Abstract
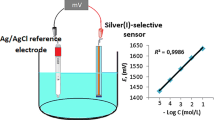
Herein, we developed a novel potentiometric sensor that exhibits high selectivity towards Ag+ ions, using a newly synthesized phenol derivative (4) molecule as an electroactive material (ionophore). The sensor was prepared by coating the surface of a conductive solid contact by a membrane containing bis(2-ethylhexyl)sebacate (BEHS) as plasticizer, poly (vinly chloride) (PVC) as a polymeric matrix, electroactive material (4), and potassium tetrakis(4-chlorophenyl)borate (KTpClPB) as an additive. The developed sensor exhibited a wide linear concentration range of 1.0 × 10–6–1.0 × 10−1 mol L−1 and a lower detection limit of 5.87 × 10−7 mol L−1. The sensor exhibited quite good selectivity over other cationic species, and its potential response remained unaffected of pH in the range of 3.0–7.0. In addition, the developed sensor had a short response time of 8 s, good repeatability, and stability. Finally, the proposed sensor was used in the direct determination of Ag+ in different water samples, and as an indicator electrode for the end point determination in the potentiometric titration of Ag+ ions against sodium chloride.
Graphical abstract









Similar content being viewed by others
References
K. Xu, C. Pérez-Ràfols, M. Cuartero, G.A. Crespo, Electrochim. Acta 374, 137929 (2021)
O. Isildak, N. Deligönül, O. Özbek, Turk. J. Chem. 43, 1149–1158 (2019)
M.A. Karimi, S.Z. Mohammadi, A. Mohadesi, A. Hatefi-Mehrjardi, M. Mazloum-Ardakani, L.S. Korani, A.A. Kabir, Sci. Iran 18, 790–796 (2011)
H.M. Abu-Shawish, S.M. Saadeh, H.M. Dalloul, B. Najri, H. Al Athamna, Sens. Actuators B Chem. 182, 374–381 (2013)
T. Zhang, Y. Chai, R. Yuan, J. Guo, Mater. Sci. Eng. C 32, 1179–1183 (2012)
J.N. Bianchin, E. Martendal, E. Carase, J. Anal. Methods. Chem. 2011, 1–7 (2011)
F. Valverde, M. Costas, F. Pena, I. Lavilla, C. Bendicho, Chem. Speciat. Bioavailab. 20, 217–226 (2008)
R.K. Saha, Orient. J. Chem. 32, 499–507 (2016)
Q. Hu, G. Yang, Y. Zhao, J. Yin, Anal. Bioanal. Chem. 375, 831–835 (2003)
B.H. Zhang, L. Qi, F.Y. Wu, Microchim. Acta 170, 147–153 (2010)
M.E.B. Mohamed, E.Y. Frag, M.H. El Brawy, Microchem. J. 164, 106065 (2021)
Ö. Isildak, O. Özbek, M.B. Gürdere, J. Anal. Test. 4, 273–280 (2020)
O. Özbek, Ö. Isildak, C. Berkel, J. Incl. Phenom. Macrocycl. Chem. 98, 1–9 (2020)
S.P. Akanji, O.A. Arotiba, D. Nkosi, Electrocatalysis 10, 643–652 (2019)
T. Tamji, A. Nezamzadeh-Ejhieh, Electrocatalysis 10, 466–476 (2019)
G. Kuzu Çelik, A.F. Üzdürmez, A. Erkal, E. Kılıç, A.O. Solak, Z. Üstündağ, Electrocatalysis 7, 207–214 (2016)
O. Özbek, Ö. Isildak, M.B. Gürdere, A. Cetin, Org. Commun. 14, 228–239 (2021)
Ö. Isildak, O. Özbek, Crit. Rev. Anal. Chem. 51, 218–231 (2021)
C. Topcu, Talanta 161, 623–631 (2016)
Ö. Isildak, O. Özbek, K.M. Yigit, Int. J. Environ. Anal. Chem. 101, 2035–2045 (2021)
O. Özbek, C. Berkel, Ö. Isildak, I. Isildak, Clin. Chim. Acta 524, 154–163 (2022)
C. Topcu, Investigation of usage of recently synthesized Schiff bases as active components in chemical sensors. MSc Thesis, Ondokuzmayis University, Samsun, Turkey (2009)
E. Findik, M. Ceylan, M. Elmastaş, Eur. J. Med. Chem. 46, 4618–4624 (2011)
Ö. Isildak, O. Özbek, K.M. Yigit, Bulg. Chem. Commun. 52, 448–452 (2020)
O. Özbek, Ö. Isildak, ChemistrySelect 7, e202103988 (2022)
O. Özbek, Ö. Isildak, I. Isildak, Biochem. Eng. J. 176, 108181 (2021)
Ö. Isildak, O. Özbek, J. Chem. Sci. 132, 29 (2020)
Y. Umezawa, P. Bühlmann, K. Umezawa, K. Tohda, A.S. Amemiya, Pure Appl. Chem. 72, 1851–2082 (2000)
R.P. Buck, E. Lindner, Pure Appl. Chem. 66, 2527–2536 (1994)
D.M. Sejmanović, B.B. Petković, M.V. Budimir, S.P. Sovilj, V.M. Jovanović, Electroanal. 23, 1849–1855 (2011)
I. Isildak, M. Yolcu, O. Isildak, N. Demirel, G. Topal, H. Hosgoren, Microchim. Acta 144, 177181 (2004)
A. Demirel, A. Doğan, G. Akkuş, M. Yılmaz, E. Kılıç, Electroanal. 18, 1019–1027 (2006)
R.K. Mahajan, M. Kumar, V. Sharma, I. Kaur, Analyst 126, 505–507 (2001)
M. Masrournia, H. Zamani, H. Mohammedzadeh, S.M. Seyedi, M.R. Ganjali, H.A. Eshghi, J. Chil. Chem. Soc. 54, 63–67 (2009)
M. Mazloum, M.S. Niassary, S.H. Mirhoseini Chahooki, M.K. Amini, Electroanal. 14, 376–381 (2002)
R.K. Mahajan, I. Kaur, V. Sharma, M. Kumar, Sensors 2, 417–423 (2002)
Acknowledgements
The authors would like to thank Research Assistant Caglar Berkel for his contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özbek, O., Çetin, A., Koç, E. et al. Synthesis and Sensor Properties of a Phenol Derivative Molecule: Potentiometric Determination of Silver(I) Ions. Electrocatalysis 13, 486–493 (2022). https://doi.org/10.1007/s12678-022-00738-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-022-00738-2