Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading cause of chronic liver disease that affects over 30% of the world’s population. For decades, the heterogeneity of non-alcoholic fatty liver disease (NAFLD) has impeded our understanding of the disease mechanism and the development of effective medications. However, a recent change in the nomenclature from NAFLD to MASLD emphasizes the critical role of systemic metabolic dysfunction in the pathophysiology of this disease and therefore promotes the progress in the pharmaceutical treatment of MASLD. In this review, we focus on the mechanism underlying the abnormality of hepatic lipid metabolism in patients with MASLD, and summarize the latest progress in the therapeutic medications of MASLD that target metabolic disorders.
Similar content being viewed by others
Introduction
According to an international expert proposal in 2020, non-alcoholic fatty liver disease (NAFLD) should be updated to metabolic dysfunction associated with fatty liver disease (MAFLD) [1]. Two of the seven criteria for metabolic dysfunction must be met for the patient to be diagnosed with MAFLD [2]. Recent multi-society Delphi consensus statements have replaced the nomenclature of NAFLD with metabolic dysfunction-associated steatotic fatty liver disease (MASLD) [3]. The diagnosis of MASLD includes evidence of hepatic steatosis along with at least one of the following five cardiometabolic criteria: the presence of overweight or obesity, impaired glucose regulation or type 2 diabetes, hypertension, increased plasma triglycerides, or decreased high-density lipoprotein cholesterol (HDL-c) [3]. The new nomenclature of MASLD not only emphasizes the critical role of systemic metabolic dysfunction in the pathogenic process leading to MASLD, but also enhances the clinicians’ awareness to the concomitant metabolic dysfunction in patients with MASLD.
The global prevalence of NAFLD is approximately 25% according to previous studies [4]. Since the use of the new nomenclature of MASLD, the nationwide prevalence of MASLD in the United States has been found to be 32.45% [5]. More recently, a community-based study among East Asians in Hong Kong showed that the prevalence of MASLD was 26.7%, and the difference between the prevalence of NAFLD and MASLD in the same population was minimal [6]. Other recent statistical data show that MASLD affects more than 30% of adults globally and causes a heavy economic burden of over $100 billion in the USA [7]. Therefore, the prevalence of MASLD is estimated to be 25–30%, similar to that of NAFLD. The estimated pooled all-cause mortality rate for patients with NAFLD was 12.60 per 1000 person years (PYs). This rate included 4.20 per 1000 PYs for mortality specific to cardiac disease, 2.83 per 1000 PYs for mortality specific to extrahepatic cancer, and 0.92 per 1000 PYs for mortality specific to liver disease [8]. In the latest third National Health and Nutrition Examination Surveys 1988–1994 (NHANES III) study including 13,856 individuals, patients with MASLD was proved to be associated with significantly higher all-cause mortality (adjusted HR 1.127, 95% CI 1.056–1.201) and diabetes-related mortality (adjusted HR 1.911, 95% CI 1.418–2.574) than those without during follow-up [6]. Therefore, patients with MAFLD/MASLD even have worse clinical outcomes than those with NAFLD but not metabolic dysfunction [9]. However, the concomitant metabolic disorders are often overlooked in patients with NAFLD, thus leading to many adverse cardiovascular and liver-related outcomes. In comparison, the diagnosis of MASLD requires the presence of metabolic dysfunction, and the excessive liver fat accumulation in MASLD specifically originates from the state of systemic metabolic dysfunction. It enables better risk stratification and personalized treatment of fatty liver disease [10]. In this review, we discussed the critical role of metabolic dysfunction in the development and progression of MASLD, and summarized the latest progress in the drug treatment of MASLD from the perspective of systemic metabolic dysfunction.
Role of metabolic dysfunction in the pathophysiology of MASLD
Metabolic dysfunction refers to the presence of obesity, hyperglycemia, hypertension or dyslipidemia clinically. The primary histological characteristic of MASLD is hepatocellular steatosis, which is thought to be the hepatic manifestation of metabolic syndrome [11]. According to the classic "two-hit theory" of fatty liver, the first hit involves excessive hepatic lipid deposition, and the second hit activates inflammatory cascades and fibrogenesis in hepatocytes after that [12], which results in non-alcoholic steatohepatitis (NASH) assessed by NAS scores and liver fibrosis classified as F1 to F4 by Metavir scores. However, subsequent studies over the last two decades have demonstrated that the pathogenesis of MASLD is much more complex than the two hits, and the "multiple-hit theory” has been widely accepted that multiple risk factors, including insulin resistance, nutritional factors, and lipid metabolism disorders, act together with genetic (e.g., patatin-like phospholipase domain containing 3 (PNPLA3), transmembrane 6 superfamily 2 (TM6SF2), and membrane bound O-acyltransferase domain containing 7 (MBOAT7) gene variants) and epigenetic (e.g., DNA methylation, histone modification, and m6A RNA methylation) factors to induce liver steatosis and progress to NASH and liver fibrosis [13]. Multiple risk factors jointly contribute to the progression of MASLD with dynamic changes from hepatic steatosis and inflammation, nonlinear progression of fibrosis to the recompensation of NAFLD-related cirrhosis, and novel pathophysiological mechanisms, such as impaired partial collagen degradation and hepatocyte regeneration, vascular remodeling and systemic inflammation enhancement, which are involved in the updated natural course of MASLD [14]. However, the latest theories on the pathogenesis of MASLD have not changed the important role of hepatic lipid accumulation as the initial and critical stage of this disease.
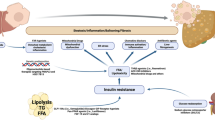
Hepatic fat accumulation arises when liver triglycerides acquisition exceeds removal. The mechanism of hepatic fat accumulation caused by metabolic dysfunction is shown in Fig. 1. Hepatic fat is derived from hepatic de novo lipogenesis (DNL), fatty acids released from the adipose tissue and dietary fat taken up in the intestine, and is metabolized through mitochondrial fatty acid β-oxidation (FAO) or exported out of the liver via very low density lipoprotein (VLDL) [15]. Metabolic dysfunction in any of the above hepatic lipid metabolism pathways could lead to MASLD.
Overview of hepatic triglycerides metabolism. TG, triglycerides; SREBP, sterol regulatory element–binding protein; ChREBP, carbohydrate response element binding protein; FFA, free fatty acid; IR, insulin resistance; CD36, cluster of differentiation 36; FATP, fatty acid transport proteins; VLDL, very-low density lipoprotein; MTTP, Microsomal TG transfer protein; FAO, fatty acid β-oxidation; CPT, carnitine palmitoyl transferase; DNL, de novo lipogenesis
Insulin resistance (IR)
IR plays a pivotal role in the pathophysiology of metabolic syndrome, which might be crucial for the development of MAFLD [16] or MASLD [17]. Carbohydrate intake increases circulating insulin and glucose levels. In individuals with insulin resistance, postprandial glucose and insulin are usually higher than those in metabolically healthy individuals. In the liver, glucose and insulin act as important regulators of DNL and are discussed in the following section. Meanwhile, the impaired ability of insulin to suppress lipolysis in peripheral adipose tissue leads to excess release of free fatty acids (FFAs) and hyperlipidaemia, which promotes the uptake of FFAs and the accumulation of intrahepatic lipids [18, 19].
Increased DNL
Insulin promotes the expression of sterol regulatory element–binding protein–1c (SREBP1c) [20], and glucose and fructose promote the translocation of carbohydrate response element binding protein (ChREBP) to the nucleus [21]. Both SREBP1c and ChREBP increase the expression of multiple enzymes that catalyze lipogenesis, including acetyl-CoA carboxylase (ACC), ATP citrate lyase (ACLY), fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD1), as demonstrated in gene knockout mice [20, 21]. The states of cellular energy excess inhibit AMP-activated protein kinase (AMPK), a Ser/Thr protein kinase and an essential cellular energy sensor [22]. It has been recognized that AMPK activation inhibits DNL by down-regulating the level of ACC phosphorylation and SREBP1c expression [23]. Therefore, AMPK might be an important mediator that regulates hepatic lipogenesis under the metabolic dysfunction status. On the other hand, the excessive liver fat will in turn exacerbate IR through the production of excess ceramides and diacylglycerols (DAGs) [24]. Hepatic insulin resistance is caused by the activation of protein kinase Cε (PKCε) in high-fat diet mice due to an increase in hepatic plasma membrane sn-1,2-DAG content [25]. This inhibition of insulin receptor kinase (IRK) is the result of the interaction between hepatic DNL and IR, which creates a vicious cycle that aids in the development and progression of MASLD.
Increased FFAs uptake
Chronic overnutrition is the fundamental reason of peripheral IR [24]. Circulating insulin functions to increase the uptake of fatty acids and enhance the synthesis of triglycerides in peripheral adipose tissue. On the other hand, in overfed individuals, high triglyceride accumulation triggers increased release of inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) as well as macrophage M1 activation in the adipose tissue [26]. Adipocytes in the periphery release FFAs more readily when low level of chronic inflammation and persistent stress in the adipose tissue activate stress-related signal transduction pathways such as inhibitor of kappa-B kinase beta (IKKB) and c-Jun N-terminal kinase (JNK) [27]. This results in the aberrant phosphorylation of insulin receptor substrate (IRS) and peripheral IR. Transporters like cluster of differentiation 36 (CD36) and fatty acid transport proteins (FATP) allow FFAs to enter hepatocytes [28]. In patients with metabolic dysfunction, the localization of CD36 as well as its palmitoylation level are significantly increased to facilitate the transport of FFAs into hepatocytes [29]. While inhibition of CD36 palmitoylation reduces its hydrophobicity, thus decreasing its localization on the plasma membrane and lipid rafts, and inhibiting hepatic FFAs uptake [30].
Increased dietary fat and gut-liver axis
Dietary fat is absorbed in the intestine, packaged into chylomicrons and delivered into the systemic circulation. About 20% of the triglycerides in chylomicrons are delivered to the liver [31]. It is estimated that the common daily diet will furnish the liver with about 10 g of fat each day, while in individuals with high fat diet (such as typical American diet), the amount of fat entering the liver from daily diet doubles. Moreover, dietary fat, especially cholesterol, can modulate gut microbiota and bile acid profiles, thus driving the progression of MASLD [32]. The human gut is colonized by a large number of microorganisms. Alterations in the type and amount of gut microbiota are known as dysbiosis. Dysbiosis leads to the development and progression of MASLD through gut-liver axis that is regulated by bile acids (BA) receptors [33], such as farnesoid X receptor (FXR) and Takeda G-coupled protein receptor 5 (TGR5). Deactivation of FXR promotes DNL, and inhibits fatty acid oxidation (FAO) and VLDL triglycerides clearance [33], while TGR5 in small intestinal cells leads to the release of glucagon-like peptide 1 (GLP-1), which regulates food intake and glucose metabolism [34].
Reduced mitochondrial FAO
Hepatic FAO and mitochondrial turnover are compromised in patients with MASLD [35]. It is necessary for carnitine palmitoyl transferase (CPT) to allow fatty acids to enter mitochondria. CPT1 and CPT2 are found in the two layers of the mitochondrial membrane respectively. CPT is reportedly upregulated [36] and CPT2 is inhibited [37] in patients with MASLD. Overexpression of CPT1A enhances hepatic FAO and lipid autophagy, thus reducing hepatic steatosis in high-fat-diet mice [38]. The expression of CPT1 is regulated by peroxisome proliferator-activated receptor (PPAR)-α [39], while the CPT2 expression is decreased in FXR deficiency, thus leading to the increase of SREBP1c-mediated FAS expression [40]. A typical example on the close correlation between mitochondrial dysfunction and MASLD is the MASLD patients carrying homozygous PNPLA3 I148M variant. The most potent genetic risk factor for MASLD is the PNPLA3 I148M variant [41]. Protein accumulation on lipid droplets inhibits the activity of adipose triglyceride lipase (ATGL), which leads to the accumulation of triglycerides in hepatic lipid droplets and a subsequent decrease in hepatic FAO [42]. Under fasting or ketogenic conditions, there is a decrease in I148M protein levels, which can cause excess hepatic triglyceride lipolysis and increase mitochondrial redox state; this can inhibit hepatic citrate synthase flux and ultimately result in liver injury [43].
Abnormal VLDL secretion
Hepatocytes export excess triglycerides into the circulation by secreting apolipoprotein B-100 (ApoB100) containing very low density lipoprotein (VLDL) [44]. It has been suggested that the VLDL secretion is increased in patients with MASLD, but does not counteract the accumulation of excess triglycerides (TGs) [45]. Impairment in the VLDL secretion can lead to the development of MASLD in individuals with relatively good metabolic status. In TM6SF2 knockout mice, TG and cholesterol contents in VLDLs secreted into the blood were significantly reduced, which contributed to the accumulation of lipids in the liver [46, 47]. Similarly, in individuals with the TM6SF2 E167K variant, the VLDL assembly is inhibited, thus leading to the accumulation of hepatic fat and reduction in plasma TG concentrations [48]. As a result, MASLD patients with the TM6SF2 E167K mutation have a more reduced risk of cardiovascular disease (CVD) and more severe hepatic fat accumulation [49]. Microsomal TG transfer protein (MTTP) catalyzes the lipidation of ApoB100 and is necessary for the assembly and secretion of VLDL. In MASLD mice, MTTP overexpression effectively reduces triglyceride levels in hepatocytes [50].
Treatment of MASLD from the perspective of metabolic dysfunction
Given the close causal relationship between metabolic disorders and MASLD, therapies targeting systemic glucose and lipid metabolism have shown promising effects. Lifestyle interventions (calorie restriction and physical exercise) have been proven to be effective in treating MASLD [51], but many patients struggle to adhere to the lifestyle intervention programs due to poor long-term compliance. Bariatric surgery has been proven to be powerful tool for sustainable weight loss and great improvement in liver steatosis in patients with MASLD and morbid obesity [52]. However, the majority of patients with MASLD neither accept invasive surgery nor meet the minimum body mass index (BMI) requirements for bariatric surgery. Therefore, an effective medication for the treatment of MASLD is of great clinical significance. Currently, novel medications targeting metabolic disorders have shown promising results for the treatment of MASLD, as illustrated in Fig. 2.
Medications targeting at metabolic dysfunction of MASLD. DNL, de novo lipogenesis; FAO, fatty acid β-oxidation; IR, insulin resistance; GLP, glucagon-like peptide; GIP, glucose-dependent insulinotropic peptide; FXR, farnesoid X receptor; FGF, fibroblast growth factor; TG, triglycerides; FFA, free fatty acid; THR, thyroid hormone receptor; PPAR, proliferator-activated receptor
Insulin sensitizers
Thiazolidinediones (TZD)
Thiazolidinediones is a kind of insulin sensitizers with thiazolidinedione ring, which act as potent activators of the nuclear receptor PPARγ. Thiazolidinediones cause decreased liver lipid accumulation and FFA plasma levels by inducing the release of adipokines, encouraging TG storage in adipose tissue, and strengthening the suppressive effect of insulin on lipolysis [53]. As summarized in Table 1, clinical trials of pioglitazone showed significant improvement in IR, liver steatosis and inflammation compared with placebo [54, 55]. Rosiglitazone showed similar a beneficial effect on liver steatosis, but its adverse effects of detrimental weight gain and edema are severe [56]. The mitochondrial pyruvate carrier (MPC) is another target of thiazolidinediones. MPC is responsible for transporting pyruvate from the cytosol across the inner membrane of mitochondrion [57]. MSDC-0602 K, a PPARγ-sparing thiazolidinedione targeting to MPC, ameliorates hepatic steatosis, circulating liver enzymes and insulin sensitivity in phase IIb trials as well as in mouse models [58]. More importantly, MSDC-0602 K tended to have fewer side effects on bone density and mesenchymal stem cell properties in obese mice compared to pioglitazone [59].
Metformin
Metformin inhibits hepatic gluconeogenesis and improves IR in patients with type 2 diabetes. Previous studies indicated that metformin effectively improves systemic inflammation and insulin sensitivity, and reduces body weight [60]. However, it also increases hepatocyte DNL that contributes to hepatic TG accumulation [61]. Although it is clear that metformin could not improve liver histological steatosis [62], it’s more often used in combination with other medications at present, such as GLP-1 receptor (GLP-1R) agonists, thiazolidinediones or sodium-dependent glucose transporter 2 (SGLT2) inhibitors [63].
Lipogenesis inhibitors
ACLY inhibitor
When excess citrate is available in cells, ACLY catalyzes the conversion of citrate to acetyl-CoA for lipogenesis. Bempedoic acid (BemA, ETC-1002) is a liver-specific ACLY competitive inhibitor that reduces hepatic steatosis through various pathways [64]. In mouse models that recapitulate different stages of the disease, BemA is proved significant reduction of hepatic TG accumulation, as well as genetically modulation of inflammation and fibrosis [65]. Clinical trials have showed positive outcomes on reduction of low-density lipoprotein (LDL) levels and cardiovascular risk [66, 67], while the efficacy among patients with MASLD remains to be studied.
ACC inhibitor
The first and committed step in DNL is catalyzed by ACCs, which convert acetyl-CoA to malonyl-CoA. Additionally, malonyl-CoA is a signaling molecule that inhibits FAO. ACC inhibitors have been proved efficacious to improve liver steatosis in animal models [68]. While in clinical trials, firsocostat (GS-0976) showed benefit in the improvement of liver lipid accumulation, stiffness and serum liver enzymes, but also led to an increase in serum triglycerides [69, 70]. Another three-part randomized phase 1 study showed similar efficacy on PF-05221304 [71]. The safety and tolerance of ACC inhibitors might limit their use in clinical practice and still need to be assessed further.
FAS inhibitor
FASs catalyze malonyl-CoA, synthesized by ACC, to saturated long-chain fatty acids. In obese mice, it’s demonstrated that the inhibition of FAS improves hepatic steatosis and IR [72]. Denifanstat (TVB-2640), a FAS targeted inhibitor, remarkably reduces hepatic lipid accumulation and serum alanine transaminase (ALT) without significantly increasing circulating triglycerides [73]. Consistently, a phase 2a trial also found that the FAS inhibitor significantly suppressed the lipid accumulation in the liver assessed by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) and serum biomarkers compared to placebo group [74].
SCD1 inhibitor
SCD1 functions to convert saturated fatty acids to monounsaturated fatty acids. The activity of SCD1 is increased in patients with MASLD [75]. In mouse models, aramchol (arachidyl-amido cholanoic acid) prevented steatohepatitis and fibrosis by blocking SCD1 and boosting the flow via the transsulfuration pathway, which kept the cellular redox balance stable [76]. Clinical trials among MASLD patients also indicated that hepatic SCD1 inhibitors dose-dependently improved liver steatosis, steatohepatitis and fibrosis, as measured by MRI-PDFF, serum liver enzymes and liver histology [77]. However, other studies found that 12-week aramchol treatment did not reduce LFC measured by MRI-PDFF or stiffness measured by magnetic resonance elastography (MRE) and vibration-controlled transient elastography (VCTE)[78].
DGAT2 inhibitor
The last step in DNL is that diacylglycerol acyltransferase (DGAT) catalyzes fatty acyl-CoA to diacylglycerol. Previous studies showed that lower level of DGAT2 expression leads to reduced steatosis in diabetic mice, but hepatocyte damage is exacerbated by lipotoxicity from FFAs [79]. Phase 1 studies indicated that selective DGAT2 inhibitor (PF-06427878) is well tolerated and significantly improves markers of liver function [80]. IONIS-DGAT2Rx is an antisense oligonucleotide inhibitor of DGAT2 expression which prevents LFC in a phase 2 trial [81]. Another DGAT2 inhibitor named ervogastat (PF-06865571) presented similar efficacy on liver steatosis, without serious gastrointestinal adverse events [82]. It's noting worth that co-administration of ACC inhibitor PF-05221304 and DGAT2 inhibitor PF-06865571 has a stacked efficacy and successfully overcomes the obstacle of ACC [83], although ACC inhibitors alone have obvious adverse effects of elevating serum TG and activating SREBP1c. Apart from a decreased likelihood of dose-dependent elevation in serum lipids, the total incidence of adverse events did not rise as PF-05221304 dose increased [83].
Currently, several novel hepatic lipogenesis inhibitors have shown promising effects in treating MASLD. We summarized the major results of these clinical trials in Table 2.
Fatty acid oxidation activators
Thyroid hormone receptor (THR) β agonists
Mitochondrial dysfunction is involved in the pathophysiology of MASLD, and in patients with steatohepatitis exhibit decreased activity of respiratory chain complexes and fatty acid oxidation [84]. It is an appealing therapeutic target for MASLD to stimulate mitochondria function according to recent studies [85]. The thyroid hormone receptor consists of 2 isoforms, namely THRα and THRβ. The THR mediates important functions for growth and metabolism at the transcriptional and post-translational levels and via autophagy [86]. THRβ increases hepatic FAO and reduces liver steatosis in rodent models of liver steatosis [87]. Resmetirom (MGL-3196) is a liver-targeted selective THRβ agonist. It resulted in significant reduction in hepatic steatosis and serum lipid metabolic products such as LDL, TG and ApoB and improvement in MRI-PDFF among patients with MASLD, with adverse events of transient mild diarrhoea and nausea [88]. Recently, the published phase 3 trials showed that resmetirom significantly improved liver fibrosis and inflammation, and reduced LFC as well as serum LDL-c, ApoB, and TG concentrations [89, 90].
PPAR α/γ/δ agonists
The PPAR transcription factors (PPARα, PPARδ and PPARγ) regulate lipid metabolism through gene transcription. PPARα and PPARδ involve in mitochondrial biogenesis and FAO, as well as fatty acid uptake and TG turnover [91]. PPAR agonists, such as lanifibranor (IVA337), elafibranor (GFT505) and saroglitazar, improved hepatic steatosis, inflammation and fibrosis in MASLD animal models [92]. The pan-PPAR agonist lanifibranor, which acts on three different PPAR isotypes, significantly improves hepatic steatosis, ballooning and inflammation with a relatively low coincidence of adverse events in phase 2b trials [93]. Elafibranor, a co-agonist of PPARα and PPARδ, improved liver enzymes, lipid and glucose metabolism, and systemic inflammation markers in adults and reduced of ALT in children with MASLD, while the efficacy on histological endpoints of liver steatosis remains to be studied [94]. While saroglitazar, another dual PPARα/γ agonist, could also effectively improve LFC assessed by MRI-PDFF and several metabolic parameters [95].
Incretins and intestinal FXR agonists
GLP-1 modulators
GLP-1 is an endogenous intestinal hormone that can lower food intake and peripheral fat mobilization by promoting the synthesis and release of insulin and preventing the secretion of glucagon. It does this by acting through the G protein-coupled GLP-1R. In studies on obese diabetic mice, rats, and rhesus monkeys, GLP-1R agonists enhanced indicators of hepatic steatosis and liver damage [96]. In patients with MASLD, GLP-1R agonists, including dulaglutide [97], exenatide [98], liraglutide [99] or semaglutide [100], have shown beneficial effects on hepatic fat content and liver histological inflammation and fibrosis, as listed in Table 3. Compared with liraglutide and dulaglutide, semaglutide has more pronounced effects on reducing body weight and blood glucose, while dulaglutide has less gastrointestinal symptoms [101, 102]. The benefit of GLP-1R agonists is strongly associated with weight loss. Combination of glucose-dependent insulinotropic peptide (GIP) or glucagon (GCG) with GLP-1R enhances the anti-obesity effect [103, 104]. In high-fat diet-fed mice, GIP increases the activity of feeding centers in hypothalamic, which leads to weight loss and less food intake [105]. Meanwhile, GIP reduces GLP-1R-mediated adverse gastrointestinal events [106]. While GCG increases energy consumption and ameliorates overnutrition and excessive fat accumulation [107]. In patients with obesity and diabetes mellitus type 2 (T2DM), GLP-1/GIP co-agonist tirzepatide showed a significant reduction in body weight, as well as LFC [108] and liver inflammation and fibrosis biomarkers [109]. More recently, a triple agonist retatrutide showed clinically meaningful improvements in patients with obesity or T2DM [110], but its effect on MASLD still requires further investigation.
FXR agonist
FXR is a bile acid receptor that mediates lipid signaling and reduces blood levels of glucose and lipids in mice [111]. Multi-center clinical trial showed obeticholic acid (OCA), an FXR agonist, can improve the fibrosis and inflammatory activity of liver [112]. However, OCA therapy has a negative impact on serum lipoprotein profile, that increases VLDL and LDL and reduces HDL [113]. Secondary analysis of FLINT trials demonstrated the correlation between 30% relative reduction in MRI-PDFF and histologic improvements such as steatosis and ballooning [114, 115]. Currently, several new-type FXR agonists are also undergoing clinical trials for MASLD. Tropifexor (LJN452) and nonsteroidal cilofexor (GS-9674) led to reduction of liver biochemistry and hepatic steatosis in patients with MASLD compared to placebo [116, 117]. Vonafexor and structurally optimized FXR agonist, MET409 showed similar reduction [118, 119].
Fibroblast growth factor (FGF) analogues
FGF19
At the intersection of the gut, liver, brain, and white adipose tissue, FGF19 is a gastrointestinal hormone that controls the synthesis of bile acid and acts as a transversal metabolic coordinator. Dysregulation of FGF19 may be linked to illnesses that impact lipid metabolism and the gut-liver axis. [120]. FGF19 analogue aldafermin (NGM 282) reduced liver fat and produced a trend toward fibrosis improvement in phase 2 trials with a generally good tolerance [121,122,123]. Furthermore, elevation of cholesterol associated with aldafermin can be effectively overcome through the co-administration with rosuvastatin, which is considered a reasonable strategy to optimize the cardiovascular risk [124].
FGF21
FGF21 acts as a hormone to enhance energy expenditure, diminish the DNL associated enzymes and regulate food preference, though patients with MASLD tend to have elevated FGF21 inversely correlated with IR [125]. High doses of recombinant FGF21 effectively decreased liver lipid content in obese mice and rhesus macaques [126]. A long-acting Fc-FGF21 fusion protein efruxifermin [127, 128] and recombinant FGF21 analog pegozafermin [129, 130] and pegbelfermin (BMS-986036) [131] have shown benefits in reducing hepatic steatosis and inflammation grades in clinical trials, all of which are well tolerated, with acceptable coincidence of diarrhoea and nausea.
Others targeting at whole-body energy balance
Modular of Leptin/Adiponectin axis
Leptin and adiponectin are secreted by white adipose tissue. When overnourished, serum levels of the two adipokine are elevated, which inhibit food intake and accelerate lipid metabolism. Adiponectin combats hepatic steatosis by activating the AMPK pathway to inhibit DNL and activating the PPAR-α pathway to promote FAO, while improves IR in the liver by activating glucose transporter proteins and inhibiting key enzymes of gluconeogenesis [132]. Recombinant leptin, metreleptin showed efficacy among patients with MASLD associated with relative leptin deficiency and partial lipodystrophy, which was considered by stimulating hepatic VLDL-TG secretion through brain-vagus-liver axis according to a recent study [133].
SGLT2 inhibitor
SGLT2 is responsible for more than 90% of filtered glucose reabsorption [134]. In a mouse model of T2DM, SGLT2 inhibitor significantly improved liver steatosis and fibrosis [135]. Clinical trials with canagliflozin [136], dapagliflozin [137], and ipragliflozin [138] showed consistent effect in reducing LFC and liver histological steatosis. Because SLGT2 is not expressed in the liver, weight loss brought on by treatment and improvements in IR may cause a decrease in liver steatosis [134].
Conclusion
MASLD is a global health problem with no medications licensed for its treatment currently. Due to the close association between metabolic dysfunction and MASLD, many medications targeting at hepatic lipid and glucose metabolism have shown promising results in patients with liver steatosis and metabolic disorders. The summary of the pathogenesis and latest medications of MASLD in this review will help physicians and researchers update the latest achievements in the field. The new nomenclature of MASLD strictly divides the patients with liver steatosis into groups according to the presence of metabolic dysfunction, and can remarkably reduce the heterogeneity of NAFLD. Further well-designed clinical trials are still required to evaluate the possibility and efficacy to treat patients with MASLD by targeting their common metabolic dysfunction. Additionally, MASLD is still a heterogeneous disease with complex and multiple causes [1]. Therefore, with understanding of the heterogeneity of MASLD, a proper clinical classification of MASLD may facilitate the choice of medications for every patient with MASLD. More importantly, since MASLD is a complex phenotype shaped by the dynamic interaction of multiple risk factors, including genetic predisposition, environmental factors and metabolic disorders, a combination of medications targeting at different steps of the pathogenesis of MASLD may achieve optimal therapeutic effect in the future.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014 e1.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–9.
Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56.
Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70(3):531–44.
Kalligeros M, Vassilopoulos A, Vassilopoulos S, Victor DW, Mylonakis E, Noureddin M. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017–2020. Clin Gastroenterol Hepatol. 2023;S1542-3565:00914–X.
Zhao Q, Deng Y. Comparison of mortality outcomes in individuals with MASLD and/or MAFLD. J Hepatol. 2024;80(2):e62–4.
Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;S1043-2760:00036–5.
Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–47.
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(10):2172-81 e6.
Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612.
Paul B, Lewinska M, Andersen JB. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022;4(6):100479.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–5.
Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64.
Feng G, Valenti L, Wong VW, et al. Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2024;21(1):46–56.
Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–23.
Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J Mol Sci. 2021;22(8):4165.
Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): beyond insulin resistance. J Hepatol. 2023;79(6):1524–41.
Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–31.
Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–75.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31.
Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4(2):107–10.
Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313.
Fang C, Pan J, Qu N, et al. The AMPK pathway in fatty liver disease. Front Physiol. 2022;13:970292.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223.
Lyu K, Zhang Y, Zhang D, et al. A membrane-bound Diacylglycerol species induces PKCϵ-mediated hepatic insulin resistance. Cell Metab. 2020;32(4):654-64.e5.
Govaere O, Petersen SK, Martinez-Lopez N, et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J Hepatol. 2022;76(5):1001–12.
Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–53.
Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36 FATP and FABPm. Biochim Biophys Acta. 1999;1441(1):4–13.
Rada P, González-Rodríguez Á, García-Monzón C, Valverde ÁM. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020;11(9):802.
Zhao L, Zhang C, Luo X, et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol. 2018;69(3):705–17.
Redgrave TG. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970;49(3):465–71.
Zhang X, Coker OO, Chu ES, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–74.
Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–95.
van Nierop FS, Scheltema MJ, Eggink HM, et al. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017;5(3):224–33.
Moore MP, Cunningham RP, Meers GM, et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022;76(5):1452–65.
Yang H, Deng Q, Ni T, et al. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int J Biol Sci. 2021;17(15):4207–22.
Liu X, Zhang J, Ming Y, Chen X, Zeng M, Mao Y. The aggravation of mitochondrial dysfunction in nonalcoholic fatty liver disease accompanied with type 2 diabetes mellitus. Scand J Gastroenterol. 2015;50(9):1152–9.
Weber M, Mera P, Casas J, et al. Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD. Faseb j. 2020;34(9):11816–37.
Hinds TD Jr, Hosick PA, Chen S, et al. Mice with hyperbilirubinemia due to Gilbert’s syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARα. Am J Physiol Endocrinol Metab. 2017;312(4):E244–52.
Lee Y, Kim BR, Kang GH, et al. The effects of PPAR agonists on atherosclerosis and nonalcoholic fatty liver disease in ApoE-/-FXR-/- mice. Endocrinol Metab (Seoul). 2021;36(6):1243–53.
Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5.
Wang Y, Kory N, BasuRay S, Cohen JC, Hobbs HH. PNPLA3, CGI-58, and inhibition of hepatic triglyceride hydrolysis in mice. Hepatology. 2019;69(6):2427–41.
Luukkonen PK, Porthan K, Ahlholm N, et al. The PNPLA3 I148M variant increases ketogenesis and decreases hepatic de novo lipogenesis and mitochondrial function in humans. Cell Metab. 2023;35(11):1887-96 e5.
Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35(4):898–904.
Pereira IV, Stefano JT, Oliveira CP. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2011;5(2):245–51.
Luo F, Smagris E, Martin SA, et al. Hepatic TM6SF2 is required for lipidation of VLDL in a pre-Golgi compartment in mice and rats. Cell Mol Gastroenterol Hepatol. 2022;13(3):879–99.
Luo F, Oldoni F, Das A. TM6SF2: a novel genetic player in nonalcoholic fatty liver and cardiovascular disease. Hepatol Commun. 2022;6(3):448–60.
Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J Biol Chem. 2016;291(20):10659–76.
Borén J, Adiels M, Björnson E, et al. Effects of TM6SF2 E167K on hepatic lipid and very low-density lipoprotein metabolism in humans. JCI Insight. 2020;5(24):e144079.
Shindo N, Fujisawa T, Sugimoto K, et al. Involvement of microsomal triglyceride transfer protein in nonalcoholic steatohepatitis in novel spontaneous mouse model. J Hepatol. 2010;52(6):903–12.
Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–46.
Verrastro O, Panunzi S, Castagneto-Gissey L, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet. 2023;401(10390):1786–97.
Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51(3):797–802.
Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85.
Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307.
Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135(1):100–10.
Bricker DK, Taylor EB, Schell JC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100.
Harrison SA, Alkhouri N, Davison BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J Hepatol. 2020;72(4):613–26.
Benova A, Ferencakova M, Bardova K, et al. Novel thiazolidinedione analog reduces a negative impact on bone and mesenchymal stem cell properties in obese mice compared to classical thiazolidinediones. Mol Metab. 2022;65:101598.
Mitrovic B, Gluvic Z, Macut D, et al. Effects of Metformin-single therapy on the level of inflammatory markers in serum of non-obese T2DM patients with NAFLD. Endocr Metab Immune Disord Drug Targets. 2022;22(1):117–24.
Green CJ, Marjot T, Walsby-Tickle J, et al. Metformin maintains intrahepatic triglyceride content through increased hepatic de novo lipogenesis. Eur J Endocrinol. 2022;186(3):367–77.
Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32(10):1211–21.
Yan J, Yao B, Kuang H, et al. Liraglutide, Sitagliptin, and insulin glargine added to Metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69(6):2414–26.
Pinkosky SL, Newton RS, Day EA, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7:13457.
Morrow MR, Batchuluun B, Wu J, et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab. 2022;34(6):919-36.e8.
Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–32.
Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64.
Kim CW, Addy C, Kusunoki J, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26(2):394-406.e6.
Lawitz EJ, Coste A, Poordad F, et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2018;16(12):1983-91.e3.
Loomba R, Kayali Z, Noureddin M, et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463-73.e6.
Bergman A, Carvajal-Gonzalez S, Tarabar S, Saxena AR, Esler WP, Amin NB. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a liver-targeting Acetyl-CoA carboxylase inhibitor (PF-05221304): a three-part randomized phase 1 study. Clin Pharmacol Drug Dev. 2020;9(4):514–26.
Wu M, Singh SB, Wang J, et al. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci U S A. 2011;108(13):5378–83.
Syed-Abdul MM, Parks EJ, Gaballah AH, et al. Fatty acid synthase inhibitor TVB-2640 reduces hepatic de novo lipogenesis in males with metabolic abnormalities. Hepatology. 2020;72(1):103–18.
Loomba R, Mohseni R, Lucas KJ, et al. TVB-2640 (FASN Inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterology. 2021;161(5):1475–86.
Kotronen A, Seppänen-Laakso T, Westerbacka J, et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58(1):203–8.
Iruarrizaga-Lejarreta M, Varela-Rey M, Fernández-Ramos D, et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol Commun. 2017;1(9):911–27.
Ratziu V, de Guevara L, Safadi R, et al. Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat Med. 2021;27(10):1825–35.
Ajmera VH, Cachay E, Ramers C, et al. MRI assessment of treatment response in HIV-associated NAFLD: a randomized trial of a Stearoyl-Coenzyme-A-Desaturase-1 inhibitor (ARRIVE Trial). Hepatology. 2019;70(5):1531–45.
Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74.
Amin NB, Carvajal-Gonzalez S, Purkal J, et al. Targeting diacylglycerol acyltransferase 2 for the treatment of nonalcoholic steatohepatitis. Sci Transl Med. 2019;11(520):eaav9701.
Loomba R, Morgan E, Watts L, et al. Novel antisense inhibition of diacylglycerol O-acyltransferase 2 for treatment of non-alcoholic fatty liver disease: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5(9):829–38.
Futatsugi K, Cabral S, Kung DW, et al. Discovery of Ervogastat (PF-06865571): a potent and selective inhibitor of Diacylglycerol Acyltransferase 2 for the treatment of non-alcoholic Steatohepatitis. J Med Chem. 2022;65(22):15000–13.
Calle RA, Amin NB, Carvajal-Gonzalez S, et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: two parallel, placebo-controlled, randomized phase 2a trials. Nat Med. 2021;27(10):1836–48.
Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21(1):57–69.
Li Y, Xu J, Lu Y, et al. DRAK2 aggravates nonalcoholic fatty liver disease progression through SRSF6-associated RNA alternative splicing. Cell Metab. 2021;33(10):2004-20 e9.
Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14(5):259–69.
Cable EE, Finn PD, Stebbins JW, et al. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49(2):407–17.
Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012–24.
Harrison SA, Bedossa P, Guy CD, et al. A phase 3, randomized, controlled trial of Resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390(6):497–509.
Harrison SA, Taub R, Neff GW, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29(11):2919–28.
Qiu YY, Zhang J, Zeng FY, Zhu YZ. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol Res. 2023;192:106786.
Lefere S, Puengel T, Hundertmark J, et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages(☆). J Hepatol. 2020;73(4):757–70.
Francque SM, Bedossa P, Ratziu V, et al. A randomized, controlled trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–58.
Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic Steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147-59.e5.
Gawrieh S, Noureddin M, Loo N, et al. Saroglitazar, a PPAR-α/γ Agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatology. 2021;74(4):1809–24.
Liu J, Yang K, Yang J, et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine. 2019;41:73–84.
Kuchay MS, Krishan S, Mishra SK, et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia. 2020;63(11):2434–45.
Liu L, Yan H, Xia M, et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev. 2020;36(5):e3292.
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90.
Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of Subcutaneous Semaglutide in nonalcoholic Steatohepatitis. N Engl J Med. 2021;384(12):1113–24.
O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–49.
Kimura T, Katakura Y, Shimoda M, et al. Comparison of clinical efficacy and safety of weekly glucagon-like peptide-1 receptor agonists dulaglutide and semaglutide in Japanese patients with type 2 diabetes: randomized, parallel-group, multicentre, open-label trial (COMING study). Diabetes Obes Metab. 2023;25(12):3632–47.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–16.
Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613–26.
Zhang Q, Delessa CT, Augustin R, et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021;33(4):833-44.e5.
Borner T, Geisler CE, Fortin SM, et al. GIP receptor Agonism attenuates GLP-1 receptor agonist-induced Nausea and Emesis in preclinical models. Diabetes. 2021;70(11):2545–53.
Kleinert M, Sachs S, Habegger KM, Hofmann SM, Müller TD. Glucagon regulation of energy expenditure. Int J Mol Sci. 2019;20(21):5407.
Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406.
Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor Agonist Tirzepatide on biomarkers of nonalcoholic Steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352–5.
Rosenstock J, Frias J, Jastreboff AM, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402(10401):529–44.
Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006–11.
Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–96.
Siddiqui MS, Van Natta ML, Connelly MA, et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2020;72(1):25–33.
Loomba R, Neuschwander-Tetri BA, Sanyal A, et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in NASH. Hepatology. 2020;72(4):1219–29.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65.
Sanyal AJ, Lopez P, Lawitz EJ, et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med. 2023;29(2):392–400.
Patel K, Harrison SA, Elkhashab M, et al. Cilofexor, a nonsteroidal FXR Agonist, in patients with Noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72(1):58–71.
Ratziu V, Harrison SA, Loustaud-Ratti V, et al. Hepatic and renal improvements with FXR agonist vonafexor in individuals with suspected fibrotic NASH. J Hepatol. 2023;78(3):479–92.
Harrison SA, Bashir MR, Lee KJ, et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75(1):25–33.
Gadaleta RM, Moschetta A. Metabolic messengers: fibroblast growth factor 15/19. Nat Metab. 2019;1(6):588–94.
Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic Steatohepatitis. Hepatology. 2020;71(4):1198–212.
Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of Aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic Steatohepatitis. Gastroenterology. 2021;160(1):219-31.e1.
Harrison SA, Abdelmalek MF, Neff G, et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2022;7(7):603–16.
Rinella ME, Trotter JF, Abdelmalek MF, et al. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J Hepatol. 2019;70(4):735–44.
Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15(1):51–69.
Véniant MM, Komorowski R, Chen P, et al. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153(9):4192–203.
Harrison SA, Ruane PJ, Freilich BL, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27(7):1262–71.
Harrison SA, Frias JP, Neff G, et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8(12):1080–93.
Loomba R, Sanyal AJ, Kowdley KV, et al. Randomized, controlled trial of the FGF21 analogue Pegozafermin in NASH. N Engl J Med. 2023;389(11):998–1008.
Loomba R, Lawitz EJ, Frias JP, et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. Lancet Gastroenterol Hepatol. 2023;8(2):120–32.
Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392(10165):2705–17.
Boutari C, Mantzoros CS. Adiponectin and leptin in the diagnosis and therapy of NAFLD. Metabolism. 2020;103:154028.
Metz M, Beghini M, Wolf P, et al. Leptin increases hepatic triglyceride export via a vagal mechanism in humans. Cell Metab. 2022;34(11):1719-31.e5.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–72.
Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of SGLT2 inhibitors: Part 3. Effects on diabetic complications in type 2 diabetic mice. Eur J Pharmacol. 2017;809:163–71.
Koshino A, Oshima M, Arnott C, et al. Effects of canagliflozin on liver steatosis and fibrosis markers in patients with type 2 diabetes and chronic kidney disease: a post hoc analysis of the CREDENCE trial. Diabetes Obes Metab. 2023;25(5):1413–8.
Latva-Rasku A, Honka MJ, Kullberg J, et al. The SGLT2 inhibitor Dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care. 2019;42(5):931–7.
Ito D, Shimizu S, Inoue K, et al. Comparison of Ipragliflozin and Pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label active-controlled trial. Diabetes Care. 2017;40(10):1364–72.
Funding
We have obtained financial supports from the National Key Research and Development Program of China (2021YFC2700403), the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), the Science and Technology Commission of Shanghai Municipality (23XD1423300,23ZR1411000), the National Natural Science Foundation of China (81873660, 32371333).
Author information
Authors and Affiliations
Contributions
Y.J. and L.W. drafted the manuscript; H.B. and X.Z. critically read the manuscript; M.X. provided the outline, organized and revised the manuscript; X.G. provided the core topic and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Y., Wu, L., Zhu, X. et al. Advances in management of metabolic dysfunction-associated steatotic liver disease: from mechanisms to therapeutics. Lipids Health Dis 23, 95 (2024). https://doi.org/10.1186/s12944-024-02092-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02092-2