, primarily aposematism, have evolved in the more derived Endomychidae and Coccinellidae, families that include the largest members of Coccinelloidea.
Material and Methods
Specimens of Catapotia laevissima were collected at night on 31 July 2013, in Parque El Haya, a peri-urban ecological reserve, in the southwest part of Xalapa City, Veracruz, Mexico. The predominant vegetation in the area is secondary cloud forest or mesophilous mountain forest (bosque mesófilo de montaña) dominated by Platanus mexicana, Liquidambar styraciflua, Inga spp., and other less common native and introduced tree species. Beetles were found under cut logs, which were likely L. styraciflua. Three adults and 6 larvae were discovered grazing among the partially concealed surfaces of the logs which had an abundant growth of unidentified fungi. Other mycophagous coccinelloids found in the same microhabitat were Epipocus rufitarsis (Chevrolat) (Endomychidae) and Discoloma sp. (Discolomatidae). Specimens were collected in 70% EtOH and were deposited in the Museum and Institute of Zoology, Polish Academy of Sciences, Warsaw, Poland (MIZ PAS) and in the CZUG (Colección Entomológica del Centro de Estudios en Zoología, Universidad de Guadalajara, Zapopan, Mexico). Additional material examined were deposited in these collections and the New Zealand Arthropod Collection, Auckland (NZAC).
Colour photographs were taken using a 4 K Ultra-high accuracy microscope VHX-7000 Series by KEYENCE in the MIZ PAS. The sample for the scanning electron microscopy (SEM) was dried using hexamethyldisilazane (HMDS) for 10 min. The material was then gold-coated using a CCU-010VL ion sputtering machine. The subsequent observations were carried out using a Hitachi S3400-N scanning electron microscope in the SEM laboratory of the MIZ PAS. Terminology used in the description follows Leschen and Carlton (1993), Shockley and Tomaszewska (2007), and Tomaszewska (2010). The gut content of a second-stage larva specimen cleared in KOH was extracted, studied, and photographed using an Olympus BX43F microscope.
Behavioural traits, defensive strategies, and ecologies were plotted over the reference tree proposed by Robertson et al. (2015) to infer origin of defensive traits. As defined by Robertson et al. (2015), the coccinellid group of Coccinelloidea is monophyletic and consists of a clade comprising Latridiidae, Akalyptoischidae, Alexiidae, Anamorphidae, Corylophidae, Endomychidae, Mycetaeidae, Eupsilobiidae, and Coccinellidae. Species-level terminals were collapsed by subfamily except for Anamorphidae which were collapsed to genus-level (Symbiotes Redtenbacher, 1849; Papuella Strohecker, 1956; Clemmus Hampe, 1850; Mychothenus Strohecker, 1853; Micropsephodes Champion, 1913; Catapotia Thomson, 1860; Bystus Guérin–Méneville, 1857; Geoendomychus Lea, 1922; Idiophyes Blackburn, 1895; and Anamorphus LeConte, 1878) and presented in Fig. 5.
Information on the feeding biology and defensive mechanisms was taken from Ślipiński and Tomaszewska 2010a, b, Tomaszewska (2010), Hartley and McHugh (2010), Ślipiński et al. (2010), Shockley et al. (2009b), Pakaluk (1984), Leschen and Carlton (1993), McHugh and Pakaluk, (1997), Burakowski and Ślipiński (2000), Escalona et al. (2017), Lord et al. (2010), Tomaszewska (2002), Hayashi (1984), and Tomaszewska (2022). These observations were substantiated by our personal observations and those of Karol Szawaryn (MIZ PAS).
Defensive mechanisms mapped on the phylogenetic tree are as follows: aposematic colouration, debris-cloaking, gregarism, reflex bleeding, autotomy, and stridulation. These are coded as unknown (?) for Geoendomychus, Idiophyes, Pleganophorinae, Leiestinae, Mycetaeidae, and Eupsilobiidae.
With respect to aposematic colouration, we used a simplistic approach, assuming that highly contrasting colour patterns would be recognizable to predators. Putatively aposematic colouration was coded as present when the dorsal surfaces of pronotum and elytra, or thorax and abdomen in larvae, have at least a third of the area coloured by marks of distinctly contrasting colours. Unpalatability by toxic or distasteful secretions has been confirmed in Coccinellidae (Aslam, 2020; de Jong et al., 1991) and is often found in aposematic species. However, even when contrasting colouration is scored for many groups of Coccinelloidea, the unpalatability of the organisms should be confirmed by behavioural or chemical observations and tests (Harvey & Paxton, 1981).
Diet and feeding strategy (fungal sporophagy, fungal hyphae feeding and predation) were scored separately for adults and larvae, even if both life stages utilized similar food because their feeding locations (cryptic vs. surface) may differ. Traits related to feeding strategies were colour coded in Fig. 5. Diet in Pleganophorinae is unknown (?).
Polymorphisms were represented proportionately by bicoloured squares. A half-coloured square represents an approximate distribution of a trait between ca. 25 and 75% of the known cases within a clade, and a quarter-coloured square represents an occurrence of a trait from 24% to fewer cases.
The range of body lengths was measured in millimetres and represented as horizontal grey bars in Fig. 5.
We optimized character traits employing ACCTRAN and DELTRAN parsimony-based criteria (Maddison et al., 1984) and treated question marks as unknown. Taxa coded with unknown defensive mechanisms are plotted with behaviours that are present in their closest relatives.
Results
Catapotia Thomson.
Catapotia Thomson, 1860: 13.
Type species: Catapotia laevissima Thomson, 1860.
Adult generic diagnosis
Catapotia can be distinguished from other Neotropical Anamorphidae by the following combination of characters: antenna with 11 antennomeres and sub-symmetrical not serrate club (Fig. 1c), pronotum with posterior margin distinctly lobed medially, without longitudinal or basal sulci and lateral carinae (Fig. 1a), procoxal cavities open externally (Fig. 1b), elytra smooth, without series of punctation (Fig. 1a), tarsi 4–4-4 (Fig. 1g), and tarsal claw simple (Fig. 1h).
Catapotia laevissima Thomson, 1860: 14.
Distribution: Mexico, Guatemala, Costa Rica, Nicaragua, Panama, Ecuador, Peru.
Larval diagnosis
Body hardly sclerotized, elongate-oval, moderately dorsoventrally flattened (Fig. 2). Antennomere II very long, about 5 times as long as wide and about four times as long as segment III (Fig. 3e); head without stemmata; last two abdominal segments without genitalic tuberculate lobes (Fig. 3a); maxilla long with stipes about 1.5 times longer than palpus (Fig. 3b); dorsal surface of thorax and abdomen with long setae having a shaft without barbs, and tips with small spoon-like structure (Fig. 3j). Of all the described anamorphid mature larvae, Catapotia laevissima most closely resembles Bystus spp. in having a very elongate antennomere II, the thorax and abdomen with large tergal and/or pleural lobes, and the dorsal vestiture with modified setae with the apical spoon-like form. However, the long maxilla with stipes about 1.5 times longer than palpus can distinguish C. laevissima larvae.
Catapotia laevissima Thomson, larval morphology. a–h, i–k fourth instar; h third instar. a Fourth instar, dorsal with head removed. b Ventral view of head. c Apex of maxilla. d Left mandible, ventral. e Antenna. f Ventral view of labrum. g Vestiture of head. h Ventral view of labial complex. i Apex of thoracic setae. j Detail of spoon-like structure on thoracic setae. k Bifid setae of maxilla
Description of mature larva (Figs. 2d, e and 3a–g, i–k)
Dimensions of all four instars (L = length, HW = head width, PW = prothoracic width, n = number examined). First instar (Fig. 2a) (n = 1): L = 1.59; HW = 0.46, PW = 0.73. Head without egg bursters. Second instar (Fig. 2b) (n = 1): L = 1.7; HW = 0.62, PW = 1.12. Third instar (Fig. 2c) (n = 2): L = 2.182.23; HW = 0–74-0.79, PW = 1–35-1.38. Fourth instar (Fig. 2D) (n = 1): L = 3.3; HW = 0.9, PW = 1.85.
Mature larva (n = 1): length 3.3 mm; head width 0.9 mm; maximum width of thorax 1.85 mm; maximum width of abdomen 1.94 mm. Body hardly sclerotized, elongate-oval, moderately dorsoventrally flattened; widest across abdominal segment I, rounded anteriorly and posteriorly; weakly constricted between segments; urogomphi absent (Fig. 3a). Dorsum and venter whitish. Vestiture composed of dense tiny, decumbent, simple setae (Fig. 3g) and very long socketed setae with apical spoon-like structure (Fig. 3i), setae longer and denser on tergal and pleural lobes; ventral surfaces with dense tiny, non-socketed setae; femora and tibiae with few sparse long, pointed setae.
Head hypognathous, strongly transverse, flattened dorsally; dorsally about 1.5 as wide as long and 0.5 times as wide as prothorax (Fig. 3b), covered by moderately long setae, with a pointed tip and normal apex (Fig. 3g). Frontal arms absent. Stemmata absent. Hypostomal rods long and stout, divergent posteriorly. Fronto-clypeal suture absent. Labrum almost twice as wide as long, sclerotized, free with anterior margin deeply roundly emarginate, covered with moderately long decumbent setae; anterior part of epipharynx with thick acute spine-like setae laterally (Fig. 3f). Antenna 3-segmented, long, moderately stout (Fig. 3e); inserted postero-laterally in a membranous pocket far behind mandibular articulations. Antennomere I hardly concealed, without long setae, about 0.1 times as long as antennomere II and nearly 0.5 times as long as III; antennomere II long, about 5 times as long as wide and about 4 times as long as antennomere III, with surface microsetose, without long setae; sensorium tapering towards apex, about as long as antennomere III; antennomere III small, without long terminal setae Mandibles transverse, symmetrical, each with two dorsolateral setae and bearing a hyaline, acute, prostheca-like process anterior to mola; incisor lobe absent; mola with rows of transverse, sclerotized small ridges (Fig. 3d). Maxillolabial complex well developed (Fig. 3b). Maxilla large, with cardo subtriangular; stipes elongate, about 1.5 times longer than palpus, with 1 small mesial seta in anterior half; mala apically produced, bearing 2 stout, curved spurs and 3–4 setose projections with flattened spoon-like apices, sometimes bifurcate; maxillary palpi comparatively long, 3-segmented; palpomeres I and II elongate, palpomere II about 1.5 times longer than I; terminal palpomere about as long as II, very weakly tapering, subrounded at apex, and bearing a group of short, apical sensory processes (Fig. 3c). Labium undivided; labial palpomere 2-segmented; palpomere I without setae, about as long as terminal palpomere; terminal palpomere subcylindrical, weakly rounded at apex with a group of apical sensillae. Hypopharynx with sclerotized hypopharyngeal sclerome, bracon, and parallel hypopharyngeal rods.
Thorax about 2/5 as long as total body length, widest across mesothorax; mesothorax longer than metathorax. Thoracic and abdominal terga without median ecdysial lines (Fig. 3a). Prothorax and mesothorax with well-developed tergal lobes; metathoracic lobe reduced to bulbose projection. Prothoracic lobe about twice as large as the mesothoracic one, rounded in outline, wider posteriad. Mesothoracic lobe rounded. Metathoracic lobe about 1/4 the size of mesothoracic one.
Abdomen widest at segment I, then rounded apically (Fig. 2d, e); pleural lobes present on abdominal segments I–VIII, rounded; lobe on segment I about as long and nearly half as wide as prothoracic lobe; abdominal segment IX 2/3 as wide as segment VIII, with posterior margin rounded and covered with long setae, urogomphi absent; segment X short, directed ventrad (Fig. 2d, e). Spiracles small, annular, anterodorsal of pleural lobes.
Legs with coxae widely separated basally, coxa slightly shorter than trochanter and femur combined, covered with a few pointed setae; trochanter elongate, with 2 mesal setae; femur cylindrical, slender, about 5 times as long as wide, with 2 inner and 2 outer setae; tibiotarsus almost 1.5 times as long as femur, weakly narrowing towards apex, covered by minuscule decumbent setae and bearing few sparse, pointed setae; tarsungulus with a single, long, sharp claw; claw with apical half strongly sclerotized, bearing single, short seta ventrally.
Material examined
Catapotia laevissima. Mexico: Veracruz, Xalapa, Parque El Haya, secondary cloud forest-bosque mesófilo de montaña. Night collecting on mouldy wood and bark. 1450 masl. 19.5199° N, 96.9434° W, 31.vii.2013. Leg. E. Arriaga-Varela (3 adults, 5 larvae: MIZ PAS, CZUG). Catapotia spp. indet. (slide mounted, NZAC). Mexico: Hidalgo, 6.6 mi. north of Jacala, Hwy. 85, 1620 m, 11 July 1990, ex encrusting fungi on pine log, R. Leschen (1 larva); Mexico: Hidalgo, 30.2 mi. S of Tamazunchale, Hwy. 85, 1030 m, 10 July 1990, ex Xylaria, R. Leschen (1 larva); Mexico: Veracruz, 11.7 mi. S. Huatusco, Hwy. 125, E. on rd to Ixhuatlán, 17 July 1990, 1130 m, fungusy log, R. Leschen (1 larva); Costa Rica: Puntarenas, Monte Verde, 1550 m, ex Xylaria, #450, R. Leschen (1 larva); same, but 23.v.89, #421 (1 larva).
Biology
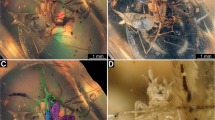
Little is published about Catapotia and the habits of C. laevissima in particular. Arriaga-Varela et al. (2007) reported the occurrence of C. laevissima in orchids, based on label data of a specimen collected in San Luis Potosí, Mexico. Leschen and Carlton (1993) reported the debris-cloaking behaviour of two unidentified species of Anamorphidae collected in Mexico on encrusting fungi on pine logs and other fungus-infested logs, as well as on Xylaria for the specimens from Costa Rica (see “Examined material”). Here we confirm that these specimens mentioned by Leschen and Carlton (1993) belong to Catapotia. The specimens identified here as Catapotia laevissima were found grazing on the partially exposed surface of rotten wood and bark with unidentified fungi, none of which formed large fleshy sporocarps like mushrooms and polypores but instead formed a resupinate layer on the dead wood. Larvae were found covered by debris particles that included parts of lichens and hyphae as well as indeterminate material (Fig. 4a). In some cases, the exuvial skin of the younger instar was still attached to the body of the larva (Fig. 2c). The exuvia held extraneous material as well, forming part of the debris packet covering the body of the larva. The gut content of a second-stage larva confirms a diet of predominantly fungal spores (Fig. 4c, d), with a smaller proportion of undetermined hyphae. Therefore, the predominantly sporophagous habits of Catapotia are confirmed as suggested by the shape of the mandibles (Fig. 3d) and as it is typical of other anamorphids.
Natural history of members of the coccinellid group. a, b debris-cloaked Anamorphidae larvae; c, d gut content of second instar Catapotia laevissima; e, f aposematic colouration in Stenotarsus globosus Guérin–Méneville. a Catapotia laevissima third instar larva with exuvial skin attached and covered with debris (Xalapa, Mexico). b Bystus aff. fibulatus (Gorham) first instar larva with debris (Xalapa, Mexico). c Gut content of second instar Catapotia laevissima showing fungal spores, fragments of hyphae. d Gut content of second instar Catapotia laevissima showing fungal spores, fragments of hyphae. e Stenotarsus globosus adult on fungus covered log (Los Chimalapas, Mexico). f Stenotarsus globosus third or fourth instar larva on fungus covered log (Los Chimalapas, Mexico)
Defensive strategies and habits among members of the coccinellid group of Coccinelloidea
Optimizations of character traits under ACCTRAN and DELTRAN were similar, except for debris-cloaking. We chose to illustrate character evolution in Fig. 5 using DELTRAN, which favours parallelisms over reversals for the same traits (changes are assigned along branches as close to the tips as possible) (see Agnarsson & Miller, 2008), and may be more representative of true patterns because we coded supraspecific taxa as terminals. Our reconstructions suggests that aposematism, gregarism, and reflex bleeding may have evolved multiple times in subfamilies containing species with a larger body size relative to other members of the coccinellid group, especially among members of “higher Endomychidae” (sensu Tomaszewska, 2005; Cyclotominae, Endomychinae, Epipocinae and Lycoperdininae) and Coccinellidae. In a more refined study, we would expect that aposematic colouration would have evolved repeatedly among many lineages, as is shown in many other groups (Arriaga-Varela et al., 2013; Robertson et al., 2004). Debris-cloaking may have originated once in Anamorphidae, within a small-bodied ancestor that had surface-feeding larvae, though debris-cloaking has not been reported in Anamorphus and Geoendomychus.
Graphical summary of defensive strategies and habits in the coccinellid group of Coccinelloidea arranged following the phylogenetic framework of Robertson et al. (2015). Clades are collapsed to subfamily level and to genera for Anamorphidae. Variation within a group was indicated with bicolored squares and represented within each by relative proportion. For the defensive strategies a scoring with question mark, “?”, indicates that a trait is unknown but is considered likely for a taxon based on the biology of its relatives. For the feeding habits a question mark “?”, indicates complete lack of information for that particular taxon. Likely origins of defensive traits following a DELTRAN criterion are signalled with a colour circle over the nodes. Origins of traits under ACCTRAN criterion are signalled with a colour rhombus shape. Loss of traits under ACCTRAN is shown with an empty rhombus shape. The known range of body length in millimetres for all organisms within a clade is provided as horizontal grey bars
With respect to the diet, we presume sporophagy is likely the plesiomorphic state within the coccinellid group, with macrophagy derived in members of the “higher Endomychidae”. Predation has evolved at least twice within the group, most notably in Coccinellidae. Although it probably developed within a clade of fungivorous ancestors, the exact evolutionary path leading to the predatory habits of the very diverse ladybirds is still unclear (Giorgi et al., 2009). This remains a key evolutionary question that would shed light on the processes behind the diversification of Coleoptera (Escalona et al., 2017).
Our results indicate that larvae and adults of the coccinellid group share similar microenvironments, either cryptic or on exposed surfaces. Cryptic habits were predominantly found in groups feeding on spores, e.g., Latridiidae, Akalyptoischiidae, and most Anamorphidae. Species that inhabit exposed surfaces of mushrooms or fungus-infested logs are principally found in Endomychidae, especially the “higher Endomychidae” clade. Surface dwelling also occurs in those active on foliage, i.e., most ladybirds.
Discussion
Debris-cloaking in Anamorphidae
The diversity of immature stages in Anamorphidae has been poorly documented. Of the 35 genera known in the family, larvae have been described for only five genera (Shockley & Tomaszewska, 2007) and observations about behaviour and functional morphology are scarcer. Larval behaviour of C. laevissima was not observed, and though we now can place the unidentified larvae observed by Leschen and Carlton (1993) into Catapotia, this information assists little in developing a complete behavioural repertoire of loading and manipulation of debris onto the dorsal surface of the body, or whether behavioural traits are common throughout Anamorphidae. We can, however, confirm that the materials used are variable (exuviae, detritus, plant, and fungal tissue) and describe morphology associated with debris-cloaking.
Larval Catapotia and Bystus share a similar body form. The setose lateral thoracic lobes may function similarly with setae for capturing debris and centring it on the body. The dorsal setae, however, differ. Bystus decorator Leschen & Carlton, 1993, and Catapotia spp. share the spoon-like apical structure of the setae, but a barbed shaft occurs only in Bystus. Though Idiophyes niponensis retains exuviae and debris like Bystus and Catapotia, the setal structure was not described by Hayashi (1984). Contrary to lacewings where nude, non-camouflaged larvae lack textured setae (Tauber et al., 2014), having barbed setae is not required for debris capture or retainment in anamorphids. It is worth noticing that the larvae of Symbiotes, an anamorphid genus in which debris-cloaking has not been observed, lack the lateral thoracic lobes (Shockley & Tomaszewska, 2007). This reinforces the idea that the presence of these lobes in Anamorphidae is associated with debris manipulation.
How anamorphid larvae choose debris, manipulate the material onto their bodies, retain, and use it in defence remains mysterious. While the function of debris-cloaking in chrysopids has been studied experimentally (Eisner et al., 1978; Hayashi & Nomura, 2011; Nakahira & Arakawa, 2006), no studies exist for anamorphids, and the actual defensive use of a covering with debris and exuvial skins has yet been explored. It is also not confirmed that debris-cloaking and exuvial retention in Anamorphidae is used for camouflage or crypsis (sensu Endler, 1981) or as a type of extrinsic autotomy (Yoshida & Leschen, 2020), where potential predators are left with a mouthful of debris or exuvia. Additionally, the actual interactions with predators and parasiotoids have not been observed or explored under natural conditions or in the lab. This can be done experimentally, by exposing debris covered and nude larvae to predators and evaluating survival or aggression rates between these classes.
Evolution of debris-cloaking in Anamorphidae
Reconstructions for the evolution of debris-cloaking between ACCTRAN and DELTRAN differ. DELTRAN shows three parallel acquisitions, while ACCTRAN reconstructs a single origin with two reversals in Geoendomychus and Anamorphus. The principal caveat for any attempt to understanding the distribution of this defensive mechanism within the family and its evolution is the lack of observations. This is true for the whole diversity in the family as well as for the 10 anamorphid genera included in the taxonomic sampling of Robertson et al. (2015), representing less than half of anamorphid genera. Furthermore, there is uncertainty whether the morphological and behavioural traits associated with debris-cloaking have evolved once or independently in Bystus (Fig. 4b), Catapotia and Idiophyes. Adults of anamorphid genera are generally setose, but a few genera include glabrous species, like New World Catapotia and Micropsephodes (see Leschen & Carlton, 2000) which do not group together as a clade in Robertson et al. (2015). Thus, we can infer that, since adult morphology may reflect evolutionary parallelisms and reversals, the same may be true to larval features, such as presence of setae with barbed shafts. Larvae of other members of the coccinellid group of Coccinelloidea show complex setae that frequently hold debris, like those in subfamily Epipocinae (see McHugh & Pakaluk, 1997). We hypothesize that larval Anamorphidae had taken advantage of the physical inability to groom their dorsal surfaces and have co-opted the debris-holding properties of their setae and used it as a defence mechanism. How and when active selection and manipulation of debris particles evolved remain mysterious.
We hypothesize that debris-cloaking larvae has evolved from a spore-feeding, small-bodied ancestor (Fig. 5). Debris-cloaking may also be an ancient behaviour. It has been recorded in insects embedded in Cretaceous amber (Kiesmuller et al., 2021; Pérez-de la Fuente et al., 2018; Wang et al., 2016). Fossil Anamorphidae have been described from earlier Eocene (Alekseev & Tomaszewska, 2018), and we have examined a putative anamorphid from Burmese amber (ca. 99 mya) (EAV, WT, personal observation). Molecular studies for a broad sampling of Coleoptera indicate that anamorphids could have originated over 100 mya (Cai et al., 2022; McKenna et al., 2019; Toussaint et al., 2017). A time-calibrated phylogenetic hypothesis for Anamorphidae that includes a more comprehensive taxonomic sampling is warranted, as is the confirmation and formal description of the Burmese fossil.
Defensive strategies in relation to feeding location and body size
The ancestor of the coccinellid group may have had cryptic habits, which subsequently gave rise to feeding on open surfaces. Following this shift in feeding location there was an increase in the number of defensive strategies among Endomychidae and Coccinellidae in relation to body size and feeding habit (Fig. 5). Within Coccinelloidea, Endomychidae (Lycoperdininae), and Coccinellidae (Coccinellini) include species having the largest body sizes reaching lengths of ca. 20 mm. The Lycoperdininae has the largest number of defensive strategies in the family which includes larval body autotomy (Tomaszewska, 2002) and stridulation in adults (Tomaszewska, 2005), which are unique to the group. We note that Robertson et al. (2015) did not sample the endomychid subfamilies Xenomycetinae and Danascelinae, and likewise, neither was the recently re-instated family Cerasommatidiidae (Arriaga-Varela et al., 2023), but we predict that the same patterns would hold true.
While predation is the main habit of the hyperdiverse Coccinellidae, the most speciose family in the group, fungi are the main food source of other members of the coccinellid group. Like other mycophagous beetles, they have evolved a diversity of defensive mechanisms for protection (Leschen, 1994). Represented in nine clades, putative aposematic colouration (Fig. 4e, f) is the most frequent defensive strategy of the coccinellid group. Determining the origin and number of times aposematism has arisen within these groups is complicated by intrageneric, and even intraspecific, colour variation (see Arriaga-Varela et al., 2013). The majority of the mycophagous coccinelloids are predominantly surface dwellers. Exceptions include cases like Mycetaea spp. (Mycetaeaidae) or Holoparamecus spp. (Endomychidae), which are usually found in humid forest leaf-litter (Arriaga-Varela et al., 2018; Shockley et al., 2009b), and the endophagous adults and larvae of Lycoperdina spp. (Endomychidae) that predominantly feed inside puffballs (Pakaluk, 1984). Members of these genera have, however, generally plain-coloured bodies. Therefore, aposematism may have little relationship to diet, but rather, it is linked to where the beetles are most active or where they are exposed to visual predators.
The presence of aposematic colouration may be linked to reflex bleeding and gregarism, both of which has been studied in vertebrates (Gamberale & Tullberg, 1998; Riipi et al., 2001). Reflex bleeding is a well-known defence in coccinellid larvae and adults (de Jong, 1991; Knapp et al., 2018) and has been reported in adults of different subfamilies in Endomychidae. Reflex bleeding mainly occurs from the tibio-femoral joints (e.g. Leschen & Carlton, 1988, Skelley & Leschen, 2001). The toxicity to potential predators is unknown. Gregarism has been widely documented in ladybirds that are aposematic (Roberge et al., 2016). Among Endomychidae, a well-documented case of aggregation from the lowland rainforest of Panama has been recorded for Stenotarsus subtilis Arrow, 1920 (Endomychinae) (Roubik & Skelley, 2001; Tanaka et al., 1987; Yoder et al., 1992). This species forms massive aggregations of adults and undergo prolongated diapause. Gregarism, adult and pupal, is a common feature among other species of neotropical Stenotarsus like S. latipes Arrow, 1920 and S. parallelicornis Arriaga-Varela et al., 2013 (Arriaga-Varela, personal observation). Some of these species are brightly orange-coloured and do not exhibit contrasting markings, yet aposematism or crypsis cannot be ruled out. Experimental treatments with similarly coloured stinkbugs show that some of these species can be aposematic for predatory birds and on the other hand are cryptic for predatory insects, such as mantids (Fabricant & Herberstein, 2015).
The balance between detectability and warning-signalling is a complex phenomenon in which body size plays an essential role (Barnett et al., 2016). Physical size will impact visual appearance and thus potentially influence crypsis (Pembury Smith & Ruxton, 2021). We hypothesize that species having a larger body size is at greater risk of being detected by predators than smaller members of the coccinellid group, particularly in “higher Endomychidae” and Coccinellinae which have the highest number of defensive strategies, mostly linked to putatively aposematic colouration.
While an increase in body size has been accompanied by a higher number of defensive strategies, members of Anamorphidae show a comparatively smaller set of features related to the predator avoidance, with debris-cloaking the most widespread. We hypothesize that the evolution of debris-cloaking in Anamorphidae may be related to their comparatively smaller body size. Unlike aposematism, which relies on conveying the message to predators on the potential toxicity or distastefulness of prey, cryptic camouflage is dependent on visually matching a portion of the background (Stevens & Merilaita, 2009). While there are studies suggesting a selective pressure for larger organisms to show aposematic signals, for example larger caterpillars are selected for the presence of dissuasive conspicuous eye spots (Hossie et al., 2015), smaller prey develop cryptic traits as in the case of decoration and debris-cloaking (Pembury Smith & Ruxton 2021; Barnett et al., 2022). This may explain different colours amongst size class in weevils (e.g., Hespenheide, 1995). Anamorphidae, having larvae 1.0 to 4.0 mm in length, fall within a size range for them to develop cryptic behaviour that may be energetically efficient. Our preliminary findings align with the results of Hossie et al. (2015), Pembury Smith and Ruxton (2021), and Barnett et al. (2022) on the effects that body size has on the dichotomy between visual undetectability and dissuasion via aposematic signals.
Experiments are required to confirm hypotheses of possible correlation amongst any of these defensive traits. Additionally, a time-calibrated phylogenetic hypothesis will shed light on the age of appearance of such characters. An integrative phylogenetic approach that considers molecular, morphological, and behavioural evidence of living and fossil taxa of the Anamorphidae and its relatives would help to determine key factors in the defensive strategies and when they evolved (Arriaga-Varela et al. in prep.).
Conclusions
-
Debris-cloaking may be more prevalent in family Anamorphidae than previously recorded.
-
Catapotia larvae are confirmed as using debris-cloaking for camouflage and having adaptations in their dorsal vestiture for holding particles. Whether or not all species are active or passive debris-cloakers remains unknown.
-
Larvae of Catapotia laevissima are most similar to those of Bystus, sharing the elongate antennomere II, the thoracic and abdominal tergal and pleural lobes, and the apical spoon like structure in thoracic setae. Catapotia larvae differ from Bystus in the long maxilla with the stipes about 1.5 times longer than the palpus.
-
Members of the coccinellid group of Coccinelloidea have developed a series of defensive strategies to avoid predation, which are probably linked to their feeding habits and body size.
-
Potentially aposematic colour patterns and likely associated behaviours like gregarism and reflex bleeding are the most common defensive strategies in the coccinellid group. These are predominantly present in lineages with surface dwelling habits and larger body sizes, like the “higher Endomychidae” clade and Coccinellinae.
-
Within Anamorphidae, larval debris-cloaking is the most prevalent defensive strategy for avoiding predation. However, it is not present in all the known larvae of the family. We hypothesize that debris-cloaking larvae has evolved from a spore-feeding, small-bodied ancestor.
-
We hypothesize that body size is likely the most important factor driving the selection forces that shaped the distribution of defensive strategies among the coccinellid group.
Data availability
All data generated or analysed during this study are included in this published article.
References
Agnarsson, I., & Miller, J. A. (2008). Is ACCTRAN better than DELTRAN? Cladistics, 24(6), 1032–1038.
Alekseev, V. I., & Tomaszewska, W. (2018). New handsome fungus beetles (Coleoptera: Coccinelloidea: Anamorphidae, Endomychidae) from European amber of the Upper Eocene. Palaeontologia Electronica, 21(1), 1–23.
Arriaga-Varela, E., Tomaszewska, K. W., & Navarrete-Heredia, J. L. (2007). A synopsis of the Endomychidae (Coleoptera: Cucujoidea) of Mexico. Zootaxa, 1594(1), 1–38.
Arriaga-Varela, E., Tomaszewska, W., Huo, L., & Seidel, M. (2018). On Neotropical Merophysiinae with descriptions of a new genus and new species (Coleoptera, Endomychidae). ZooKeys, 736, 1–41.
Arriaga-Varela, E., Tomaszewska, W., Szawaryn, K., Robertson, J., Seidel, M., Ślipiński, A., & Fikáček, M. (2023). The resurrection of Cerasommatidiidae, an enigmatic group of coccinelloid beetles (Coleoptera: Coccinelloidea) based on molecular and morphological evidence. Zoological Journal of the Linnean Society, 197, 1078–1115. https://doi.org/10.1093/zoolinnean/zlac082
Arriaga-Varela, E., Zaragoza-Caballero, S., Tomaszewska, W., & Navarrete-Heredia, J. L. (2013). Preliminary review of the genus Stenotarsus Perty (Coleoptera: Endomychidae) from México, Guatemala and Belize, with descriptions of twelve new species. Zootaxa, 3645, 1–79.
Arrow, G. J. (1920). A contribution to the classification of the coleopterous family Endomychidae. Transactions of the Entomological Society of London, 1920, 1–83.
Aslam, M. (2020). Conspicuousness and toxicity of Coccinellidae: An aposematic review. Arthropods, 9(3), 85–91.
Bameul, F. (1989). Description du comportement de camouflage d’un Coléoptère: le déguisement actif de Georissus crenulatus (Coleoptera Georissidae), et proposition d’une nouvelle classification des déguisements chez les Invertébrés. Comptes Rendus Académie des Sciences du Paris, 309 (Série III), 351–356.
Barnett, J. B., Scott-Samuel, N. E., & Cuthill, I. C. (2016). Aposematism: Balancing salience and camouflage. Biology Letters, 12(8), 20160335.
Barnett, J. B., Yeager, J., McEwen, B. L., Kinley, I., Anderson, H. M., & Guevara, J. (2022). Size-dependent colouration balances conspicuous aposematism and camouflage. Journal of Evolutionary Biology, 2022, 1–10.
Berke, S. K., Miller, M., & Woodin, S. A. (2006). Modelling the energy–mortality trade-offs of invertebrate decorating behaviour. Evolutionary Ecology Research, 8(8), 1409–1425.
Brandt, M., & Mahsberg, D. (2002). Bugs with a backpack: The function of nymphal camouflage in the West African assassin bugs Paredocla and Acanthaspis spp. Animal Behaviour, 63(2), 277–284.
Burakowski, B., & Ślipiński, S. A. (2000). The larvae of Leiestinae with notes on the phylogeny of Endomychidae (Coleoptera: Cucujoidea). Annales Zoologici, 50, 559–573.
Cai, C., Tihelka, E., Giacomelli, M., Lawrence, J. F., Ślipiński, A., Kundrata, R. Y., & S., Thayer, M. K., Newton, A. F., Leschen, R. A. B., Gimmel, M. L., Lü, L., Engel, M. S., Bouchard, P., Huang, D, Pisani, D., & Donoghue, P. C. J. (2022). Integrated phylogenomics and fossil data illuminate the evolution of beetles. Royal Society Open Science, 9(3), 211771.
Chaboo, C. S., & McHugh, J. V. (2010). Maternal care by a species of Pselaphacus percheron (Coleoptera: Erotylidae: Erotylinae) from Peru. The Coleopterists Bulletin, 64(2), 116–118.
Champion, G. C. (1913). Notes on various Central American Coleoptera, with descriptions of new genera and species. Transactions of the Entomological Society of London, 1913, 58–169.
de Jong, P. W., Holloway, G. J., Brakefield, P. M., & de Vos, H. (1991). Chemical defence in ladybird beetles (Coccinellidae). II. Amount of reflex fluid, the alkaloid adaline and individual variation in defence in 2-spot ladybirds (Adalia bipunctata). Chemoecology, 2, 15–19.
Drilling, K., & Dettner, K. (2010). First insights into the chemical defensive system of the erotylid beetle. Tritoma Bipustulata. Chemoecology, 20(4), 243–253.
Eisner, T., Hicks, K., Eisner, M., & Robson, D. S. (1978). “Wolf-in-sheep’s-clothing” strategy of a predaceous insect larva. Science, 199(4330), 790–794.
Endler, J. A. (1981). An overview of the relationships between mimicry and crypsis. Biological Journal of the Linnean Society, 16(1), 25–31.
Escalona, H. E., Zwick, A., Li, H. S., Li, J., Wang, X., Pang, H., Hartley, D., Jermiin, L. S., Nedvěd, O., Misof, B., Niehuis, O., Ślipiński, A., & Tomaszewska, W. (2017). Molecular phylogeny reveals food plasticity in the evolution of true ladybird beetles (Coleoptera: Coccinellidae: Coccinellini). BMC Evolutionary Biology, 17, 1–11.
Fabricant, S. A., & Herberstein, M. E. (2015). Hidden in plain orange: Aposematic colouration is cryptic to a colorblind insect predator. Behavioral Ecology, 26(1), 38–44.
Gamberale, G., & Tullberg, B. S. (1998). Aposematism and gregariousness: The combined effect of group size and colouration on signal repellence. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1399), 889–894.
Giorgi, J. A., Vandenberg, N. J., McHugh, J. V., Forrester, J. A., Ślipiński, S. A., Miller, K. B., Shapiro, L. R., & Whiting, M. F. (2009). The evolution of food preferences in Coccinellidae. Biological Control, 51(2), 215–231.
Gorham, H. S. (1874). Descriptions of new species of Coleoptera from Japan. Entomologist’s Monthly Magazine, 10, 224–226.
Gressitt, J. L., Samuelson, G. A., & Vitt, D. H. (1968). Moss growing on living Papuan mossforest weevils. Nature, 217(5130), 765–767.
Guérin–Méneville, F. E. (1857). Matériaux pour une Monographie des Coléoptères du groupe des Eumorphides, et plus. spécialement du genre Eumorphus. Archives Entomologiques, 1, 237–280 + pl. 13.
Guinot, D., & Wicksten, M. K. (2015). Camouflage: carrying behaviour, decoration behaviour, and other modalities of concealment in Brachyura. In Castro, P., Davie, P., Guinot, D., Schram, F., & Klein, C.V., (Eds.), Treatise on zoology-anatomy, taxonomy, biology. The Crustacea, 9, 583–638. Brill, The Netherlands.
Hanley, R. S., Goodrich, M., & A. (1995). Review of mycophagy, host relationships and behavior in the new world oxyporinae (Coleoptera: Staphylinidae). The Coleopterists Bulletin, 49(3), 267–280.
Hanski, I. (1989). Fungivory: Fungi, insects and ecology. In N. Wilding, N. Collins, P. Hammond, & J. Webber (Eds.), Insect–fungus Interactions (pp. 25–68). Academic Press.
Hartley & McHugh, J. V. (2010). Latridiidae Erichson, 1842. In R. A. B. Leschen, R. G. Beutel & J. F. Lawrence (Eds.), Coleoptera, beetles. Volume 2: Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (pp. 481–486). In N. P. Kristensen, & R. G. Beutel, (Eds.), Handbook of zoology. Arthropoda: Insecta. Walter de Gruyter.
Harvey, P. H., & Paxton, R. J. (1981). The evolution of aposematic colouration. Oikos, 37, 391–393.
Hayashi, N. (1984). The endomychid species Idiophyes niponensis (Gorham) occurring in a house and its larva (Coleoptera). House and Household Insect Pests, 21–22, 17–20. [in Japanese, with English title]
Hayashi, M., & Nomura, M. (2011). Larvae of the green lacewing Malladades jardinsi (Neuroptera: Chrysopidae) protect themselves against aphid-tending ants by carrying dead aphids on their backs. Applied Entomology and Zoology, 46(3), 407–413.
Hespenheide, H. A. (1995). Mimicry in the Zygopinae (Coleoptera: Curculionidae). Memoirs of the Entomological Society of Washington, 14, 145–154.
Hossie, T. J., Skelhorn, J., Breinholt, J. W., Kawahara, A. Y., & Sherratt, T. N. (2015). Body size affects the evolution of eyespots in caterpillars. Proceedings of the National Academy of Sciences, 112(21), 6664–6669.
Hultgren, K. M., & Stachowicz, J. J. (2008). Alternative camouflage strategies mediate predation risk among closely related co-occurring kelp crabs. Oecologia, 155, 519–528.
Kiesmüller, C., Haug, J. T., Müller, P., & Hörnig, M. K. (2021). Debris-carrying behaviour of bark lice immatures preserved in 100 million years old amber. PalZ, 96(2), 231–258.
Knapp, M., Dobeš, P., Řeřicha, M., & Hyršl, P. (2018). Puncture vs. reflex bleeding: Haemolymph composition reveals significant differences among ladybird species (Coleoptera: Coccinellidae), but not between sampling methods. European Journal of Entomology, 115, 1–6.
Lea, A. M. (1922) On Australian Coleoptera. Part IV. Records of the South Australian Museum, 2, 271–308, pl. 4.
Leschen, R. A. B. (1994). Ecological and behavioral correlates among mycophagous Coleoptera. Folia Entomologica Mexicana, 92, 9–19.
Leschen, R. A. B., & Carlton, C. E. (1988). Immature stages of Endomychus biguttatus Say (Coleoptera: Endomychidae) with observations on the alimentary canal. Journal of the Kansas Entomological Society, 61, 321–327.
Leschen, R. A., & Carlton, C. E. (1993). Debris cloaking in Endomychidae: A new species from Peru (Coleoptera). Zoological Journal of the Linnean Society, 109(1), 35–51.
Leschen, R. A., & Carlton, C. E. (2000). A New Species of Micropsephodes From Southern United States (Coleoptera: Endomychidae: Anamorphinae. The Coleopterists Bulletin, 54(2), 232–238.
Lord, N. P., Hartley, C. S., Lawrence, J. F., McHugh, J. V., Whiting, M. F., & Miller, K. B. (2010). Phylogenetic analysis of the minute brown scavenger beetles (Coleoptera: Latridiidae), and recognition of a new beetle family, Akalyptoischiidae fam. n. (Coleoptera: Cucujoidea). Systematic Entomology, 35(4), 753–763.
Maddison, W. P., Donoghue, M. J., & Maddison, D. R. (1984). Outgroup analysis and parsimony. Systematic Biology, 33(1), 83–103.
McHugh, J. V., & Kiselyova, T. G. (2003). First descriptions for larval stages of Eurysphindus (Coleoptera: Cucujoidea: Sphindidae). The Coleopterists Bulletin, 57(1), 17–25.
McHugh, J. V., & Pakaluk, J. (1997). Review of the larval stages of Epipocinae (Insecta: Coleoptera: Endomychidae). Annales Zoologici, 47(1), 59–77.
McKenna, D. D., Shin, S., Ahrens, D., Balke, M., Beza-Beza, C., Clarke, D. J., Donath, A., Escalona, H. E., Friedrich, F., Letsch, H., Liu, S., Maddison, D., Mayer, C., Misof, B., Murin, P. J., Niehuis, O., Peters, R. S., Podsiadlowski, L., Pohl, H., … Beutel, R. G. (2019). The evolution and genomic basis of beetle diversity. Proceedings of the National Academy of Sciences, 116(49), 24729–24737.
Nakahira, K., & Arakawa, R. (2006). Defensive functions of the trash-package of a green lacewing, Malladades jardinsi (Neuroptera: Chrysopidae), against a ladybird, Harmonia axyridis (Coleoptera: Coccinellidae). Applied Entomology and Zoology, 41(1), 111–115.
Navarrete-Heredia, J. L., Tokareva, A., Arriaga-Varela, E., Newton, A. F., & Solodovnikov, A. (2021). Possible stridulatory organs in oxyporine rove beetles (Coleoptera: Staphylinidae), their biological role and systematic significance. Journal of Natural History, 55(33–34), 2129–2143.
Pakaluk, J. (1984). Natural history and evolution of Lycoperdina ferruginea (Coleoptera: Endomychidae) with descriptions of immature stages. Proceedings of the Entomological Society of Washington, 86(2), 312–325.
Pembury Smith, M. Q., & Ruxton, G. D. (2020). Camouflage in predators. Biological Reviews, 95(5), 1325–1340.
Pembury Smith, M. Q., & Ruxton, G. D. (2021). Size-dependent predation risk in cryptic prey. Journal of Ethology, 39, 191–198.
Pérez-de la Fuente, R., Delclòs, X., Peñalver, E., Speranza, M., Wierzchos, J., Ascaso, C., & Engel, M. S. (2012). Early evolution and ecology of camouflage in insects. Proceedings of the National Academy of Sciences, 109(52), 21414–21419.
Pérez-de la Fuente, R., Peñalver, E., Azar, D., & Engel, M. S. (2018) A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Abstract Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-34870-1
Rider, S. D., & Hostetler, H. A. (2022). Reflex bleeding in tonically immobilized larvae causes debris-based camouflage in the blue death-feigning beetle, Asbolus verrucosus LeConte (Coleoptera: Tenebrionidae). The Coleopterists Bulletin, 76(2), 237–247.
Riipi, M., Alatalo, R. V., Lindström, L., & Mappes, J. (2001). Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature, 413(6855), 512–514.
Roberge, C., Fréchette, B., Labrie, G., Dumont, F., & Lucas, E. (2016). Gregarious pupation act as a defensive mechanism against cannibalism and intraguild predation. Insect Science, 23(4), 612–620.
Robertson, J. A., McHugh, J. V., & Whiting, M. F. (2004). A molecular phylogenetic analysis of the pleasing fungus beetles (Coleoptera: Erotylidae): Evolution of colour patterns, gregariousness and mycophagy. Systematic Entomology, 29(2), 173–187.
Robertson, J. A., Ślipiński, A., Moulton, M., Shockley, F. W., Giorgi, A., Lord, N. P., Mckenna, D. D., Tomaszewska, W., Forrester, J., Miller, K. B., Whiting, M. F., & McHugh, J. V. (2015). Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Systematic Entomology, 40(4), 745–778.
Roubik, D. W., & Skelley, P. E. (2001). Stenotarsus subtilis Arrow, the aggregating fungus beetle of Barro Colorado Island nature monument, Panama (Coleoptera: Endomychidae). The Coleopterists Bulletin, 55(3), 249–263.
Ruxton, G. D., & Stevens, M. (2015). The evolutionary ecology of decorating behaviour. Biology Letters, 11, 20150325.
Shockley, F. W., & Tomaszewska, K. W. (2007). First larval description for Symbiotes gibberosus (Lucas) (Coleoptera: Endomychidae). Annales Zoologici, 57, 741–755.
Shockley, F. W., Tomaszewska, K. W., & McHugh, J. V. (2009a). An annotated checklist of the handsome fungus beetles of the world (Coleoptera: Cucujoidea: Endomychidae). Zootaxa, 1999, 1–113.
Shockley, F. W., Tomaszewska, K. W., & McHugh, J. V. (2009b). Review of the natural history of the handsome fungus beetles (Coleoptera: Cucujoidea: Endomychidae). Insecta Mundi, 72, 1–24.
Skelley, P., & Leschen. R. A. B. (2001). Endomychidae Leach, 1815. In R. H. Arnett Jr. & M. Thomas, (Eds). American beetles, 2, 366–370. CRC Press.
Stevens, M., & Merilaita, S. (2009). Animal camouflage: Current issues and new perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1516), 423–427.
Strohecker, H. F. (1956). Notes on Papuan Endomychidae with the description of a new genus and species (Coleoptera). Annales Historico-Naturales Musei Nationalis Hungarici, 7, 69–70.
Ślipiński, A. & Tomaszewska, W. (2010a). Coccinellidae Latreille, 1802. In R. A. B. Leschen, R. G. Beutel & J. F. Lawrence (Eds.), Coleoptera, beetles. Volume 2: Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (pp. 454–472). In N. P. Kristensen, & R. G. Beutel, (Eds.). Handbook of Zoology. Arthropoda: Insecta. Walter de Gruyter.
Ślipiński, A. & Tomaszewska, W. (2010b). Alexiidae Imhoff, 1856. In R. A. B. Leschen, R. G. Beutel & J. F. Lawrence (Eds.), Coleoptera, beetles. Volume 2: Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (pp. 432–434). In N. P. Kristensen, & R. G. Beutel, (Eds.), Handbook of Zoology. Arthropoda: Insecta. Walter de Gruyter.
Ślipiński, A., Lawrence, J. F., & Cline, A. R. (2010). Corylophidae LeConte, 1852. Pp. 472–481. In R. A. B. Leschen, R. G. Beutel & J. F. Lawrence (Eds.), Coleoptera, beetles. Volume 2: Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (pp. Pp. 472–481). In N. P. Kristensen, & R. G. Beutel, (Eds.), Handbook of zoology. Arthropoda: Insecta. Walter de Gruyter.
Tanaka, S., Wolda, H., & Denlinger, D. L. (1987). Abstinence from mating by sexually mature males of the fungus beetle, Stenotarsus rotundus, during a tropical dry season. Biotropica, 19, 252–254.
Tauber, C. A., Tauber, M. J., & Albuquerque, G. S. (2014). Debris-carrying in larval Chrysopidae: Unravelling its evolutionary history. Annals of the Entomological Society of America, 107(2), 295–314.
Thomson, J. (1860). Musée Scientifique ou recueill d’histoire naturelle, Heft 1.
Tomaszewska, K. W. (2002). A review of the genus Archipines Strohecker (Coleoptera: Endomychidae), with descriptions of new taxa and immature stages of Archipines championi Gorham. Annales De La Société Entomologique De France, 38(4), 363–383.
Tomaszewska, W. (2005). Phylogeny and generic classification of the subfamily Lycoperdininae with a re-analysis of the family Endomychidae (Coleoptera: Cucujoidea). Annales Zoologici, 55(supplement), 1–172.
Tomaszewska, W. (2010). Endomychidae Leach, 1815. In R. A. B. Leschen, R. G. Beutel & J. F. Lawrence (Eds.), Coleoptera, beetles. Volume 2: Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (pp. 442–454). In N. P. Kristensen, & R. G. Beutel, (Eds.), Handbook of zoology. Arthropoda: Insecta. Walter de Gruyter.
Tomaszewska, W. (2022). A contribution to the knowledge of immature stages of Endomychidae (Coleoptera: Coccinelloidea)–Description of larva of Trochoideus dalmani westwood. Annales Zoologici, 72(2), 277–283.
Toussaint, E. F., Seidel, M., Arriaga-Varela, E., Hájek, J., Král, D., Sekerka, L., Short, A. E. Z., & Fikáček, M. (2017). The peril of dating beetles. Systematic Entomology, 42, 1–10.
Wang, B., Xia, F., Engel, M. S., Perrichot, V., Shi, G., Zhang, H., Chen, J., Jarzembowski, E. A., Wappler, T., & Rust, J. (2016). Debris-carrying camouflage among diverse lineages of Cretaceous insects. Science Advances, 2(6), e1501918.
Yoder, J. A., Denlinger, D. L., & Wolda, H. (1992). Aggregation promotes water conservation during diapause in the tropical fungus beetle. Stenotarsus Rotundus. Entomologia Experimentalis Et Applicata, 63(2), 203–205.
Yoshida, T., & Leschen, R. A. B. (2020). Larval descriptions and exuvial retention of Toramini (Coleoptera: Erotylidae: Cryptophilinae). The Coleopterists Bulletin, 74(1), 1–14.
Acknowledgements
We thank Magdalena Kowalewska-Groszkowska (MIZ) for the help with the habitus photographs and SEM illustrations. Karol Szawaryn (MIZ) provided valuable feedback on the biology of Coccinellidae and Kelly Greig (University of Auckland, New Zealand) commented on a draft prior to submission. Ibeth Rodríguez Gutiérrez (Tecnológico de Estudios Superiores de Huixquilucan, Mexico) confirmed the fungal nature of the material in the gut contents of the larvae. We are deeply thankful to Joseph V. McHugh (University of Georgia, USA) and an anonymous reviewer for their valuable comments and suggestions.
Funding
This work was partly supported by the National Science Centre of Poland (Narodowe Centrum Nauki; grant No. 2020/36/C/NZ8/00584 to Emmanuel Arriaga-Varela). Richard A. B. Leschen was supported in part by Marsden Fund Te Pūtea Rangahau (Contract LCR1902).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arriaga-Varela, E., Leschen, R.A.B. & Tomaszewska, W. The debris-cloaking larva of Catapotia laevissima and the origin of defensive strategies in Anamorphidae and other Coccinelloidea (Coleoptera). Org Divers Evol 23, 901–915 (2023). https://doi.org/10.1007/s13127-023-00622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-023-00622-x