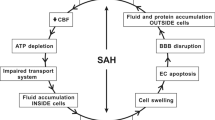
Abstract
Increasingly, experimental research in subarachnoid hemorrhage (SAH) has investigated early brain injury and the microcirculation. A number of pathophysiological changes occur in the cerebral microvessels after SAH including altered vasoreactivity, vasoconstriction, inflammation, blood–brain barrier impairment, increased microthrombi, and inversion of neurovascular coupling. This focused review looks at the current state of knowledge regarding the changes that occur in the microcirculation and the neurovascular unit after SAH.

Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ASA:
-
Acetylsalicylic acid
- ATP:
-
Adenosine triphosphate
- BBB:
-
Blood–brain barrier
- cGMP:
-
Cyclic guanosine monophosphate
- DCI:
-
Delayed cerebral ischemia
- eNOS:
-
Endothelial nitric oxide synthase
- FITC:
-
Fluorescein isothiocyanate
- IgG:
-
Immunoglobulin G
- IHC:
-
Immunohistochemistry
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MMP-9:
-
Matrix metalloproteinase-9
- NFkB:
-
Nuclear factor-kB
- NMDA:
-
N-methyl-d-aspartate
- NO:
-
Nitric oxide
- PAR-1:
-
Protease-activated receptor-1
- PUMA:
-
p53 upregulated modulator of apoptosis
- SAH:
-
Subarachnoid hemorrhage
- siRNA:
-
Small interfering RNA
- TNFα:
-
Tumor necrosis factor alpha
- tPA:
-
Tissue plasminogen activator
- uPA-T:
-
Urokinase-type plasminogen activator
- VDCC:
-
Voltage-dependent calcium channels
References
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–21.
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–46.
Macdonald RL. History and definition of delayed cerebral ischemia. Acta Neurochir Suppl. 2013;115:3–7.
Ecker A, Riemenschneider PA. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951;8:660–7.
Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–21.
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10:618–25.
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012;43:1463–9.
Herz DA, Baez S, Shulman K. Pial microcirculation in subarachnoid hemorrhage. Stroke. 1975;6:417–24.
Kniesel U, Wolburg H. Tight junctions of the blood–brain barrier. Cell Mol Neurobiol. 2000;20:57–76.
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–61.
Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–4.
Fenstermacher J, Gross P, Sposito N, Acuff V, Pettersen S, Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529:21–30.
Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood–brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–17.
Sedlakova R, Shivers RR, Del Maestro RF. Ultrastructure of the blood–brain barrier in the rabbit. J Submicrosc Cytol Pathol. 1999;31:149–61.
Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85.
Grotta JC, et al. (2002) Report of the Stroke Progress Review Group. National Institute of Neurological Disorders and Stroke. [online], http://www.ninds.nih.gov/find_people/groups/stroke_prg/StrokePRGreport-4-23-02.pdf. Accessed 1 Oct 2013.
Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf). 2011;203:47–59.
Peppiatt C, Attwell D. Neurobiology: feeding the brain. Nature. 2004;431:137–8.
Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50.
Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci U S A. 2012;109:E1387–95.
Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–9.
Allan S. The neurovascular unit and the key role of astrocytes in the regulation of cerebral blood flow. Cerebrovasc Dis. 2006;21:137–8.
Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32:1207–21.
Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. The vascular neural network—a new paradigm in stroke pathophysiology. Nat Rev Neurol. 2012;8:711–6.
Sehba FA, Friedrich V. Early micro vascular changes after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:49–55.
Rosenblum WI. Pial arteriolar responses in the mouse brain revisited. Stroke. 1976;7:283–7.
Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–25.
Cipolla MJ (2009) “The cerebral microcirculation”. In: Granger DN, Granger J, editors. Integrated systems physiology: From molecule to function #2. San Rafael: Morgan & Claypool Life Sciences.
Britz GW, Meno JR, Park IS, Abel TJ, Chowdhary A, Nguyen TS, et al. Time-dependent alterations in functional and pharmacological arteriolar reactivity after subarachnoid hemorrhage. Stroke. 2007;38:1329–35.
Wiernsperger N, Schulz U, Gygax P. Physiological and morphometric analysis of the microcirculation of the cerebral cortex under acute vasospasm. Stroke. 1981;12:624–7.
Friedrich B, Muller F, Feiler S, Scholler K, Plesnila N. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab. 2012;32:447–55.
Sun BL, Zheng CB, Yang MF, Yuan H, Zhang SM, Wang LX. Dynamic alterations of cerebral pial microcirculation during experimental subarachnoid hemorrhage. Cell Mol Neurobiol. 2009;29:235–41.
Ishikawa M, Kusaka G, Yamaguchi N, Sekizuka E, Nakadate H, Minamitani H, et al. Platelet and leukocyte adhesion in the microvasculature at the cerebral surface immediately after subarachnoid hemorrhage. Neurosurgery. 2009;64:546–53.
Kajita Y, Dietrich HH, Dacey Jr RG. Effects of oxyhemoglobin on local and propagated vasodilatory responses induced by adenosine, adenosine diphosphate, and adenosine triphosphate in rat cerebral arterioles. J Neurosurg. 1996;85:908–16.
Katusic ZS, Milde JH, Cosentino F, Mitrovic BS. Subarachnoid hemorrhage and endothelial L-arginine pathway in small brain stem arteries in dogs. Stroke. 1993;24:392–9.
Park KW, Metais C, Dai HB, Comunale ME, Sellke FW. Microvascular endothelial dysfunction and its mechanism in a rat model of subarachnoid hemorrhage. Anesth Analg. 2001;92:990–6.
Park KW, Dai HB, Metais C, Comunale ME, Sellke FW. Isoflurane does not further impair microvascular vasomotion in a rat model of subarachnoid hemorrhage. Can J Anaesth. 2002;49:427–33.
Park IS, Meno JR, Witt CE, Chowdhary A, Nguyen TS, Winn HR, et al. Impairment of intracerebral arteriole dilation responses after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg. 2009;111:1008–13.
Vollmer DG, Takayasu M, Dacey Jr RG. An in vitro comparative study of conducting vessels and penetrating arterioles after experimental subarachnoid hemorrhage in the rabbit. J Neurosurg. 1992;77:113–9.
Nystoriak MA, O’Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–12.
Koide M, Bonev AD, Nelson MT, Wellman GC. Subarachnoid blood converts neurally evoked vasodilation to vasoconstriction in rat brain cortex. Acta Neurochir Suppl. 2013;115:167–71.
Ohkuma H, Manabe H, Tanaka M, Suzuki S. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2000;31:1621–7.
del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998;65:1–9.
Tso MK, Macdonald RL. Acute microvascular changes after subarachnoid hemorrhage and transient global cerebral ischemia. Stroke Res Treat. 2013;2013:425281.
Titova E, Ostrowski RP, Zhang JH, Tang J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol Res. 2009;31:568–81.
Koide M, Wellman GC. SAH-induced suppression of voltage-gated K(+) (K (V)) channel currents in parenchymal arteriolar myocytes involves activation of the HB-EGF/EGFR pathway. Acta Neurochir Suppl. 2013;115:179–84.
Cach R, Smock T, Popejoy S. Blood-borne factors regulating microvascular constriction in the rat hippocampal slice. Brain Res. 1987;414:1–7.
Zubkov AY, Tibbs RE, Aoki K, Zhang JH. Prevention of vasospasm in penetrating arteries with MAPK inhibitors in dog double-hemorrhage model. Surg Neurol. 2000;54:221–7.
Ohkuma H, Itoh K, Shibata S, Suzuki S. Morphological changes of intraparenchymal arterioles after experimental subarachnoid hemorrhage in dogs. Neurosurgery. 1997;41:230–5.
Asano T, Sano K. Pathogenetic role of no-reflow phenomenon in experimental subarachnoid hemorrhage in dogs. J Neurosurg. 1977;46:454–66.
Ohkuma H, Suzuki S. Histological dissociation between intra- and extraparenchymal portion of perforating small arteries after experimental subarachnoid hemorrhage in dogs. Acta Neuropathol. 1999;98:374–82.
Ohkuma H, Suzuki S, Ogane K. Phenotypic modulation of smooth muscle cells and vascular remodeling in intraparenchymal small cerebral arteries after canine experimental subarachnoid hemorrhage. Neurosci Lett. 2003;344:193–6.
Sabri M, Ai J, Lakovic K, D’abbondanza J, Ilodigwe D, Macdonald RL. Mechanisms of microthrombi formation after experimental subarachnoid hemorrhage. Neuroscience. 2012;224:26–37.
Johshita H, Kassell NF, Sasaki T, Ogawa H. Impaired capillary perfusion and brain edema following experimental subarachnoid hemorrhage: a morphometric study. J Neurosurg. 1990;73:410–7.
Sehba FA, Friedrich Jr V, Makonnen G, Bederson JB. Acute cerebral vascular injury after subarachnoid hemorrhage and its prevention by administration of a nitric oxide donor. J Neurosurg. 2007;106:321–9.
Friedrich V, Flores R, Muller A, Sehba FA. Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res. 2010;1354:179–87.
Uhl E, Lehmberg J, Steiger HJ, Messmer K. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery. 2003;52:1307–15.
Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, & van Gijn J (2007) Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. CD000277.
Meyer R, Deem S, Yanez ND, Souter M, Lam A, Treggiari MM. Current practices of triple-H prophylaxis and therapy in patients with subarachnoid hemorrhage. Neurocrit Care. 2010;14:24–36.
Ostergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1825–37.
Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative–nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–7.
Nihei H, Kassell NF, Dougherty DA, Sasaki T. Does vasospasm occur in small pial arteries and arterioles of rabbits? Stroke. 1991;22:1419–25.
Perkins E, Kimura H, Parent AD, Zhang JH. Evaluation of the microvasculature and cerebral ischemia after experimental subarachnoid hemorrhage in dogs. J Neurosurg. 2002;97:896–904.
Josko J. Cerebral angiogenesis and expression of VEGF after subarachnoid hemorrhage (SAH) in rats. Brain Res. 2003;981:58–69.
Zhou N, Xu T, Bai Y, Prativa S, Xu JZ, Li K, et al. Protective effects of urinary trypsin inhibitor on vascular permeability following subarachnoid hemorrhage in a rat model. CNS Neurosci Ther. 2013;19:659–66.
Ansar S, Edvinsson L. Subtype activation and interaction of protein kinase C and mitogen-activated protein kinase controlling receptor expression in cerebral arteries and microvessels after subarachnoid hemorrhage. Stroke. 2008;39:185–90.
Friedrich V, Flores R, Muller A, Bi W, Peerschke EI, Sehba FA. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J Neuroinflammation. 2011;8:103.
Moore KL. Structure and function of P-selectin glycoprotein ligand-1. Leuk Lymphoma. 1998;29:1–15.
Yatsushige H, Ostrowski RP, Tsubokawa T, Colohan A, Zhang JH. Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J Neurosci Res. 2007;85:1436–48.
Erdo F, Erdo SL. Bimoclomol protects against vascular consequences of experimental subarachnoid hemorrhage in rats. Brain Res Bull. 1998;45:163–6.
Germano A, Costa C, DeFord SM, Angileri FF, Arcadi F, Pike BR, et al. Systemic administration of a calpain inhibitor reduces behavioral deficits and blood–brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2002;19:887–96.
Germano A, Caffo M, Angileri FF, Arcadi F, Newcomb-Fernandez J, Caruso G, et al. NMDA receptor antagonist felbamate reduces behavioral deficits and blood–brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–44.
Imperatore C, Germano A, d’Avella D, Tomasello F, Costa G. Effects of the radical scavenger AVS on behavioral and BBB changes after experimental subarachnoid hemorrhage. Life Sci. 2000;66:779–90.
Suzuki H, Ayer R, Sugawara T, Chen W, Sozen T, Hasegawa Y, et al. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38:612–8.
Yan J, Manaenko A, Chen S, Klebe D, Ma Q, Caner B, et al. Role of SCH79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke. 2013;44:1410–7.
Doczi T, Joo F, Adam G, Bozoky B, Szerdahelyi P. Blood–brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery. 1986;18:733–9.
Doczi T, Joo F, Sonkodi S, Adam G. Increased vulnerability of the blood–brain barrier to experimental subarachnoid hemorrhage in spontaneously hypertensive rats. Stroke. 1986;17:498–501.
Germano A, d’Avella D, Imperatore C, Caruso G, Tomasello F. Time-course of blood–brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir (Wien). 2000;142:575–80.
Wang Z, Zuo G, Shi XY, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-kappaB signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm. 2011;2011:848309.
Smith SL, Scherch HM, Hall ED. Protective effects of tirilazad mesylate and metabolite U-89678 against blood–brain barrier damage after subarachnoid hemorrhage and lipid peroxidative neuronal injury. J Neurosurg. 1996;84:229–33.
Yatsushige H, Calvert JW, Cahill J, Zhang JH. Limited role of inducible nitric oxide synthase in blood–brain barrier function after experimental subarachnoid hemorrhage. J Neurotrauma. 2006;23:1874–82.
Scholler K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, et al. Characterization of microvascular basal lamina damage and blood–brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–46.
Yan J, Chen C, Hu Q, Yang X, Lei J, Yang L, et al. The role of p53 in brain edema after 24 h of experimental subarachnoid hemorrhage in a rat model. Exp Neurol. 2008;214:37–46.
Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, et al. Blood–brain barrier disruption following subarachnoid hemorrhage may be facilitated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol. 2011;230:240–7.
Gules I, Satoh M, Nanda A, Zhang JH. Apoptosis, blood–brain barrier, and subarachnoid hemorrhage. Acta Neurochir Suppl. 2003;86:483–7.
Friedrich V, Flores R, Muller A, Sehba FA. Escape of intraluminal platelets into brain parenchyma after subarachnoid hemorrhage. Neuroscience. 2010;165:968–75.
Sehba FA, Friedrich V. Cerebral microvasculature is an early target of subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:199–205.
Sehba FA, Mostafa G, Knopman J, Friedrich Jr V, Bederson JB. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J Neurosurg. 2004;101:633–40.
Sehba FA, Flores R, Muller A, Friedrich V, Chen JF, Britz GW, et al. Adenosine A(2A) receptors in early ischemic vascular injury after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg. 2010;113:826–34.
Friedrich V, Flores R, Sehba FA. Cell death starts early after subarachnoid hemorrhage. Neurosci Lett. 2012;512:6–11.
Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Delayed cell death related to acute cerebral blood flow changes following subarachnoid hemorrhage in the rat brain. J Neurosurg. 2005;102:1046–54.
Peterson EW, Cardoso ER. The blood–brain barrier following experimental subarachnoid hemorrhage. Part 1: Response to insult caused by arterial hypertension. J Neurosurg. 1983;58:338–44.
Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–7.
Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–32.
Zhang S, Wang L, Liu M, Wu B. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010. CD006778.
Stein SC, Browne KD, Chen XH, Smith DH, Graham DI. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery. 2006;59:781–7.
Sehba FA, Mostafa G, Friedrich Jr V, Bederson JB. Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg. 2005;102:1094–100.
Sabri M, Ai J, Lass E, D’abbondanza J, Macdonald RL. Genetic elimination of eNOS reduces secondary complications of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1008–14.
Pisapia JM, Xu X, Kelly J, Yeung J, Carrion G, Tong H, et al. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp Neurol. 2012;233:357–63.
Ramakrishna R, Sekhar LN, Ramanathan D, Temkin N, Hallam D, Ghodke BV, et al. Intraventricular tissue plasminogen activator for the prevention of vasospasm and hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2010;67:110–7.
van den Bergh WM, Algra A, Dorhout Mees SM, van Kooten F, Dirven CM, van Gijn J, et al. Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH Study. Stroke. 2006;37:2326–30.
Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–47.
Nicoletti C, Offenhauser N, Jorks D, Major S, Dreier JP “Assessment of neurovascular coupling”. In: Chen J, Xu X-M, Xu ZC, Zhang JH, editors. Animal models of acute neurological injuries II: Injuries and mechanistic assessments. Springer Protocols Handbooks. Totowa, NJ: Humana; 2012. Volume I. pp. 353–72
Acknowledgments
MT receives funding from the Vanier Canada Graduate Scholarship, Canadian Institutes of Health Research, Neurosurgery Research and Education Foundation, and the Joint Cerebrovascular Section of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons. RLM receives grant support from the Brain Aneurysm Foundation, Heart and Stroke Foundation of Canada, and is a stockholder of Edge Therapeutics. RLM is Chief Scientific Officer of Edge Therapeutics.
Conflict of Interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: “Phenotypic transformation of smooth muscle in cerebrovascular disease”
Rights and permissions
About this article
Cite this article
Tso, M.K., Macdonald, R.L. Subarachnoid Hemorrhage: a Review of Experimental Studies on the Microcirculation and the Neurovascular Unit. Transl. Stroke Res. 5, 174–189 (2014). https://doi.org/10.1007/s12975-014-0323-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-014-0323-4