Abstract
Guidelines recommend shorter duration (1–12 months) for dual antiplatelet therapy (DAPT) in the second-generation drug-eluting stent (DES) era. However, whether shorter DAPT duration affects stent strut conditions and neointimal characteristics at mid-term follow-up remains uncertain. Therefore, we studied the relation between DAPT duration and vascular healing response as assessed by optical coherence tomography (OCT). This study was retrospective observational study. Participants comprised 64 patients who underwent serial OCT at both 9 and 18 months after DES implantation. All patients received DAPT until the 9-month follow-up then were divided into two groups: 49 patients who continued DAPT (longer DAPT group); and 15 patients who stopped taking the P2Y12 inhibitor and were treated with aspirin alone (shorter DAPT group) at the 18-month follow-up. Using OCT, we evaluated and compared stent strut conditions and neointimal characteristics between groups at both 9 and 18 months after stent implantation. Baseline clinical and procedural parameters were mostly similar between groups. At the 18-month follow-up, no in-stent thrombus assessed by OCT was observed in either group. No significant differences in OCT characteristics or measurements of neointima were seen between groups at 9- or 18-month follow-ups. Neointimal volume increased from 9 to 18 months in both groups, with a similar degree of neointimal proliferation in both groups (shorter DAPT group, 0.23 ± 0.29 mm3/mm; longer DAPT group, 0.19 ± 0.27 mm3/mm; P = 0.56). In conclusion, interrupting DAPT 9 months after second-generation DES implantation did not affect the development of in-stent thrombus, neointimal proliferation or stent strut coverage at 18-month follow-up compared with continuing DAPT.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Stent implantation has developed as a treatment for coronary artery disease. Bare metal stents (BMS) successfully restore the acute vessel lumen, but long-term outcomes are often compromised by in-stent restenosis (ISR). Drug-eluting stents (DES) markedly reduce ISR compared with BMS and indications for percutaneous coronary intervention (PCI) have widened. In the second-generation DES era, some clinical trials suggested the superiority of DES over BMS even with in-stent thrombosis and patients with the acute coronary syndrome (ACS) [1,2,3].
A previous study showed that longer administration of dual antiplatelet therapy (DAPT) prevented thrombotic adverse events, but increased the risk of hemorrhagic complications [4]. On the other hand, some recent papers have shown that shorter DAPT duration did not increase thrombotic events, particularly with the use of second-generation DES [1, 5, 6]. The guideline thus recommends a shorter duration of DAPT after DES implantation, particularly for patients at high risk of bleeding [7, 8]. However, differences in stent strut condition between patients with shorter and longer DAPT administration at mid-term after DES implantation remain unclear. The purpose of this study was to evaluate the influence of DAPT duration on neointimal condition after second-generation DES implantation using optical coherence tomography (OCT).
Methods
Study population
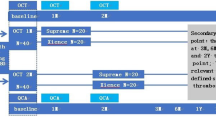
Participants comprised 64 consecutive patients who underwent PCI with second-generation DES as a treatment for de novo lesions and were subsequently followed-up with OCT examinations at Kawasaki Medical School Hospital from November 2011 to February 2014 (Fig. 1). The interventional strategy and stent selection were left to the discretion of the operator. Follow-up OCT (ILUMIEN or ILUMIEN OPTIS OCT Intravascular Imaging System; St. Jude Medical, St. Paul, MN) were performed at 9 months and 18 months after DES implantation. Second-generation DES included everolimus-eluting stents (CoCr-EES, Xience™; Abbott Vascular, Santa Clara, CA or PtCr-EES, PROMUS™; Boston Scientific, Natick, MA), biolimus-eluting stents (BES, Nobori®; Terumo Corporation, Tokyo, Japan), and zotarolimus-eluting stents (ZES: Resolute™, Medtronic, Santa Rosa, CA).
DAPT with 100 mg of aspirin and either 75 mg of clopidogrel or 200 mg of ticlopidine was started at PCI and continued at least until the 9-month follow-up angiography. We classified patients into two groups: the shorter DAPT group with interruption of DAPT after the 9-month follow-up; and the longer DAPT group with the continuation of DAPT until the 18-month follow-up. The duration of DAPT was left to the doctor’s discretion. In addition, we calculated the PRECISE-DAPT score at index PCI and the DAPT score at 9-month follow-up [9, 10]. Patients with unclear OCT images due to insufficient blood removal or artifacts at follow-up coronary angiographies were excluded from analysis. In-stent restenosis (ISR) was defined angiographical more than 50% stenosis of the stented segment at follow-up periods.
This study was performed in compliance with the Declaration of Helsinki with regard to investigations involving human participants. The study protocol was approved by the ethics committee at Kawasaki Medical School, and written informed consent was obtained from each patient prior to enrolment.
OCT imaging and analysis
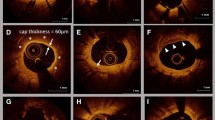
At follow-up angiographies, OCT was performed after intracoronary administration of nitroglycerin (0.2–0.3 mg). The OCT catheter was placed > 10 mm distal to the stented lesion and pulled back using an automatic pullback system. Cross-sectional OCT images of stented segments at 1-mm intervals were analyzed. We evaluated the neointimal coverage of each stent strut, neointimal volume, neointimal tissue characteristics and presence of thrombus. A covered strut was defined as a neointimal thickness (NIT), representing the distance between the center of the strut and the neointimal surface, > 30 μm (Fig. 2) [11, 12]. Neointimal volume was calculated by adding each neointimal area (stent area minus lumen area) throughout the stented segment. Neointimal volume index was determined as the neointimal volume divided by the length of the stented lesion. We evaluated the neointimal characteristics at the minimum lumen area (MLA) site of the stented segment and classified into four patterns: homogeneous, heterogeneous, layered and neoatherosclerosis (NA) [13]. NA was defined as the presence of lipid, calcification, thin-cap fibroatheroma, neovascularization and intimal rupture in the neointima, as mentioned in previous studies [14, 15]. Thrombus was defined as a mass protruding into the lumen with an irregular surface [16]. These OCT findings were compared between shorter DAPT and longer DAPT groups.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile range and were compared using a two-tailed unpaired t test or Wilcoxon rank-sum test. Categorical variables are expressed as percentages and were compared using the Chi-square test. Values of P < 0.05 were considered statistically significant. All statistical analyses were conducted using JMP for Mac version 11.0 (SAS Institute, Cary, NC).
Results
A total of 64 patients (64 lesions) were studied, with 15 patients in the shorter DAPT group and 49 patients in the longer DAPT group. This study was retrospective observational study and guidelines of the Japanese circulation society recommended that DAPT duration after DES implantation was more than 12 months when analyzed cases underwent PCI [17]. Therefore, we continued DAPT for most of the cases without revascularization at 9-month follow-up angiography. However, recent studies reported the safety and effectiveness of short DAPT duration after DES implantation [1, 5, 6] and the duration of DAPT was left to the doctor’s discretion in this study.
Baseline clinical characteristics are shown in Table 1. In terms of risk factors, no significant differences were evident between groups, but a tendency was seen toward hypertension being more frequent in the longer DAPT group than in the shorter DAPT group. DAPT score and PRICISE-DAPT score did not differ significantly between groups. Pharmacotherapies excluding antiplatelet drugs at the 18-month follow-up are shown in Table 2. Angiotensin II receptor blockers, β-blockers and calcium channel blockers were used more frequently in the longer DAPT group than in the shorter DAPT group because of the patients’ comorbidity of hypertension. All patients in the shorter DAPT group stopped taking a P2Y12 inhibitor (not aspirin) when changing from DAPT to SAPT. In longer DAPT group, there were three patients (6%) who took ticlopidine and the remaining patients took clopidogrel as a P2Y12 inhibitor.
Lesion and procedural characteristics are listed in Table 3. Target vessel, selected stent type and stent size were similar in both groups. Frequency of ISR did not differ significantly between groups at either follow-up.
OCT findings at follow-up
OCT findings at 9- and 18-month follow-ups are shown in Tables 4 and 5. As for neointimal tissue characteristics at MLA site, no significant differences were evident between groups during these periods, with each group predominantly showing a homogeneous pattern. Most stent struts were covered with neointima in both groups. Evidence of thrombus was detected in one case from the longer DAPT group at 9-month follow-up but had disappeared by the 18-month follow-up.
Changes in OCT findings between 9- and 18-month follow-ups are shown in Table 6 and Fig. 3. In both groups, neointimal volume and stent strut coverage tended to be increased (no significant difference) and increments in neointimal proliferation were similar.
Discussion
The main finding in this study was that interrupting DAPT after the 9-month follow-up did not influence stent strut coverage, the existence of thrombus or neointimal volume at 18-month follow-up as assessed by OCT.
Insufficient neointimal proliferation and incomplete healing were often observed in DES compared with BMS, and have been associated with late stent thrombosis in previous autopsy reports [18,19,20]. DAPT has thus been continued for a longer duration after DES implantation to prevent thrombotic events. A previous DAPT study supported the effect of longer DAPT administration for > 1 year in preventing these adverse events of DES [4]. On the other hand, hemorrhage is one disadvantage of continuing DAPT for longer periods.
Recently, DES have seen improvements in stent design, polymers, drug dose, and so on, creating the so-called second generation of DES. Some OCT studies have shown better stent strut coverage and vascular healing response for implanted second-generation DES than for first-generation DES at mid-term follow-up [21, 22]. This improvement allows DAPT duration to be shorter without increasing thrombotic events. In fact, first-generation DES have mainly been used in DAPT studies and most implanted DES in recent papers that have recommended shorter DAPT duration have been second-generation DES [6, 23,24,25]. In our study, vascular healing and appearance of thrombus after second-generation DES at mid-term follow-up were almost uniform regardless of the duration of DAPT. In the shorter DAPT group, neointimal volume increase due to thrombus formation in the period from 9-month to 18-month follow-up were not observed in these serial OCT examinations. Our data support shorter DAPT duration as acceptable, especially for second-generation DES.
As for changes in OCT findings during the period from 9- to 18-month follow-up, percentage stent strut coverage and neointimal volume increased in both groups numerically, but not statistically significant. The rate of covered struts in our study was not markedly different from that in previous papers (87.5–99.7%) on mid-term OCT after second-generation DES implantation [21, 26, 27]. These results showed that neointimal proliferation continued overtime in the mid-term after second-generation DES implantation. However, the increase in neointimal volume was very low and was considered to be of little clinical relevance. These findings suggest favorable neointimal proliferation for second-generation DES.
This study had several limitations. First, it was a single-center, retrospective, observational study with no randomization. Second, the sample size was relatively limited, raising the possibility of selection bias. These limitations meant that no definitive conclusions have been reached. Third, we did not evaluate patients with target lesion failure or adverse events during follow-up. Finally, the follow-up period was limited to 18 months. We therefore could not assess correlations between OCT findings and clinical outcomes such as stent thrombosis or late catch-up ISR. Long-term follow-up in a larger population is needed to confirm our results.
Conclusion
Interrupting DAPT at 9 months after second-generation DES implantation might not affect the development of in-stent thrombus, neointimal proliferation or stent strut coverage compared with DAPT continued for up to 18 months.
References
Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabate M, Valgimigli M, et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496–504.
Bonaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygard O, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–52.
Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–402.
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66.
Vranckx P, Valgimigli M, Juni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940–9.
Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321:2414–27.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152:1243–75.
Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–34.
Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–49.
Sawada T, Shite J, Negi N, Shinke T, Tanino Y, Ogasawara D, et al. Factors that influence measurements and accurate evaluation of stent apposition by optical coherence tomography. Assessment using a phantom model. Circ J. 2009;73:1841–7.
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72.
Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia-Garcia HM, van Soest G, et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–93.
Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954–63.
Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–22.
Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97:1713–7.
JCS Joint Working Group. Guidelines for elective percutaneous coronary intervention in patients with stable coronary artery disease (JCS 2011) published in 2012--digest version. Circ J 2013;77:1590–1607.
Chieffo A, Foglieni C, Nodari RL, Briguori C, Sangiorgi G, Latib A, et al. Histopathology of clinical coronary restenosis in drug-eluting versus bare metal stents. Am J Cardiol. 2009;104:1660–7.
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202.
Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–41.
Choi HH, Kim JS, Yoon DH, Hong KS, Kim TH, Kim BK, et al. Favorable neointimal coverage in everolimus-eluting stent at 9 months after stent implantation: comparison with sirolimus-eluting stent using optical coherence tomography. Int J Cardiovasc Imaging. 2012;28:491–7.
Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, et al. Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart. 2009;95:1907–12.
Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65:1298–310.
Sheyin O, Perez X, Pierre-Louis B, Kurian D. The optimal duration of dual antiplatelet therapy in patients receiving percutaneous coronary intervention with drug-eluting stents. Cardiol J. 2016;23:307–16.
Natsuaki M, Morimoto T, Yamamoto E, Shiomi H, Furukawa Y, Abe M, et al. One-year outcome of a prospective trial stopping dual antiplatelet therapy at 3 months after everolimus-eluting cobalt-chromium stent implantation: ShortT and OPtimal duration of Dual AntiPlatelet Therapy after everolimus-eluting cobalt-chromium stent (STOPDAPT) trial. Cardiovasc Interv Ther. 2016;31:196–209.
Goryo Y, Kume T, Ueda T, Watanabe M, Yamada R, Neishi Y, et al. Vascular healing response after everolimus-eluting stent implantation in acute coronary syndrome culprit lesions: comparison with implantation in stable angina pectoris. Acta Cardiol Sin. 2018;34:124–9.
Kim JS, Kim BK, Jang IK, Shin DH, Ko YG, Choi D, et al. Comparison of neointimal coverage between zotarolimus-eluting stent and everolimus-eluting stent using optical coherence tomography (COVER OCT). Am Heart J. 2012;163:601–7.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goryo, Y., Kume, T., Okamoto, H. et al. Influence of dual antiplatelet therapy duration on neointimal condition after second-generation drug-eluting stent implantation. Cardiovasc Interv and Ther 37, 101–108 (2022). https://doi.org/10.1007/s12928-021-00765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-021-00765-8