Abstract
Metabolic dysfunction–associated fatty liver disease (MAFLD) is nowadays considered the liver manifestation of metabolic syndrome. Its prevalence is increasing worldwide in parallel to the epidemic of diabetes and obesity. MAFLD includes a wide spectrum of liver injury including simple steatosis and non-alcoholic steatohepatitis (NASH) that may lead to serious complications such as liver cirrhosis and liver cancer. The complexity of its pathophysiology and the intricate mechanisms underlying disease progression explains the huge variety of molecules targeting diverse biological mechanisms that have been tested in preclinical and clinical settings in the last two decades. Thanks to the large number of clinical trials of the last few years, most of them still ongoing, the pharmacotherapy scenario of MAFLD is rapidly evolving. The three major components of MAFLD, steatosis, inflammation, and fibrosis seem to be safely targeted with different agents at least in a large proportion of patients. Likely, in the next few years more than one drug will be approved for the treatment of MAFLD at different disease stages. The aim of this review is to synthesize the characteristics and the results of the most advanced clinical trials for the treatment of NASH to evaluate the recent advances of pharmacotherapy in this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Definition and epidemiology
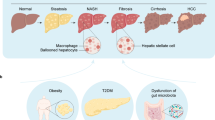
Metabolic dysfunction–associated fatty liver disease (MAFLD) has become one of the most relevant forms of chronic liver disease worldwide due to the progressively increased in obesity rates over the past 30–40 years [1]. Obesity is defined as a body mass index ≥ 30 kg/m2 and the World Health Organization estimates that over 13% of the world population (> 600 million people) suffer from it, with more prevalent rates among children and adolescents [2]. The metabolic syndrome includes three out of the following conditions: abdominal obesity, hypertriglyceridemia, low HDL cholesterol, arterial hypertension, and/or hyperglycemia. MAFLD is not only associated to those comorbidities but also to insulin resistance. Indeed, the nomenclature of non-alcoholic fatty liver disease (NAFLD) has been updated to MAFLD. This term describes better the liver disease associated with known metabolic dysfunction. In fact, the new definition of MAFLD refers to hepatic steatosis in addition to one of the following three criteria: overweight/obesity, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation. The exclusion of other liver diseases including alcoholic, autoimmune, or viral hepatitis is not a prerequisite for the diagnosis of MAFLD [3].
Within the general population, the overall global prevalence of NAFLD (defined using imaging criteria) is estimated to be 25% [4]. However, important variability was observed according to geographic regions, being up to 31.8% in the Middle East, 30.4% in South America, and 13.5% in Africa [4]. NAFLD incidence has rarely been measured.
Given the common risk factors between NAFLD and cardiovascular risk factors, cardiac-related death is one of the leading causes of death for NAFLD patients [5]. Other causes include liver-related death, malignancy, or other causes such as infections, type 2 diabetes mellitus, or pulmonary embolism.
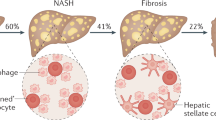
NAFLD is defined as the presence of steatosis in > 5% of hepatocytes in the absence of other competing chronic liver diseases and without significant alcohol consumption (< 20 g/day in women and < 30 g/day in men). NAFLD includes non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), the latter being more severe as it includes hepatocyte damage with the risk of subsequent development of significant fibrosis, cirrhosis, and/or hepatocellular carcinoma [6]. In this context, NASH has become one of the leading causes of cirrhosis in adults in the USA and NASH-related cirrhosis is currently the second indication of liver transplantation in the USA [7].
Whereas the definitive diagnosis of both MAFLD and NASH requires a liver biopsy, imagine techniques such as magnetic resonance imaging (MRI) (MRI proton density fat fraction [MRI-PDFF] or magnetic resonance spectroscopy), computed tomography (CT), ultrasound (US) with controlled attenuation parameter (CAP), and/or elastography may provide a valuable assessment of fat deposition and significant fibrosis [6]. Although no specific treatments other than the control of the associated metabolic disorders is available, follow-up to control comorbidities is recommended to detect disease progression.
Genetics and pathogenesis
Most individuals with MAFLD have no symptoms, and the disease may remain silent until it has progressed to cirrhosis [8]. Recent data suggest that the transition from NAFL to NASH is quite dynamic whereas fibrosis development is significantly slower in NAFLD than in NASH, needing even up to 14 years [9]. Up to 20% of patients with NASH are considered as “rapid progressors” where, although predictors are largely unknown, genetic susceptibility might play a role [10]. The relevance of the genetic determinants is just beginning to be elucidated [11], but they may involve intrahepatic lipolysis, triglyceride export, hepatic mitochondrial oxidation, or glucokinase activity. For instance, a significant association of a variant in “patatin-like phospholipase domain-containing 3” (PNPLA3) gene on chromosome 22 leading to modifications in retinol metabolism [12]; or in “transmembrane 6 superfamily member 2” (TM6SF2) gene on chromosome 19 leading to an impaired lipid transporter, have been associated with fatty liver disease [13]. Polymorphism in “ectoenzyme nucleotide pyrophosphate phosphodiesterase 1” (ENPP1 or PC1) and in “insulin receptor substrate-1” (IRS1) genes, which are related to insulin resistance, have also been described in NAFLD patients [14]. Even, a polymorphism in “membrane bound O-acyltransferase domain-containing 7” (MBOAT7-TMC4) gene, involved in oxidative stress, increases the risk of fibrosis in patients with NAFLD [15]. Genetic variants in “glucokinase regulatory protein” (GCKR) gene [16], “solute carrier family 2-member 1” (SLC2A1) gene [17], and “17-beta hydroxysteroid dehydrogenase 13” (HSD17B13) gene [18], have also been identified in patients with fatty liver disease.
NASH is the result of multiple intracellular signals derived either from proinflammatory molecules and contact with immune cells and/or from external stimulation through visceral adipose tissue and gut microbiome, the last two conditioned by diet. The outcome of these interactions on hepatocytes includes modified insulin signaling, lipogenesis, mitochondrial dysfunction, and activation or abnormal functioning of nuclear receptors such as bile acid receptors farnesoid X receptor (FXR) [19], liver X receptor (LXR) [20], pregnane X receptor (PXR) [21], and vitamin D receptor (VDR) [22] leading to hepatic inflammation by different downstream effects. Hepatic stellate cells are activated by these signals may trigger a fibrogenic response with extracellular matrix production [23].
Diet with excess carbohydrates and saturated fat lead to an energy metabolism imbalance with lipid deposition in skeletal muscle. Subsequent muscle insulin resistance due to increased intramyocellular lipid content impedes the storage of ingested glucose as muscle glycogen [24]. Glucose is then rerouted to the liver where insulin resistance stimulates sterol regulatory element–binding protein 1c (SREBP1c) [25] which increases the expression of hepatic enzymes that regulate de novo lipogenesis with higher VLDL production and hypertriglyceridemia. Other transcription factors such as carbohydrate-responsive element–binding protein (ChREBP) [26], peroxisome proliferator–activated receptor gamma coactivator 1-beta (PPARg coactivator 1-b) [27], and LXR [28] also foster liver lipogenesis.
Although visceral adipose tissue has been related to the pathogenesis of NASH, visceral adipose tissue may just be an additional site for lipid accumulation when subcutaneous adipose tissue capacity is surpassed [29]. Circulating triglyceride-enriched lipoproteins are cleared by peripheral lipoprotein lipase (Lpl) [30]. However, an increase in endogenous Lpl inhibitors, such as apolipoprotein C3 (ApoC3) [31], angiopoietin-like proteins 3/8 (ANGPTL3/8) complex, and ANGPTL4 [32], impedes clearance of these circulating triglyceride-enriched lipoproteins and therefore, induces an increased hepatic triglyceride uptake. Increases in endogenous Lpl inhibitors have been described in NASH patients.
All in all, NASH is a heterogeneous disease with a complex pathophysiology that makes finding a single effective treatment a major challenge.
Drug therapy in NASH
It is well-known that the first approach to treat NAFLD patients is the nutritional intervention with a change in lifestyles. However, the complexity of NASH pathophysiology and the interplay of multiple genetic and environmental factors in disease progression explains why the pharmacotherapy of this clinical condition includes a variety of molecules with different biological targets, from drugs used to treat of the diseases that contribute to NASH development and progression (i.e., antidiabetic agents) to drugs targeting liver inflammation and fibrosis. Its long natural history and wide spectrum of severity, from mild inflammation to end-stage cirrhosis, are challenges in the evaluation of pharmacological interventions and requires specific interventions for each disease stage.
In the last 10 years, more than 20 molecules have been tested for the treatment of NASH. Most drugs were discarded after unsuccessful clinical trials, others are still in early stage of clinical development (phase 1 and 2 clinical trials) and a few have reached phase 3 trials. So far, drugs at most advanced clinical development include antidiabetic drugs, FXR agonists, PPAR agonists, and thyroid hormone receptor (THR) agonists (Fig. 1). Table 1 includes a synthesis of the clinical trials (phase II/III) in treatment of NASH.
Antidiabetic drugs
As insulin resistance and type 2 diabetes mellitus are closely associated with NASH, the efficacy of several antidiabetic agents has been studied in NASH. Pioglitazone showed histological benefits (improved NAFLD activity score, also called NAS or improvement in single histological components of NASH) in three randomized trials in diabetic and prediabetic patients with NASH. However, the effect on liver fibrosis was not significant, although worsening of liver fibrosis was not observed [33,34,35].
Newer antidiabetic agents including SGLT1/2 (sodium-glucose co- transporter-1/2) inhibitors and GLP-1R (glucagon-like peptide 1 receptor) agonists are being tested in NASH. Dapagliflozin and semaglutide have reached late-stage clinical development.
Dapagliflozin is an oral SGLT2 inhibitor which impedes glucose reabsorption in the proximal tubule leading to glucosuria and plasma glucose reduction. Between 2018 and 2021, many studies regarding the use of dapagliflozin in NASH patients were published. A meta-analysis of 7 trials showed that treatment with 10 mg dose dapagliflozin compared to the placebo or control group in patients with NASH (image-based diagnosis) significantly lowered ALT (weighted mean difference (WMD): − 6.62U/L; 95%CI: − 12.66, − 0.58; p = 0.03) and AST levels (WMD: − 4.20U/L; 95%CI: − 7.92,-0.47; p = 0.03). Gamma-glutamyl transferase (GGT) levels were non-significantly decreased whereas homeostatic model assessment of insulin resistance (HOMA-IR) was significantly affected (WMD: − 0.88; 95%CI: − 1.43,-0.33; p = 0.002). Although levels of total cholesterol increased under dapagliflozin treatment, safety profile between groups had no significant difference [36]. The DEAN study (NCT03723252) is a phase 3 trial which compares dapagliflozin vs. placebo among patients with histologically confirmed NASH with the primary endpoint of improvement in scored liver histology at 12 months. Other endpoints include resolution of NASH, changes in fibrosis score and changes in metabolic features such as body weight, hemoglobin A1 (Hb1Ac) or insulin resistance.
Other SGLT1/2 inhibitors are in earlier phases of clinical testing in NASH. The ELIVATE study (NCT04065841) is assessing if licogliflozin alone or in combination with tropifexor, an agonist of the bile acid receptor FXR, improves fibrosis and/or NAS score in patients with NASH and fibrosis stage 2 or 3. A phase 2a study to evaluate the safety and tolerability of MET409 (a synthetic FXR agonist) alone or in combination with empagliflozin in patients with type 2 diabetes mellitus and NASH has finalized recruitment and the results will be reported soon.
Semaglutide is a GLP-1R agonist which enhances insulin secretion to regulate blood glucose level. This GLP-1R agonist was first assessed in a 72-week phase 2 clinical trial [37] in patients with biopsy-confirmed NASH and liver fibrosis stages 1, 2, or 3. Patients were randomly assigned to receive semaglutide at a dose of 0.1 mg (80 patients), 0.2 mg (78 patients), or 0.4 mg (82 patients) or placebo (80 patients). Primary outcome was NASH resolution without worsening of fibrosis and only patients with stage 2 or 3 fibrosis levels were assessed in this cohort. This outcome was achieved in 40% of patients in the 0.1-mg group, 36% in the 0.2-mg group, 59% in the 0.4-mg group, and 17% in the placebo group (p < 0.001 for semaglutide 0.4 mg vs. placebo). However, no significant differences were observed between the different doses used and the percentage of patients with an improvement in fibrosis staging (43% with 0.4 mg vs. 33% with placebo, p = 0.48). Gastrointestinal and gallbladder disorders occurred in a higher percentage of patients in the semaglutide groups. Increases in amylase and lipase from baseline were also observed in the treated groups without any acute pancreatitis episode. A phase 3 clinical trial (ESSENCE, NCT04822181) is recruiting non-cirrhotic NASH patients to evaluate the potential resolution of steatohepatitis and the improvement in fibrosis with this GLP-1R agonist.
The LEAN trial [38], a phase 2 trial evaluating the effect of liraglutide (1.8 mg daily) compared to placebo in patients with biopsy-confirmed NASH, showed histological resolution of NASH (39% with liraglutide vs. 9% with placebo, p = 0.019). Fibrosis progression was observed in 36% of patients receiving placebo vs in 9% of liraglutide-treated patients (p = 0.04). Adverse events reported were mainly gastrointestinal disorders or administration site reactions.
Others GLP1R such as cotadutide (NCT04019561), tirzepatide (NCT04166773), or efinopegdutide (NCT04944992) have been tested or are still being tested in phase 2 trials and the results have not been published yet.
PPAR modulators
The peroxisome proliferator-activator receptor (PPAR) family is formed by PPARα, PPARγ, and PPARδ which are mainly located in liver, brown adipose tissue, and macrophage. They activate fatty acid oxidation, lower synthesis of triglycerides, and increase insulin sensitivity. Saroglitazar is a dual PPARα and PPARγ agonist, that was first approved in India for the treatment of type 2 diabetes mellitus patients with hypertriglyceridemia [39]. Since 2020, it is widely used as treatment for NASH patients in India. Among patients with NAFLD (stages 0–3) diagnosed by imaging (ultrasound, CT scan, or MRI) or liver biopsy showing NASH or simple steatosis, and alanine aminotransferase (ALT) > 1.5 upper limit of normal recruited into a phase 3 trial (EVIDENCES II), saroglitazar reduced ALT levels after 16 weeks of therapy [40]. Patients receiving saroglitazar 4 mg reduced the most their levels of ALT (− 45.8% vs. 3.4%, p < 0.001). Histological improvement of NASH after 16 weeks of treatment was observed with 4 mg of saroglitazar (reduction in liver fat content − 19.7% vs. 4.1% with placebo (p = 0.004)). Safety and tolerability of saroglitazar were further assessed in a phase 2 study (EVIDENCES IV), and the most frequent adverse effects were diarrhea and cough [41].
After a successful phase 2 b trial, elafibranor, a dual PPAR-alpha/delta agonist, was tested in a phase 3 clinical trial in patients with non-cirrhotic NASH. In this trial, elafibranor did not meet histological endpoints (NASH resolution without worsening of fibrosis) nor the key secondary endpoint (fibrosis improvement at least one stage) [42].
Lanifibranor, the only pan-PPAR agonist, was assessed in non-cirrhotic biopsy-confirmed highly active NASH (stages 0–3) patients in a phase 2b trial (NATIVE study) were 1200 mg showed better results than 800 mg dose or placebo in histological resolution of NASH (49% and 39%, respectively, vs. 22%), histological improvement of fibrosis (48% and 34%, respectively, vs. 29%), or both (35% and 25%, respectively, vs. 9%) were observed [43]. Nausea, diarrhea, peripheral edema, anemia, and weight gain occurred more frequently with lanifibranor than with placebo.
The PPARα agonist pemafibrate has been tested in NASH patients (MRI diagnosis with ALT elevation) [44]. The primary endpoint of percentage change in liver fat content measured by MRI at week 24 was not met (− 5.3% vs − 4.2%; treatment difference − 1.0%, p = 0.85). However, liver stiffness measured by MRI decreased at week 48 (treatment difference − 5.7%, p = 0.036), and was maintained at week 72 (treatment difference − 6.2%, p = 0.024).
FXR agonists
FXR modulation may have multiple implications in the treatment of NASH since, besides bile acid (BA) synthesis, this receptor is involved in glucose and lipid metabolism, and in the regulation of inflammation [45]. FXR activation downregulates bile acid synthesis through the upregulation of fibroblast growth factor (FGF)-19 expression and by reducing the expression of CYP7A1, the rate-limiting enzyme of the BA synthesis. This results in a protective effect against the toxic accumulation of BAs through increased conjugation in the liver and secretion into the bile canaliculi. FXR activation improves glucose tolerance by reducing hepatic gluconeogenesis and increasing glycogen synthesis [46]. Additionally, FXR activation reduces fat accumulation in the liver by targeting SHP expression and CYP7A1 activity [47]. Obeticholic acid (OCA) is a semi-synthetic bile acid analog of chenodesoxicholic acid with a potent agonist activity on the FXR in the liver and in the intestine. OCA, as the other members of FXR agonists family, may exert multiple therapeutic effects on NASH by improving hepatic lipid and glucose metabolism and through its anti-inflammatory and anti-fibrotic activity. OCA lowers plasma triglycerides by downregulating the expression of SREBP1c expression (48) and increases hepatic fatty acid oxidation through upregulation of pyruvate dehydrogenase kinase 4 (PDK4) and modulating glucose-dependent lipogenic genes [49]. It has been shown that OCA reduces insulin resistance and improves glucose homeostasis in diabetic patients with NASH [50]. OCA may also contribute to reduce portal pressure by increasing nitric oxide synthases (iNOS) and decreasing inflammation mediators.
After a phase 2b clinical trial in patients with biopsy-proven NASH without cirrhosis (FLINT trial) in which OCA displayed a beneficial impact on fibrosis without resolution of advanced fibrosis and at least of 2 points improvement in NASH compared to placebo [51], this drug is now being tested in a large phase 3 randomized, placebo-controlled trial (REGENERATE trial, NCT2548351) to evaluate the long-term effects on NASH and fibrosis in patients with stage 1–3 fibrosis. The results of an interim analysis showed a significant improvement of fibrosis in approximately 20% of patients treated with OCA vs. 12% of placebo [52]. The NASH resolution endpoint was not met in this preliminary analysis. The most significant side effect was a dose-dependent pruritus, that in some cases (< 10%) required treatment discontinuation. Increased cholesterol levels were frequently observed, and statins were newly prescribed in almost 50% of patients receiving OCA [52].
At the same time, the antifibrogenic effect of OCA is being tested in a phase 3 trial including patients with compensated cirrhosis due to NASH (REVERSE trial, NCT03439254).
MET642 and MET 409 are other structurally optimized synthetic FXR agonists. Preliminary reports suggest that these compounds produce less pruritus than OCA. MET642 is now being tested in a phase 2 clinical trial in patients with NASH (NCT0477396). After the results of a phase 1b trial in which MET409 showed its ability to reduce the liver fat content in monotherapy, this drug is now being tested in a phase 2b trial in combination with empagliflozin.
Tropifexor, another highly potent non-bile acid FXR agonist, is being tested in a phase 2 clinical trial (FLIGHT FXR, NCT02855164) in patients with stage 1–3 fibrosis due to NASH. In a preliminary analysis, treatment with tropifexor lead to a significant reduction of hepatic fat, liver transaminases, and body weight compared to placebo with a favorable safety profile. Complete results are expected to be released in the next few months [53].
Cilofexor is another nonsteroidal FXR agonist that showed efficacy in decreasing liver steatosis a phase 2 trial [54]. The preliminary results of a phase 2 trial testing cilofexor in combination with firsocostat (an acetyl-CoA carboxylase inhibitor) and semaglutide suggest a synergistic activity of this combination in improving liver steatosis and liver biochemistry [55].
EDP305 is another FXR agonist. A phase 2 randomized, double-blind, placebo-controlled, dose-ranging trial (ARGON-1) has shown that when administered to non-cirrhotic biopsy-proven NASH patients, a 12-week course of EDP305 reduced liver fat content and decreased ALT [56]. The most frequent adverse event was pruritus and changes in lipid parameters were milder than with first-in-clasee FXR agonists. ARGON-2 is a phase 2b study testing the efficacy of EDP305 in NASH patients with stage 2–3 fibrosis (NCT04378010).
Finally, TERN-101 is a nonsteroidal FXR agonist with enhanced liver distribution. In a preliminary analysis of a phase 2 trial (LIFT study) in patients with stage 1–3 liver fibrosis, a 12-week course of TERN-101 rapidly decreased ALT and GGT and seemed to have positive effect on inflammation and fibrosis measured by using a non-invasive composite marker assessed by MRI (cT1) and MRI-PDFF [57].
FGF analogs
FGF19 and FGF21 are members of FGF superfamily that exert similar but not identical beneficial effects on lipid and glucose metabolism. Indeed, the administration of both FGF19 and FGF21 in animal models improve insulin sensitivity, improve body weight and fat mass, lipid levels, and liver steatosis. This latter effect probably depends on the inhibition of SREBP1 and reduced expression of genes involved in triglyceride synthesis [58].
In the last years, different FGF21 analogs and one FGF19 analog have been tested in phase 1 and 2 trials for the treatment of NASH.
In a phase 2b trial, the FGF19 analog aldafermin failed to improve liver fibrosis among patients with NASH-related stage 2 or 3 fibrosis. Other FGF21 analogs are pegbelfermin and efruxifermin. In a phase 2 trial in patients with stage 1–3 liver fibrosis, efruxifermin reduced liver fat content, improved liver function tests, and fibrosis and inflammation markers (including NAS score). Fibrosis stage improvement of at least 1 point was observed in almost half of the patients and NASH resolution in almost one third. Safety profile was favorable [59].
THR beta agonists
THR-β is involved in the regulation of lipid metabolism, plays a role in insulin sensitivity, and promotes liver regeneration and reduces hepatocyte apoptosis in the liver. In a phase 2 trial in patients with NASH and fibrosis, the THR-β agonist resmetirom decreased liver fat content and improved lipid metabolism parameters and liver function tests, with a positive impact on lipid profile and fibrosis markers, with no significant effect on body weight [60]. A phase 3 study on patients with non-invasive diagnosis of NAFLD showed that 100 mg of resmetiron administered daily for 52 weeks improved liver fat content in approximately 50% of treated patients vs 8% of placebo-treated patients; liver fibrosis in approximately 20% of treated patients vs. 10% of placebo-treated patients (both measured by a non-invasive test). Lipid metabolism parameters, liver enzymes, and inflammatory biomarkers also improved compared to placebo [61]. There were no major safety concerns. Main adverse events were gastrointestinal (diarrhea, nausea). A phase 3 study in NASH patients with fibrosis is still ongoing and data will be available soon (MAESTRO-NASH, NCT03900429).
A liver-directed THR-β agonist, VK2809, improved liver fat content compared to placebo among patients with NAFLD treated with two different doses in a preliminary analysis of a phase 2 trial [62]. No serious adverse events were reported.
Anti-fibrotic and anti-inflammatory agents
Currently, many agents with anti-fibrotic and anti-inflammatory effects are being tested in NASH. Most of them are still in early stage of clinical development: in phase 1 (as for example GB1211 targeting galectin 3, DFV890 targeting NLPR-3, or nimacimab targeting CB1) and phase 2 (as for example tipelukast, a leucotrien, or nitazoxanide, an antiparasitic agent). The description of all of them exceeds the objective of this review.
The available information from molecules in later stages of clinical development is summarized below.
Cenicriviroc (CVC) is an orally administered, small molecule antagonist that blocks chemokine 2 and 5 receptors, both with well-known roles in liver inflammation and fibrosis. In a phase 2b trial (CENTAUR), the primary objective of histological improvement in NASH was not met although CVC improved measurable liver fibrosis without worsening NASH [59]. A phase 3 trial (AURORA, NCT03028740) was prematurely stopped when an interim analysis casted doubts on the efficacy of the drug. Preliminary results of a phase 2b trial (TANDEM, NCT03517540) showed that the combination of tropifexor with CVC was safe and able to reduce body weight and ALT in patients with biopsy-proven NASH with fibrosis. However, the combination was not superior to either drug in monotherapy in terms of histological endpoints [63].
Belapectin is a complex carbohydrate that targets galectin-3, a B-galactoside-binding lectin that plays a role in inflammatory response and fibrosis [64]. A phase 2b clinical trial (NASH-CX, NCT02462967) showed that belapectin has not any significant effect on inflammation and fibrosis compared to placebo. However, it produced a significant decrease of the hepatic venous pressure gradient in patients with NASH-related cirrhosis without esophageal varices [65]. A phase 2b/3 trial (NAVIGATE, NCT04365868) is assessing the proportion of NASH patients with compensated cirrhosis who develop new esophageal varices after an 18-month course of belapectin compared to placebo, as well as the incidence of long-term clinically significant cirrhosis-related events.
Selonsertib is an oral inhibitor of the apoptosis signal–regulating kinase-1 (ASK1). Although well tolerated, it failed to show improvement in fibrosis without worsening of NASH in two different phase 3 trials (STELLAR-3, in patients with stage 3 fibrosis; and STELLAR-4, in patients with compensated cirrhosis) [66].
Other metabolic treatments
Molecules involved in other metabolic pathways are at different stage of clinical development for the treatment of NASH.
Stearoyl-coenzyme A desaturase-1 (SCD-1) is considered a mediator of liver steatosis and fibrosis because of its role in fatty acid biosynthesis [67] [68]. Aramchol is an oral SCD1 modulator which showed an ability to reduce liver fat and improve NASH and fibrosis with favorable tolerance among patients with overweight or obesity and prediabetes in a phase 2b trial (ARREST) [69]. An ongoing phase 3 trial (ARMOR study, NCT04104321) in patients with advanced fibrosis and NASH aims to evaluate the efficacy of aramchol vs. placebo on NASH resolution, fibrosis improvement, and clinical outcomes related with NASH progression.
Icosabutate is a synthetic omega 3 fatty acid (a structurally engineered eicosapentaenoic acid that resist oxidation and does not accumulate in hepatocytes) that could confer beneficial effects on hepatic oxidative stress, inflammation, and fibrosis. A phase 2b study is evaluating the efficacy of different doses of icosabutate on the resolution of NASH without worsening of fibrosis. The primary endpoint is to evaluate the percentage of patients with resolution of NASH defined as disappearance of ballooning with lobular inflammation without worsening in fibrosis (ICONA study, NCT04052516).
MSDC-0602 K is a thiazolidine-dione designed to modulate the mitochondrial pyruvate carrier (MPC), a protein complex that regulates the entry of pyruvate into the mitochondria. A phase 2b placebo-controlled randomized trial (EMMINENCE) failed to show improved histological outcomes (≥ 2-point NAS improvement without worsening fibrosis, NASH resolution, and fibrosis reduction) among patients with biopsy-proven NASH and stage 1–3 fibrosis [70]. A dose-dependent improvement in the glycemic control and liver enzymes were nevertheless observed.
A novel, deuterium-stabilized R-pioglitazone, PXL065, that lacks PPAR-gamma activity but exerts its non-genomic target activities (mitochondrial pyruvate carrier and acyl-CoA synthetase 4 inhibition) has been tested in a phase 2 trial in non-cirrhotic patients with NASH (NCT04321343). According to the preliminary results reported at AASLD 2022 meeting, this drug can reduce the liver fat content in 40% of patients and improve at least 1 fibrosis stage in 30–50% of treated patients. In this trial, up to 30% of patients showed NASH resolution after 36 weeks of treatment with a good safety profile since it lacks the PPAR-gamma side effects of glitazones [71]. The phase 3 trial has not started yet.
Other molecules targeting fatty acid synthesis or de novo lipogenesis, such as DGAT2i (NCT04399538) or TVB-2640 (NCT03938246, NCT04906421) have been tested in phase 2 trial alone or in combination with other compounds. Ad interim analysis showed encouraging results for TVB2640 but the definitive results have not been reported yet.
Conclusion
The pharmacological scenario of NASH therapy will certainly change in the next future. The results of the latest clinical trials are showing that the three major components of NASH, steatosis, inflammation, and fibrosis can be pharmacologically targeted. Although therapies on trial have acceptable tolerance and a good safety profile, a drug with potential to ameliorate all components of NASH is yet to be identified. Indeed, the results of single drug trials, most of them preliminary, showed improvement of NASH components in only a proportion of NASH patients, in general lower than 50%. Combination therapy looks a promising strategy. The rationale behind drug combination is on one hand to increase the efficacy of one single drug and on the other hand, to reduce side effects of one drug by allowing the use of a lower dose or by controlling the side effects of the first drug (72). So, the best treatment will probably be defined in the next few years not only according to different disease stages but also aiming to tailor the treatment for each patient according to the presence of comorbidities.
References
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ et al (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. The Lancet 377(9765):557–567
WHO [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M et al (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73(1):202–209
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84
Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z et al (2013) Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci 58(10):3017–3023
Marchesini G, Day CP, Dufour JF, Canbay A, Nobili V, Ratziu V et al (2016) EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64(6):1388–1402
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM et al (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148(3):547–555
Spengler EK, Loomba R (2015) Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc 90(9):1233–1246
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R (2015) Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 13(4):643-654.e9
Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen C-H et al (2017) Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Investig 127(7):2697–2704
Anstee QM, Seth D, Day CP (2016) Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology. 150(8):1728-1744.e7
Dongiovanni P (2013) PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol 19(41):6969
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A et al (2014) Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 46(4):352–356
Dongiovanni P, Valenti L, Rametta R, Daly AK, Nobili V, Mozzi E et al (2010) Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut 59(2):267–273
Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R et al (2016) The MBOAT7-TMC4 Variant rs641738 Increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 150(5):1219-1230.e6
Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PRV, Orho-Melander M et al (2009) The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet 18(21):4081–4088
Vazquez-Chantada M, Gonzalez-Lahera A, Martinez-Arranz I, Garcia-Monzon C, Regueiro MM, Garcia-Rodriguez JL et al (2013) Solute carrier family 2 member 1 is involved in the development of nonalcoholic fatty liver disease. Hepatology 57(2):505–514
Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P et al (2018) A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med 378(12):1096–1106
Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B (2017) Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152(7):1679-1694.e3
Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW et al (2013) Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab 18(1):106–117
Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ et al (1859) 2016 Nuclear receptors and nonalcoholic fatty liver disease. Biochimica et Biophysica Acta (BBA) - Gene Reg Mech 9:1083–99
Cao Y, Shu X-B, Yao Z, Ji G, Zhang L (2020) Is vitamin D receptor a druggable target for non-alcoholic steatohepatitis? World J Gastroenterol 26(38):5812–5821
Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS et al (2009) Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol-Gastrointestinal Liver Physiol 296(6):G1248-57
Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF (2012) Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 61(11):2711–2717
Brown MS, Goldstein JL (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7(2):95–96
Uyeda K, Repa JJ (2006) Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 4(2):107–110
Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D et al (2009) The role of peroxisome proliferator-activated receptor γ coactivator-1 β in the pathogenesis of fructose-induced insulin resistance. Cell Metab 9(3):252–264
Bindesbøll C, Fan Q, Nørgaard RC, MacPherson L, Ruan H-B, Wu J et al (2015) Liver X receptor regulates hepatic nuclear O-GlcNAc signaling and carbohydrate responsive element-binding protein activity. J Lipid Res 56(4):771–785
Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M et al (2017) What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes 41(6):853–865
Loomba R, Friedman SL, Shulman GI (2021) Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184(10):2537–2564
Richart C, Auguet T, Terra X (2010) Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med 363(2):193–195
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98(4):2133–2223
Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J et al (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355(22):2297–2307
Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C et al (2016) Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus. Ann Intern Med 165(5):305
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM et al (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362(18):1675–1685
Sun L, Deng C, Gu Y, He Y, Yang L, Shi J (2022) Effects of dapagliflozin in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 46(4):101876
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V et al (2021) A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 384(12):1113–1124
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R et al (2016) Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet 387(10019):679–690
Jani RH, Pai V, Jha P, Jariwala G, Mukhopadhyay S, Bhansali A et al (2014) A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRES. Diabetes Technol Ther 16(2):63–71
Gawrieh S, Noureddin M, Loo N, Mohseni R, Awasty V, Cusi K et al (2021) Saroglitazar, a PPAR-α/γ Agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatology 74(4):1809–1824
Siddiqui MS, Idowu MO, Parmar D, Borg BB, Denham D, Loo NM et al (2021) A phase 2 double blinded, randomized controlled trial of saroglitazar in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 19(12):2670–2672
Stephen A Harrison, Vlad Ratziu, Pierre Bedossa, Jean-Francois Dufour, Frederik Kruger, Jörn M Schattenberg, Sven M. Francque, Marco Arrese, Jacob George, Elisabetta Bugianesi, Helena Cortez-Pinto, Quentin M. Anstee, Adrian C. Gadano, Manuel Romero-Gomez, DWH and AJS. RESOLVE-IT phase 3 trial of Elafibranor in NASH: final results of the week 72 interim surrogate efficacy analysis. 2022.
Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ et al (2021) A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med 385(17):1547–1558
Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H et al (2021) Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 54(10):1263–1277
Caligiuri A, Gentilini A, Marra F (2016) Molecular pathogenesis of NASH. Int J Mol Sci 17(9):1575
Jiao Y, Lu Y, Li X (2015) Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol Sin 36(1):44–50
Panzitt K (1867) Wagner M 2021 FXR in liver physiology: multiple faces to regulate liver metabolism. Biochimica et Biophysica Acta (BBA) - Mol Basis Disease 7:166133
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA et al (2004) Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Investig 113(10):1408–1418
Savkur RS, Bramlett KS, Michael LF, Burris TP (2005) Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor. Biochem Biophys Res Commun 329(1):391–396
Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall H-U, Kipnes M et al (2013) Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145(3):574-582.e1
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF et al (2015) Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. The Lancet 385(9972):956–965
Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z et al (2019) Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. The Lancet 394(10215):2184–2196
Quentin M. Anstee, Kathryn Jean Lucas, Sven M. Francque, Manal F. Abdelmalek, Arun J Sanyal, Vlad Ratziu, Adrian C. Gadano, Mary E. Rinella, Michael R. Charlton, Rohit Loomba, Edward Mena, George Boon Bee Goh, Jossy Kochuparampil Li Chen, Gerardo Rodrigue SL and MCP. Safety and efficacy of tropifexor plus cenicriviroc combination therapy in adult patients with fibrotic NASH: 48 week results from the phase 2b tandem study. Hepatology. 2020 Nov 1;72(S1):1A-130A
Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE et al (2020) Cilofexor, a Nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 72(1):58–71
Alkhouri N, Herring R, Kabler H, Kayali Z, Hassanein T, Kohli A et al (2022) Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: a randomised, open-label phase II trial. J Hepatol 77(3):607–618
Ratziu V, Rinella ME, Neuschwander-Tetri BA, Lawitz E, Denham D, Kayali Z et al (2022) EDP-305 in patients with NASH: a phase II double-blind placebo-controlled dose-ranging study. J Hepatol 76(3):506–517
Rohit Loomba, Kris V Kowdley, Raj Vuppalanchi, Tarek Hassanein, Sergio E. Rojter, Muhammad Y Sheikh, Sam Moussa, Diana Chung, Clarence Eng, Tonya Marmon, Mona Qureshi, Erin Quirk DC and EJL. Liver distributed FXR agonist TERN-101 demonstrates favorable safety and efficacy profile in NASH phase 2a LIFT study. Hepatology. 2021 Oct 14;74(S1):1–156
Talukdar S, Kharitonenkov A (2021) FGF19 and FGF21. In NASH we trust Mol Metab 46:101152
Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA et al (2021) Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med 27(7):1262–1271
Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP et al (2019) Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet 394(10213):2012–2024
Harrison S, Taub R, Neff G, Moussa S, Alkhouri N, Bashir M (2022) Primary data analyses of MAESTRO-NAFLD-1 a 52 week double-blind placebo-controlled phase 3 clinical trial of resmetirom in patients with NAFLD. J Hepatol 1(77):S14
Lian B, Loomba R, Neutel J, Margaritescu C, Homer K, Luk A et al (2020) VK2809, a novel liver-directed thyroid receptor agonist, produces durable reductions in liver fat in patients with non-alcoholic fatty liver disease: results of 4-week follow-up assessment from a 12-week phase 2 randomized, placebo-controlled trial. J Hepatol 73:S53
Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M et al (2020) A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: study design of the TANDEM trial. Contemp Clin Trials 88:105889
Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR et al (2011) When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50(37):7842–7857
Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M et al (2020) Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 158(5):1334-1345.e5
Harrison SA, Wong VW-S, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z et al (2020) Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol 73(1):26–39
Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X et al (2007) Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 6(6):484–496
Iruarrizaga-Lejarreta M, Varela-Rey M, Fernández-Ramos D, Martínez-Arranz I, Delgado TC, Simon J et al (2017) Role of aramchol in steatohepatitis and fibrosis in mice. Hepatol Commun 1(9):911–927
Ratziu V, de Guevara L, Safadi R, Poordad F, Fuster F, Flores-Figueroa J et al (2021) Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat Med 27(10):1825–1835
Harrison SA, Alkhouri N, Davison BA, Sanyal A, Edwards C, Colca JR et al (2020) Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase IIb study. J Hepatol 72(4):613–626
Harrison SA, Thang C, Bozec S, Bolze S, DeWitt S, Moller DE PF (2022) PXL065 (deuterium-stabilized R-enantiomer of pioglitazone) reduces liver fat content and improves liver histology without PPARG -mediated side effects in patients with NASH: analysis of a 36 week placebo-controlled phase 2 trial (DESTINY1). [Internet]. Available from: https://www.aasld.org/the-liver-meeting/pxl065-deuterium-stabilized-r-enantiomer-pioglitazone-reduces-liver-fat-content. Accessed 24 Mar 2023
Dufour J-F, Caussy C, Loomba R (2020) Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut 69(10):1877–1884
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval
This work is not research involving humans or animals.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• For the time being, there is no specific treatment for NASH to avoid disease progression into hepatic fibrosis or to reduce established fibrosis.
• As pathophysiology of NASH is very complex and not-completely well understood, identification of biological drug targets is challenging.
• Many clinical trials are under development to try to identify drugs to diminish hepatic inflammation/fibrosis. A synthesis of the results of the most advanced clinical trials for the treatment of NASH is presented in this review.
Paloma Sangro, Manuel de la Torre Aláez Shared equal authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sangro, P., de la Torre Aláez, M., Sangro, B. et al. Metabolic dysfunction–associated fatty liver disease (MAFLD): an update of the recent advances in pharmacological treatment. J Physiol Biochem 79, 869–879 (2023). https://doi.org/10.1007/s13105-023-00954-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00954-4