Abstract
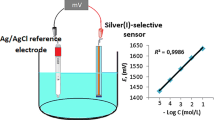
A novel poly(vinyl chloride) membrane potentiometric sensor for chromium(III) ions based on the use of 5,5′-(1,4-phenylene)bis(3-(naphthalen-1-yl)-4,5-dihydro-1H-pyrazole-1-carbothioamide) as a neutral ionophore was developed. The optimum composition of the best performing membrane contained ionophore, potassium tetrakis (p-chlorophenyl) borate (KTpClPB), dibutyl phthalate (DBP), and poly(vinyl chloride) (PVC) in the ratio of 5.5:1.5:55:38 (mg). The sensor exhibits a working concentration range of 1.0 × 10–5–1.0 × 10–1 mol L−1 and a detection limit of 1.7 × 10–6 mol L−1. The sensor shows good selectivity for chromium(III) ions over a number of cations including alkali, alkaline earth, heavy and transition metals. The response time of the sensor is 8 s. In addition, the developed sensor shows good reusability and stability. The sensor operates in the wide pH range of 5.0–11.0. The sensor could be used as an indicator electrode in the quantification of Cr3+ ions by potentiometric titration against ethylenediaminetetraacetic acid (EDTA). Finally, this sensor was successfully used for the determination of chromium(III) in commercial water, purification water and wastewater.








Similar content being viewed by others
References
Kumar P, Shim Y-B. Chromium(III)-selective electrode using p-(4-acetanilidazo)calix[4]arene as an ionophore in PVC matrix. Bull Korean Chem Soc. 2008;29:2471–6.
Lu J, Tian J, Wu H, Zhao C. Speciation determination of chromium(VI) and chromium(III) in soil samples after cloud point extraction. Anal Lett. 2009;42:1662–7.
Soomro R, Ahmed MJ, Memon N. Simple and rapid spectrophotometric determination of trace level chromium using bis (salicylaldehyde) orthophenylene diamine in nonionic micellar media. Turk J Chem. 2011;35:155–70.
Mallah A, Memon SQ, Solangi AR, Khan A, Khuhawar MY, Bhanger MI. Separation and determination of chromium (III) chromium (VI), gold (III) and arsenic (V) by capillary zone electrophoresis using 2-acetylpyridine-4-phenylthiosemicarbazone as complexing reagent. J Chem Soc Pak. 2014;36:255–62.
Zhao ZX, Zhang XS. Determination of trace amounts of chromium(III) in water samples using online flow injection catalytic spectrophotometry. J Appl Spectrosc. 2017;83:1084–8.
Mao J, He Q, Liu W. An, “off–on” fluorescence probe for chromium(III) ion determination in aqueous solution. Anal Bioanal Chem. 2010;396:1197–203.
Gupta VK, Jain AK, Kumar P, Agarwal S, Maheshwari G. Chromium(III)-selective sensor based on tri-o-thymotide in PVC matrix. Sensor Actuat B Chem. 2006;113:182–6.
Anthemidis NA, Zachariadis GA, Kougoulis JS, Strati JA. Flame atomic absorption spectrometric determination of chromium(VI) by on-line preconcentration system using a PTFE packed column. Talanta. 2002;57:15–22.
Taraba L, Krizek T, Kubickova A, Coufal P. Sample pretreatment for the capillary electrophoretic determination of organic acids in chromium(III) plating baths. J Sep Sci. 2015;38:4255–61.
Kumar P, Kumar S, Jain S, Lamba BY, Joshi G, Arora S. All solid state chromium(III) selective potentiometric sensor based on 2-(1-(2-((3-(2-hydroxyphenyl)-1H-pyrozol-1-yl)methyl)benzyl)-1H-pyrazol-3-yl)phenol. Electroanalysis. 2014;26:2161–7.
Tiglea P, Lichtig J. Determination of traces of chromium in foods by solvent extraction flame atomic absorption spectrometry. Anal Lett. 2000;33:1615–24.
Sun HW, Kang WJ, Liang SX, Ha J, Shen SG. Determination of chromium(III) and total chromium in water by derivative atomic absorption spectrometry using flow injection on-line preconcentration with a double microcolumn. Anal Sci. 2003;19:589–92.
Amin AS, Kassem MA. Chromium speciation in environmental samples using a solid phase spectrophotometric method. Spectrochim Acta. 2012;96:541–7.
Magini M. X-ray diffraction study of concentrated chromium (III) chloride solutions. I. Complex formation analysis in equilibrium conditions. J Chem Phys. 1980;73:2499–505.
Umesh B, Rajendran RM, Manoharan MT. Method for the determination of chromium in feed matrix by HPLC. Poult Sci. 2015;94:2805–15.
Zhu X, Hu B, Jiang Z, Wu Y, Xiong S. Speciation of chromium(III) and chromium(VI) by in situ separation and sequential determination with electrothermal vaporization inductively coupled plasma atomic emission spectrometry. Anal Chim Acta. 2002;471:121–6.
Martínez-Gallegos S, Bulbulian S. Neutron activation analysis for chromium(III) and (VI) in lixiviated liquid through a calcined chromium(VI) adsorbed hydrotalcite. J Radioanal Nucl Chem. 2005;266:285–7.
Elmosallamy MAF, Fathy AM, Ghoneim AK. Lead(II) potentiometric sensor based on 1,4,8,11-tetrathiacyclotetradecane neutral carrier and lipophilic additives. Electroanalysis. 2008;20:1241–5.
Kumar P, Shim Y-B. A novel cobalt(II)-selective potentiometric sensor based on p-(4-n-butylphenylazo)calix[4]arene. Talanta. 2009;77:1057–62.
Isildak Ö, Özbek O. Application of potentiometric sensors in real samples. Crit Rev Anal Chem. 2020. https://doi.org/10.1080/10408347.2019.1711013.
Isildak Ö, Deligönül N, Özbek O. A novel silver(I)-selective PVC membrane sensor and its potentiometric applications. Turk J Chem. 2019;43:1149–58.
Jiang C, Yao Y, Cai Y, Ping J. All-solid-state potentiometric sensor using single-walled carbon nanohorns as transducer. Sens Actuators B. 2019;283:284–9.
Özbek O, Isildak Ö, Berkel C. The use of porphyrins in potentiometric sensors as ionophores. J Incl Phenom Macrocycl Chem. 2020. https://doi.org/10.1007/s10847-020-01004-y.
Khairul WM, Abu Hasan MF, Daud AI, Zuki HM, Kubulat KH, Abdulkadir M. Theoretical and experimental investigation of pyridyl thiourea derivatives as ionophores for Cu(II) ion detection. Malays J Anal Sci. 2016;20:73–84.
Isildak Ö, Özbek O, Gürdere MB, Çetin A. Development of PVC membrane potentiometric sensor for the determination of potassium ion and its applications. Pamukkale Univ Muh Bilim Derg. 2020. https://doi.org/10.5505/pajes.2020.27982.
Gürdere MB, Özbek O, Ceylan M. Aluminum chloride–catalyzed C-alkylation of pyrrole and indole with chalcone and bis-chalcone derivatives. Synt Commun. 2016;46:322–31.
Ansari A, Ali A, Asif M. Review: biologically active pyrazole derivatives. New J Chem. 2017;41:16–411.
Gürdere MB, Kamo E, Budak Y, Sahin Yağlıoğlu A, Ceylan M. Synthesis and anticancer and cytotoxic effects of novel 1,4-phenylene-bis-N-thiocarbamoylpyrazole and 1,4-phenylene-bis-pyrazolylthiazole derivatives. Turk J Chem. 2017;41:179–89.
Isildak Ö, Özbek O, Yigit KM. Zinc(II)-selective PVC membrane potentiometric sensor for analysis of Zn2+ in drug sample and different environmental samples. Int J Environ Anal Chem. 2019. https://doi.org/10.1080/03067319.2019.1691542.
Isildak Ö, Özbek O. Silver(I)-selective PVC membrane potentiometric sensor based on 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine and potentiometric applications. J Chem Sci. 2020;132:29.
IUPAC. Recommendations for nomenclature of ion-selective electrodes. Pure Appl Chem. 1976;48:127–32.
Faridbod F, Ganjali MR, Dinarvand R, Norouzi P. The fabrication of potentiometric membrane sensors and their applications. Afr J Biotechnol. 2007;6:2960–87.
Ganjali MR, Mizani F, Salavati-Niasari M, Javanbakht M. Novel potentiometric membrane sensor for the determination of trace amounts of chromium(III) ions. Anal Sci. 2003;19:235–8.
Abbaspour A, Izadyar A. Chromium(III) ion-selective electrode based on 4-dimethylaminoazobenzene. Talanta. 2001;53:1009–133.
Gholivand MB, Sharifpour F. Chromium(III) ion selective electrode based on glyoxal bis(2-hydroxyanil). Talanta. 2003;60:707–13.
Kumar P, Sharma HK, Shalaan KG. Development of chromium(III) selective potentiometric sensor by using synthesized triazole derivative as an ionophore. J Chem. 2013;12:1–6.
Ma YH, Yuan R, Chai YQ, Liu XL. Potentiometric membrane electrode for Cr(III) ion based on a new aryl amide bifunctional bridging ligand as a neutral carrier. J Chin Chem Soc. 2009;56:676–82.
Sharma RK, Goel A. Development of a Cr(III)-specific potentiometric sensor using Aurin tricarboxylic acid modified silica. Anal Chim Acta. 2015;534:137–42.
Youssef AFA, Issa YM, Mohamed MS. Carbon paste electrode modified with chromium thiopental for the potentiometric flow injection analysis of chromium (III). Toxicol Environ Chem. 2012;94:220–38.
Heidari Z, Masrournia M. A novel modified carbon paste electrode for the determination of chromium(III) in water. J Anal Chem. 2018;73:824–31.
Abu-Shawish HM, Saadeh SM, Hartani K, Dalloula HM. A comparative study of chromium(III) ion-selective electrodes based on N,N-Bis(salicylidene)-o-phenylenediaminatechromium(III). J Iran Chem Soc. 2009;6:729–37.
Acknowledgements
The authors would like to thank Research Assistant Caglar Berkel (Tokat Gaziosmanpasa University, Department of Molecular Biology and Genetics) and MSc student Alper Cetin for their contributions.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Isildak, Ö., Özbek, O. & Gürdere, M.B. Development of Chromium(III)-selective Potentiometric Sensor by Using Synthesized Pyrazole Derivative as an Ionophore in PVC Matrix and its Applications. J. Anal. Test. 4, 273–280 (2020). https://doi.org/10.1007/s41664-020-00147-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-020-00147-8