Abstract
Adsorptive Stripping Voltammetric method for Nimesulide (NIM) determination was developed. As a working electrode, glassy carbon electrode (GCE) modified with carbon black and Nafion (CB-Nafion GCE) was used. All measurements were carried out in 0.1 M acetate buffer (pH 4.6). Conducted experiments allowed to optimize differential pulse voltammetry (DPV) instrumental parameters: sampling and waiting time ts = tw = 10 ms, step potential Es = 4 mV, and pulse amplitude ΔE = 50 mV. The best results were obtained for preconcentration potential and time equal to 400 mV and 20 s, respectively. Limit of detection was calculated and was equal to 0.14 µM for 20-s preconcentration time and 0.06 µM for 40-s preconcentration time. In order to prove the applicability of the developed method, concentration of nimesulide in pharmaceutical products was determined. Calculated recoveries were in the range 94–99%, which indicates that the method might be assumed as accurate. Coefficient of variation was equal to 5.0% (n = 7, NIM concentration 1 µM) Obtained results of NIM determination were in good agreement with the content declared by producers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a group of substances which are inhibitors of cyclooxygenase (COX). NSAIDs are chemically unrelated and might be divided into a few groups: salicylates, phenylacetic acids, indoles, oxicams, pyrazoles, propionic acid derivatives, and sulfonanilides. Despite differences in a structure, all NSAIDs exhibit similar therapeutic effects such as anti-inflammatory, analgesic (dental pain, menstrual pain, migraine), and antipyretic. One of the non-steroidal anti-inflammatory drugs commonly prescribed for painful conditions is nimesulide. It belongs to the group of sulfonanilides [1,2,3]. In the literature, a few methods for nimesulide determination might be found. The most common are high-performance liquid chromatography (HPLC) [4, 5], liquid chromatography with UV detection (LC-UV) [6], liquid chromatography coupled with mass spectrometry (LC–MS) [7, 8], and spectrophotometry [9,10,11]. Those methods are often complicated, require expensive equipment, and generate toxic chemical wastes. Therefore, a good alternative is voltammetry, which belongs to the electrochemical group of methods. In the literature, examples of nimesulide determination using voltammetry might be found [12,13,14,15]. Mentioned method is relatively inexpensive, generates little waste, and the process of sample preparation is easy and fast. In addition, voltammetry might be characterized by low detection limits, good accuracy, and low interferences. The most important part of each voltammetric system is the working electrode (WE). In the literature, a description of different construction solutions used for organic [16] and inorganic [17] substances determination might be found. The first type of electrodes are mercury-based electrodes [18, 19]. It is the oldest group that has evolved over the years and was very popular for a long time. Nowadays, due to mercury toxicity, are less used. The next type of working electrode are film electrodes which might be, for example, based on bismuth, mercury, and antimony [20, 21]. Glassy carbon electrode (GCE) and carbon paste electrodes (CPE) are the next group of WE. Their main advantage is the possibility of their modification with a wide group of materials. It allows to significantly improve their sensitivity and lower a limit of detection. In recent years, different groups of material have been used as modifiers of GCE and CPE; among them were, for example, carbon nanomaterials (carbon black [22], carbon nanotubes [23], graphene [24]), metals (Au [25], Ni [26]), metal oxides (CeO2 [27], Fe2O3 [28]), or conductive polymers (PEDOT [29], polyaniline [30]). Hybrid materials consisted of 2 or more different substances have become also popular. Such approach allows to combine properties of all used materials (e.g., high surface area, good electrical conductivity, electrocatalytic properties) which might lead to improvement of analytical performance of the sensor.
The aim of this work was to develop highly sensitive voltammetric method for nimesulide determination. As a working electrode, GCE modified with carbon black and Nafion was used. GCE might be characterized by good chemical and mechanical properties; therefore, it is a useful tool in chemical analysis. Carbon black and Nafion were used to improve sensitivity of GCE. The developed procedure was optimized, and interference study was carried out. The usefulness of the voltammetric method was tested by analysis of commercially available pharmaceutical products containing nimesulide.
Experimental
Apparatus
All voltammetric measurements were carried out using multipurpose electrochemical analyzer M161 and electrode stand M164 (mtm – anko, Poland). Typical three-electrode system was used: a working electrode (glassy carbon electrode modified with carbon black and Nafion CB-Nafion-GC), reference electrode (Ag/AgCl/3 M KCl), and auxiliary electrode (platinum wire). The next part of the measuring system was a quartz cell (total volume 20 mL), where solution was stirred using a magnetic bar (~ 500 rpm).
Chemical and Glassware
All chemicals used in voltammetric measurements were of analytical grade. Acetate buffer solution (pH 4.6) was made from glacial acetic acid (Suprapur, Merck) and anhydrous sodium acetate (Sigma-Aldrich) mixed in an appropriate proportion. Nimesulide stock solution was prepared by dissolving of nimesulide reference material (Sigma-Aldrich) in 96% ethanol (POCH, Poland). The remaining chemicals were purchased as follow: carbon black—3D nano, Poland; Nafion 5 wt. % solution in a mixture of lower aliphatic alcohols and water—Sigma-Aldrich; and aluminum oxide 0.3 µm—Buehler Micropolish II. During voltammetric measurements and for preparation of all chemicals, double-distilled water was used. Borosilicate glassware used in the experiments was cleaned with 5% HNO3 solution and rinsed with double-distilled water prior to use.
Sample Preparation
Samples used for nimesulide determination were in a form of powder (Nimesil) and tablets (Aulin). In the case of Aulin 3 tablets were weighed, crushed in mortar to obtain powder, and homogenized. The next steps of the preparation were the same in the case of both samples. The appropriate amount of powder was at first dissolved in ethanol and filtrated. Obtained filtrate was transferred into a volumetric flask (volume 25 mL) and filled up to the mark with ethanol.
Working Electrode Preparation
Working electrode was used in developed voltammetric procedure based on glassy carbon electrode (GCE). To obtain a highly sensitive sensor, GCE was modified with carbon nanomaterial—carbon black and fluoropolymer—Nafion. In the first step the surface modifier was prepared following the procedure (optimized proportions):
-
a.
5 mg of carbon black was transferred into the volumetric flask (volume 5 mL)
-
b.
Addition of 100 µL of 5% Nafion
-
c.
Filling up to the mark with ethanol (96%)
-
d.
Mixture sonication—30 min
In the next step, the surface of GCE was prepared in order to remove contamination and to activate the surface and then the modifier was applied:
-
a.
Polishing GCE surface using aluminum oxide (0.3 µm) suspension and polishing cloth
-
b.
Rinsing of GCE in a stream of double-distilled water (to remove the remaining aluminum oxide suspension)
-
c.
Sonication in methanol—5 min (the second step of surface cleaning)
-
d.
Drying of the electrode
-
e.
Sonication of the modifier (directly before use)—5 min
-
f.
Application of 10 µL of the modifier on the surface of GCE using a drop casting method
-
g.
Drying (room temperature)—approximately 15 min
Prepared CB-Nafion Modified Electrode is Ready to Use.
Measurement Procedure
Voltammetric nimesulide determination was conducted using DPV technique. During the DPV measurements, the instrumental parameters were as follows: sampling and waiting time: ts = tw = 10 ms, pulse time: tp = 20 ms, step potential: Es = 4 mV, and pulse amplitude: ΔE = 50 mV. The registration potential range was 400–1350 mV. Supporting electrolyte was consisted of 0.1 M acetate buffer (pH 4.6) and total volume 10 mL. Each DPV measurement was conducted as follows:
-
a.
Electrode’s surface cleaning: Ec = 1360 mV, tc = 5 s
-
b.
Preconcentration step: Eacc = 400 mV, tacc = 20 s
-
c.
Rest period: t = 3 s
-
d.
Polarization and registration of the voltammograms: potential range 400–1350 mV.
Results
Behavior of Nimesulide on CB-Nafion GCE
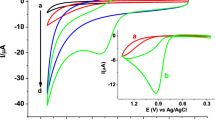
Voltammetric behavior of nimesulide on the surface of bare GCE, GCE modified with carbon black, GCE modified with carbon black and Nafion, and GCE modified with graphene and platinum are presented in Fig. 1. Measurement carried out on the surface of bare GCE clearly shows that there is practically no signal from nimesulide. Only modified electrodes were allowed to obtain signal from the analyte. Peak current and potential measured on the surface of each modified electrode were as follow: GCE modified with carbon black and Nafion 1032 mV and 3.95 µA, GCE modified with carbon black: 1038 mV and 0.83 µA, and GCE modified with graphene and platinum 1060 mV and 0.31 µA. As it can be seen the highest nimesulide peak current and the lowest potential (best catalytic properties) were registered using GCE modified with carbon black and Nafion; therefore, this electrode was used in further measurements.
Comparison of DPV voltammograms registered on bare GC (1), GC modified with graphene and platinum (2), GC modified with carbon black (3), and GC modified with carbon black and Nafion (4). All measurements were carried out in 0.1 M acetate buffer (pH 4.6); preconcentration potential and time were equal to 400 mV and 20 s, respectively; and NIM concentration was 10 µM
To investigate the mechanism of the electrode reaction, the linear sweep voltammetric (LSV) measurement was carried out. Voltammograms were registered in the potential range from 600 to 1300 mV for different scan rates from the range 12.5 mV s−1–250 mV s−1 (Fig. 2A). NIM concentration during the LSV measurement was equal to 0.5 mM. In the next step the dependence of nimesulide peak current on the square root of the scan rate was plotted (Fig. 2B). The linearity was obtained (Eq. 1) that suggests the diffusion character of the electrode process.
In the next step the number of electrons taking part in the electrode reaction was calculated. For this purpose a criterion described by Eq. 2 was used. Value of Ep (peak potential) and Ep/2 (potential where the current is at half peak value) were taken from LSV measurement (for scan rate 100 mV s−1: Ep = 1108 mV and Ep/2 = 1063 mV). Value of transfer coefficient α was assumed to be 0.5. Calculations revealed that number of electrons taking part in the nimesulide oxidation was equal to 2.
In order to obtain more data concerning electrode’s reaction, the dependence between nimesulide peak potential and pH of the supporting electrolyte was determined. In the experiment, signal from 10 µM of nimesulide was measured in electrolytes characterized by different pH from the range 4.0–6.5, which was presented in Fig. 3. It might be observed that the higher the pH value is, the lower the peak potential is, which describes Eq. 3. The slope of the Eq. 3 is equal to 55.5 mV pH−1, which is close to theoretical value ~ 59 mV pH−1 (Eq. 4). That indicates that 1 proton per 1 electron take part in the electrode reaction. Based on the obtained results a probable mechanism of NIM reaction on the surface of CB-Nafion GCE was proposed and presented in Fig. 4.
Supporting Electrolyte
The choice of the supporting electrolyte in voltammetric method is an important part of the research. It determines potential range in which measurement might be done but also has an influence on peak potential, shape, and current. In the experiment, 5 different electrolytes were tested: 0.1 M monopotassium phosphate, 0.01 M potassium chloride, 0.1 M potassium nitrate, 0.1 M acetate buffer (pH 4.6), and 0.1 M borate buffer (pH 9.1). Experiment was conducted for 10 µM of nimesulide; the preconcentration potential and time were equal 400 mV and 20 s, respectively. Results of the experiment were gathered in Table 1. Well-shaped peak from nimesulide appeared in almost all tested electrolytes (despite borate buffer). The highest peak current was obtained in 0.1 M acetate buffer (pH 4.6); therefore, further measurements were conducted in this environment.
Instrumental Parameters
To improve the sensitivity of a DPV measurement and to obtain well-shaped and high peak from the measured analyte, instrumental parameter optimization is necessary. During the experiment, the following parameters were optimized: sampling time tp, waiting time tw, step potential Es, and pulse amplitude ΔE. When certain parameter was tested, values of others were fixed. NIM concentration during all described experiments was equal to 10 µM. The influence of waiting and sampling time was tested in the range of values: 10–80 ms. In case of both parameters analogous dependence was obtained; the lower the value of the time is, the higher the register NIM signal is. Therefore, for both tp and tw, the lowest tested value (10 ms) was chosen as optimal. Measurements for step potential were carried out for values in the range 1–7 mV. The higher the Es is, the higher the peak current is. Nevertheless, when the value of parameter was changed, a shift in peak potential was observed. Considering the mentioned fact and the shape of the registered signals it was assumed that Es equal to 4 mV is optimal—for this value the peak was well-shaped and the current was relatively high. The influence of pulse amplitude was tested for values in the range 5–75 mV (positive and negative mode). The obtained dependence has shown that the higher the absolute value of ΔE is, the higher the register signal is. The highest peak current was obtained for 75 mV; nevertheless, for this value background was relatively high. Therefore, a pulse amplitude equal to 50 mV was chosen as optimal. The best conditions for nimesulide DPV measurements were as follows: tp = tw = 10 ms, Es = 4 mV, and ΔE = 50 mV.
Preconcentration Potential and Time
Preconcentration Potential
Preconcentration potential is a parameter optimized in stripping techniques where the preconcentration step is used. In the experiment NIM signal was registered for different preconcentration potentials in the range 0–850 mV. Measurements were carried out in 0.1 M acetate buffer (pH 4.6) for 10 µM of NIM. The results of the experiment were presented in Fig. 5. As it might be observed, the preconcentration potential had practically no influence on NIM peak current value. In the tested range of potentials, peak current was approximately equal to 3.80 ± 0.05 µA. In further measurements preconcentration potential equal to 400 mV was used.
Preconcentration Time
Preconcentration time not only has influence on sensitivity of the measurement but also determines the usability of the method. Shorter times are more practical and allow to perform quick measurements. Therefore, the experiment in which the influence of preconcentration time is in the of range 0–120 s on NIM signal was carried out. Measurements were performed in 0.1 M acetate buffer (pH 4.6) for 10 µM of nimesulide, the preconcentration potential was equal to 400 mV. The obtained dependence was presented in Fig. 6. As it can be seen the lowest peak current was registered when the preconcentration step was not introduced (time equal to 0 s). For 5 s and more, NIM signal was clearly higher. After exceeding 20 s the curve began to flatten; therefore, this value was chosen as optimal (the peak current was relatively high (3.90 µA) and the time was quite short which allows to perform a quick measurement).
Interferences
Interference study helps to determine which potential components of sample’s matrix might have an influence on register signal. Therefore, in the experiment, influence of different substances on NIM signal (10 µM) was tested. All measurements were conducted in 0.1 M acetate buffer (pH 4.6), and preconcentration potential and time were equal to 400 mV and 20 s, respectively. Among the tested substances were Pb(II), Cd(II), Cu(II), Mo(II), Mn(II), Zn(II) (10 µM added), Ca(II), Mg(II) (1 mM added), citric acid, ascorbic acid, glucose (100 µM added), and ibuprofen (20 µM added). The presence of 10 µM of Pb(II) and Mn(II) caused a decrease of the signal by 10% and 25%, respectively. Higher concentration of Ca(II) also had the influence of NIM signal—1 mM of Ca(II) caused a 20% decrease of the peak current. The last interfering substance was glucose which in the concentration of 100 µM caused 10% decrease of the signal. It is worth to mention that quantities of substances which were interfering were relatively high during the experiment so their presence in real samples should not be problematic during measurements.
Analytical Performance
”To investigate the analytical performance of the developed method for nimesulide determination, calibration was conducted (Fig. 7). Measurements were carried out in 0.1 M acetate buffer (pH 4.6), preconcentration potential was 400 mV, and the preconcentration time was equal to 20 s and then 40 s. First calibration for shorter preconcentration time was conducted for NIM concentration in the range 0.25–1.75 µM. Parameters of regression line were as follows: slope 0.35 ± 0.01 µA µM−1, intercept 0.11 ± 0.01 µA, and r = 0.997 (Fig. 6A). Limit of detection (LOD) calculated for this calibration was equal to 0.14 ± 0.06 µM. The second calibration conducted for a longer preconcentration time (40 s) might be characterized by the following regression line parameters: slope 0.669 ± 0.013 µA µM−1, intercept 0.103 ± 0.007 µA, and r = 0.999 (Fig. 7A). Calculated LOD was equal to 0.06 ± 0.03 µM. In Table 2, comparison of nimesulide LOD obtained using different analytical methods was presented. To verify the usefulness of developed voltammetric method for nimesulide determination, analyte concentration in commercially available pharmaceutical products was determined. Measurements were conducted in accordance with procedure described in the “Measurement Procedure” section; samples were prepared as it was described in the “Sample Preparation” section. Obtained results (Table 3) were in good agreement with the nimesulide content declared by producers. Based on obtained results, the recoveries were calculated and were in the range 94–99%, which indicates that the developed DPV method is accurate.
Conclusions
Adsorptive Stripping Voltammetric method for nimesulide determination was developed. As a working electrode, glassy carbon electrode modified with carbon black and Nafion was used. During the research, instrumental parameters were optimized as well as preconcentration potential and time. The best results were obtained in 0.1 M acetate buffer (pH 4.6). Interferences studies revealed an impact of high concentration of Pb(II), Mn(II), Ca(II), and glucose on NIM signal. Despite the fact that their presence in sample reduces the NIM signal, the measurement still can be proceed correctly. Conducted calibrations allowed to calculate a limit of detection which was equal to 0.06 µM (40-s preconcentration time). The applicability of the method was proved by analysis of pharmaceutical products. Obtained results were in good agreement with declarations of producers. Recoveries calculated based on the conducted measurements were in the range 94–99%, which means that developed method for nimesulide determination might be assumed as accurate. Considering the presented arguments, it might be concluded that Adsorptive Stripping Voltammetric method for nimesulide determination might be a useful tool in routine laboratory practice.
References
C. Carbone, T. Musumeci, R. Pignatello, Non-steroidal anti-inflammatory drugs, in Drug-Biomembrane Interaction Studies: The Application of Calorimetric Techniques. (Woodhead Publishing Limited, Catania, Italy, 2013), pp. 281–303
I. Kyrikou, S.K. Hadjikakou, D. Kovala-Demertzi et al., Effects of non-steroid anti-inflammatory drugs in membrane bilayers. Chem Phys Lipids 132, 157–169 (2004). https://doi.org/10.1016/j.chemphyslip.2004.06.005
C. Pereira-Leite, C. Nunes, S. Reis, Interaction of nonsteroidal anti-inflammatory drugs with membranes: in vitro assessment and relevance for their biological actions. Prog Lipid Res 52, 571–584 (2013). https://doi.org/10.1016/j.plipres.2013.08.003
G. Khaksa, N. Udupa, Rapid and sensitive method for determination of nimesulide in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 727, 241–244 (1999). https://doi.org/10.1016/S0378-4347(98)00567-2
V.B. Patravale, S. D’Souza, Y. Narkar, HPTLC determination of nimesulide from pharmaceutical dosage forms. J Pharm Biomed Anal 25, 685–688 (2001). https://doi.org/10.1016/S0731-7085(00)00597-5
S. Chandran, P. Ravi, P.R. Jadhav, R.N. Saha, A simple, rapid, and validated LC method for the estimation of nimesulide in human serum and its application in bioavailability studies. Anal Lett 41, 2437–2451 (2008). https://doi.org/10.1080/00032710802352480
X. Sun, K.L. Xue, X.Y. Jiao et al., Simultaneous determination of nimesulide and its four possible metabolites in human plasma by LC-MS/MS and its application in a study of pharmacokinetics. J Chromatogr B Anal Technol Biomed Life Sci 1027, 139–148 (2016). https://doi.org/10.1016/j.jchromb.2016.05.008
R.E. Barrientos-Astigarraga, Y.B. Vannuchi, M. Sucupira et al., Quantification of nimesulide in human plasma by high-performance liquid chromatography/tandem mass spectrometry. Application to bioequivalence studies. J Mass Spectrom 36, 1281–1286 (2001). https://doi.org/10.1002/jms.232
K. Upadhyay, A. Asthana, N. Tiwari, S.B. Mathew, Determination of nimesulide in pharmaceutical and biological samples by a spectrophotometric method assisted with the partial least square method. Res Chem Intermed 39, 3553–3563 (2013). https://doi.org/10.1007/s11164-012-0862-9
S. Altinöz, Ö.Ö. Dursun, Determination of nimesulide in pharmaceutical dosage forms by second order derivative UV spectrophotometry. J Pharm Biomed Anal 22, 175–182 (2000). https://doi.org/10.1016/S0731-7085(99)00264-2
S. Chandran, S. Saggar, K.P. Priya, R.N. Saha, New ultraviolet spectrophotometric method for the estimation of nimesulide. Drug Dev Ind Pharm 26, 229–234 (2000). https://doi.org/10.1081/DDC-100100350
P.B. Deroco, R.C. Rocha-Filho, O. Fatibello-Filho, A new and simple method for the simultaneous determination of amoxicillin and nimesulide using carbon black within a dihexadecylphosphate film as electrochemical sensor. Talanta 179, 115–123 (2018). https://doi.org/10.1016/j.talanta.2017.10.048
A. Wong, A.M. Santos, O. Fatibello-Filho, Determination of piroxicam and nimesulide using an electrochemical sensor based on reduced graphene oxide and PEDOT:PSS. J Electroanal Chem 799, 547–555 (2017). https://doi.org/10.1016/j.jelechem.2017.06.055
C. Wang, X. Shao, Q. Liu et al., Differential pulse voltammetric determination of nimesulide in pharmaceutical formulation and human serum at glassy carbon electrode modified by cysteic acid/CNTs based on electrochemical oxidation of l-cysteine. J Pharm Biomed Anal 42, 237–244 (2006). https://doi.org/10.1016/j.jpba.2006.03.038
N.P. Shetti, S.J. Malode, S.D. Bukkitgar et al., Electro-oxidation and determination of nimesulide at nanosilica modified sensor. Mater Sci Energy Technol 2, 396–400 (2019). https://doi.org/10.1016/j.mset.2019.03.005
M. Brycht, S. Skrzypek, A. Nosal-Wiercińska et al., The new application of renewable silver amalgam film electrode for the electrochemical reduction of nitrile, cyazofamid, and its voltammetric determination in the real samples and in a commercial formulation. Electrochim Acta 134, 302–308 (2014). https://doi.org/10.1016/j.electacta.2014.04.143
M. Korolczuk, M. Grabarczyk, I. Rutyna, An adsorptive stripping voltammetry procedure for ultra-trace determination of U (VI) using double accumulation step on two lead-film working electrodes. Talanta 130, 342–346 (2014). https://doi.org/10.1016/j.talanta.2014.07.016
L.B.O. Santos, G. Abate, J.C. Masini, Determination of atrazine using square wave voltammetry with the Hanging Mercury Drop Electrode (HMDE). Talanta 62, 667–674 (2004). https://doi.org/10.1016/j.talanta.2003.08.034
S. Abbasi, K. Khodarahmiyan, F. Abbasi, Simultaneous determination of ultra trace amounts of lead and cadmium in food samples by adsorptive stripping voltammetry. Food Chem 128, 254–257 (2011). https://doi.org/10.1016/j.foodchem.2011.02.067
M. Grabarczyk, J. Wasąg, Determination of trace amounts of Ga (III) by adsorptive stripping voltammetry with in situ plated bismuth film electrode. Talanta 144, 1091–1095 (2015). https://doi.org/10.1016/j.talanta.2015.07.083
S. Skrzypek, S. Smarzewska, W. Ciesielski, Determination of blasticidin S in spiked rice using SW voltammetry with a renewable silver amalgam film electrode. Electroanalysis 24, 1153–1159 (2012). https://doi.org/10.1002/elan.201100715
J. Smajdor, R. Piech, M. Ławrywianiec, B. Paczosa-Bator, Glassy carbon electrode modified with carbon black for sensitive estradiol determination by means of voltammetry and flow injection analysis with amperometric detection. Anal Biochem 544, 7–12 (2018). https://doi.org/10.1016/j.ab.2017.12.025
F. Zahirifar, M. Rahimnejad, R.A. Abdulkareem, G. Najafpour, Biocatalysis and agricultural biotechnology determination of diazinon in fruit samples using electrochemical sensor based on carbon nanotubes modified carbon paste electrode. Biocatal Agric Biotechnol 20, 101245 (2019). https://doi.org/10.1016/j.bcab.2019.101245
S.M. Oliveira, J.M. Luzardo, L.A. Silva et al., High-performance electrochemical sensor based on molecularly imprinted polypyrrole-graphene modified glassy carbon electrode. Thin Solid Films 699, 137875 (2020). https://doi.org/10.1016/j.tsf.2020.137875
Y. Wei, A. Wang, Y. Liu, Development of a glassy carbon electrode modified with graphene/Au nanoparticles for determination of acetaminophen in pharmaceutical preparation. Russ J Electrochem 54, 1141–1147 (2018). https://doi.org/10.1134/S1023193518140112
A. Maringa, T. Mugadza, E. Antunes, T. Nyokong, Characterization and electrocatalytic behaviour of glassy carbon electrode modified with nickel nanoparticles towards amitrole detection. J Electroanal Chem 700, 86–92 (2013). https://doi.org/10.1016/j.jelechem.2013.04.022
S. Ansari, M. Shahnawaze Ansari, N. Dev, S.P. Satsangee, CeO2 nanoparticles based electrochemical sensor for an anti-anginal drug. Mater Today Proc 18, 1210–1219 (2019). https://doi.org/10.1016/j.matpr.2019.06.583
H. Wang, P. Chen, F. Wen et al., Flower-like Fe2O3@MoS2 nanocomposite decorated glassy carbon electrode for the determination of nitrite. Sensors Actuators, B Chem 220, 749–754 (2015). https://doi.org/10.1016/j.snb.2015.06.016
J. Smajdor, B. Paczosa-Bator, R. Piech, Voltammetric electrode based on Nafion and poly(2,3–dihydrothieno–1,4–dioxin)–poly(styrenesulfonate) film for fast and high sensitive determination of metamizole. J Electrochem Soc 163, B146–B152 (2016). https://doi.org/10.1149/2.0611605jes
S.K. Ponnaiah, P. Periakaruppan, A glassy carbon electrode modified with a copper tungstate and polyaniline nanocomposite for voltammetric determination of quercetin Microchim Acta 185 (2018). https://doi.org/10.1007/s00604-018-3071-4
S.D. Bukkitgar, N.P. Shetti, R.M. Kulkarni, M.R. Doddamani, Electro-oxidation of nimesulide at 5% barium-doped zinc oxide nanoparticle modified glassy carbon electrode. J Electroanal Chem 762, 37–42 (2016). https://doi.org/10.1016/j.jelechem.2015.12.023
Acknowledgements
The publication is financed from the subsidy No. 16.16.160.557 of the Polish Ministry of Science and Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Łysoń, M., Górska, A., Paczosa-Bator, B. et al. Nimesulide Determination on Carbon Black-Nafion Modified Glassy Carbon Electrode by Means of Adsorptive Stripping Voltammetry. Electrocatalysis 12, 641–649 (2021). https://doi.org/10.1007/s12678-021-00676-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00676-5