Abstract
Background
Cluster of differentiation 24 (CD24) is a highly glycosylated glycosylphosphatidylinositol (GPI)-anchored surface protein, expressed in various tumor cells, as a "don't eat me" signaling molecule in tumor immune. This study aimed to investigate the potential features of CD24 in pan-cancer.
Methods
The correlations between 22 immune cells and CD24 expression were using TIMER analysis. R package “ESTIMATE” was used to predict the proportion of immune and stromal cells in pan-cancer. Spearman's correlation analysis was performed to evaluate the relationships between CD24 expression and immune checkpoints, chemokines, mismatch repair, tumor mutation burden and microsatellite instability, and qPCR and western blot were conducted to assess CD24 expression levels in liver hepatocellular carcinoma (LIHC). In addition, loss of function was performed for the biological evaluation of CD24 in LIHC.
Results
CD24 expression was positively correlated with myeloid cells, including neutrophils and myeloid-derived suppressor cells, in various tumors, such as BLCA, HNSC-HPV, HNSC, KICH, KIRC, KIRP, TGCT, THCA, THYM, and UCEC. In contrast, anti-tumor NK cells and NKT cells showed a negative association with CD24 expression in BRCA-Her2, ESCA, HNSC-HPV, KIRC, THCA, and THYM. The top three tumors with the highest correlation between CD24 and ImmuneScore were TGCT, THCA, and SKCM. Functional enrichment analysis revealed CD24 expression was negatively associated with various immune-related pathways. Immune checkpoints and chemokines also exhibited inverse correlations with CD24 in CESC, CHOL, COAD, ESCA, READ, TGCT, and THCA. Additionally, CD24 was overexpressed in most tumors, with high CD24 expression in BRCA, LIHC, and CESC correlating with poor prognosis. The TIDE database indicated tumors with high CD24 expression, particularly melanoma, were less responsive to PD1/PD-L1 immunotherapy. Finally, CD24 knockdown resulted in impaired proliferation and cell cycle progression in LIHC.
Conclusion
CD24 participates in regulation of immune infiltration, influences patient prognosis and serves as a potential tumor marker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Immune checkpoint inhibitor (ICI) therapy has demonstrated remarkable therapeutic outcomes across multiple cancer types, with neoantigens on cancer cell surfaces serving as the basis for this approach [1]. Nevertheless, cancer cells often express specific "don't eat me" signaling molecules to evade immune system clearance, such as programmed cell death ligand 1 (PD-L1) [2], CD47 [3], and major histocompatibility complex (MHC) class I component β-microglobulin (β2M) [4]. Monoclonal antibodies targeting these "don't eat me" signals have been utilized in cancer treatment [5]. For instance, Opdivo and Keytruda, which target PD-1, have been approved in China, offering hope to patients with over 15 types of cancer, including SKCM, COAD, NSCLC, and HNSC [6]. However, clinical evidence indicates that not all patients benefit from these agents, suggesting the presence of additional "don't eat me" signals.
CD24 is a GPI-anchored protein expressed on the surface of mature B cells, where it regulates their growth and differentiation [7]. Beyond immune cells, CD24 is also frequently expressed in various human cancers [8,9,10]. In cancer contexts, CD24 is an essential driver of tumorigenesis and metastasis, and serves as a pivotal biomarker for cancer stem cells [11,12,13]. CD24 is known to interact with p-selectin and sialic acid-binding insulin-like lectin 10 (siglec-10), with the CD24-p-selectin interaction facilitating tumor cell interactions with activated platelets and endothelial cells, thereby promoting tumor cell expansion, invasion, and metastasis [14]. Additionally, the CD24-siglec-10 pathway initiates an inhibitory signaling cascade through SHP-1 and/or SHP-2 phosphatases, protecting the host from excessive pathological cell death [15]. Research in 2019 identified that the CD24-siglec-10 interaction on macrophages inhibits phagocytosis. Monoclonal antibodies blocking this signaling can significantly enhance the clearance of CD24 + tumors, highlighting the potential of CD24 blockade in immunotherapy [16]. These findings emphasize the critical role of CD24 in tumor immunomodulation. However, its role across various cancers remains unclear.
In this study, we first assessed the association between CD24 and immune cells, immune scores, immune checkpoints, and chemokines. We explored CD24 expression profiles in pan-cancer contexts and examined their implications for patient prognosis. We also evaluated the potential of CD24 as a predictor for immunotherapy response. Finally, we validated CD24 expression levels and their impact on biological processes in liver hepatocellular carcinoma (LIHC). The primary objective was to investigate the relationship between CD24 expression and immune cell infiltration across different cancers and to assess its potential as an immunotherapy response predictor.
2 Methods:
2.1 Data collection and process
CD24 expression data from various cancer samples were retrieved from the TCGA database via UCSC Xena (https://xenabrowser.net/) (N = 60,499). The data were log2(x + 0.001) transformed and filtered to exclude values below 3. Fragments per kilobase million (FPKM) values were converted to transcripts per kilobase million (TPM) values for better comparability between samples. Normal tissue samples from both TCGA and GTEx (http://commonfund.nih.gov/GTEx/) (N = 10,535) databases were used for comparisons with cancer tissues. The R software was utilized to calculate expression differences between normal and tumor samples, with statistical significance determined using the unpaired Wilcoxon rank sum and signed rank tests.
2.2 Immune infiltration analysis
The TIMER 2.0 database was employed to analyze CD24 expression and immune cell infiltration, providing comprehensive immune infiltration levels for TCGA tumor samples based on six algorithms: CIBERSORT, TIMER, quanTIseq, xCell, MCP-counter, and EPIC. The ESTIMATE algorithm in R was used to analyze the ratios of immune and stromal matrix components in the tumor microenvironment (TME), which are reported as ImmuneScore, StromalScore, and ESTIMATEScore. These scores correlate positively with immune, matrix, and combined components, respectively. Spearman's correlation analysis was conducted to examine the relationship between CD24 expression and immune checkpoint markers [17], and the TISIDB database (cis.hku.hk/TISIDB/) was used to analyze CD24's association with various chemokines.
2.3 Gene set enrichment analyses
Gene set enrichment analyses were performed using ggplot2 and GSEA to compare biological signaling pathways between high and low CD24 expression groups [17]. The top five KEGG and HALLMARK terms were selected for this analysis.
2.4 Mismatch repair (MMR)
The MMR genes analyzed included MLH1, MSH2, PMS2, MSH6 and EPCAM, and Spearman's correlation analysis was used to assess the relationship between CD24 expression and these MMR-related genes [17].
2.5 Prognostic analysis of CD24 in pan-cancer
Kaplan–Meier analysis was employed to generate prognostic forest plots of CD24 across TCGA tumors, focusing on overall survival (OS), disease-free survival (DFS), disease-free interval (DFI), and progression-free interval (PFI).
2.6 Immunotherapy prediction analysis
The TIDE database (http://tide.dfci.harvard.edu) was used to identify biomarkers predictive of ICI efficacy by analyzing expression profiles across various tumor types. TIDE can be used to assess immune cold tumors and identify cells that inhibit T cell infiltration, which often leads to reduced benefits from ICI monotherapy. TIDE predictions are considered superior to existing biomarkers in estimating patient survival.
2.7 Cell lines and cell culture
Human HCC cell lines (L02, HCCLM3, Huh7, HepG2, and Hep3B) were obtained from the Chinese Academy of Sciences (Shanghai, China). Each cell line was authenticated by short tandem repeat analysis and cultured for fewer than six months post-testing. Cells were maintained in RPMI 1640 medium (Thermo, NY, USA) supplemented with 10% fetal bovine serum (Gibco, CA, USA) and incubated at 37 °C in a 5% CO2 environment. Small interfering RNAs (siRNAs) targeting CD24, obtained from Ribobio (Guangzhou, China), included CD24-siRNA-1 (5'-CTTCTGCATCTCTACTCTTAA-3') and CD24-siRNA-2 (5'-TGCTCCTACCCACGCAGATTT-3'). These siRNAs were transfected using lipoFiter 3.0 (Hanbio, Shanghai, China) according to the manufacturer's instructions. The siRNA used for mouse tumorigenesis was modified with 2'-O-Methyl (2'-OMe) (GenePharma, Shanghai, China).
2.8 Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed to measure CD24 mRNA expression in LIHC cells and evaluate knockdown efficiency. Relative quantification of mRNA was achieved using the ΔΔCt method, with GAPDH as the reference. Primers used were CD24 forward (5'-GCTCCTACCCACGCAGATTTA-3') and reverse (5'-GAGACCACGAAGAGACTGGC-3'), and GAPDH forward (5'-GGAAATCCCATCACCATCTTC-3') and reverse (5'-TGGACTCCACGACGTACTCAG-3').
2.9 Western blotting analysis
Total proteins from LIHC cells were extracted using protein lysates, and the protein concentration was measured using a BCA kit (Beyotime, P0010). The proteins were separated by 10% SDS-PAGE and transferred to PVDF membranes, following which the membranes were blocked with 5% bovine serum albumin in tris-buffered saline with Tween 20 for 2 h at room temperature, then incubated overnight with primary antibodies: anti-CD24 (1:1000, Novus, USA) and anti-tubulin (1:5000, M2005L; Abmart, Shanghai, China). Next, the membranes were probed with a secondary antibody (goat anti-rabbit IgG horseradish peroxidase conjugate, 1:5000, SA00001-2; Proteintech, Wuhan, China) for 2 h at room temperature. Protein bands were visualized using an ECL chemiluminescence reagent (BL520A; Biosharp, Beijing, China) and captured with a Chemiluminescent Imaging System (Tanon 4600 SF, NJ, China). The bands were quantified using Gel-Pro Analyzer version 4.0.00.001 (MediaCyberneticsLP, China).
2.10 Cell counting kit-8 (CCK-8) assay
Cell proliferation was assessed using the CCK-8 assay (Dojindo, Kumamoto, Japan). Briefly, the cells (2.5 × 103) were seeded in triplicate in 96-well plates and treated with CCK-8 solution (10 μL) at 24, 48, 72, and 96 h post-transfection, and absorbance was measured at 450 nm using a microplate reader (Thermo, Waltham, MA, USA).
2.11 Colony formation
To evaluate the impact of CD24 on long-term proliferation, 2 × 103 control or CD24-knockdown Huh7 and HepG2 cells were seeded in six-well plates. The medium was replaced every four days to maintain optimal cell growth. Cells were cultured for 15 days, after which colonies were washed with PBS, fixed in 4% paraformaldehyde for 20 min, and stained with 0.1% crystal violet for 30 min. Lastly, the colonies were photographed and counted.
2.12 Cell cycle and flow cytometry analysis
The distribution of cells across the cell cycle phases, including the G0/G1, S, and G2/M phases, was assessed using a staining kit (C1052; Beyotime). After reaching confluence, cells were adjusted to a concentration of 1 × 105 cells/mL, then seeded into a six-well culture plate with 3 mL of culture medium per well, and incubated at 37 °C with 5% CO2 for 24 h. Following transfection with siRNA or siRNA-negative control, cells were cultured for an additional 48 h. Then, 1 × 106 cells were harvested, washed once with phosphate-buffered saline, resuspended to obtain a single-cell suspension, and fixed and stained according to the manufacturer's instructions. The stained samples were analyzed by flow cytometry (BD-FACSVerse; Becton Dickinson, San Jose, CA), and the data were processed using CellQuest software version 5.1 (BD Biosciences, San Jose, CA).
2.13 Statistical analysis
Student's t-test was used to analyze relative expression between low and high CD24 expression groups. Spearman's correlation analysis was employed to evaluate two-variable correlations. These analyses were performed using R statistical software (version 4.0.3), supplemented with R packages, including ggplot2, clusterProfiler, GSVA, and enrichPlot. A significance level of P < 0.05 was considered statistically significant.
3 Results
3.1 Immune infiltration analysis
CIBERSORT, TIMER, quanTIseq, xCell and MCP-counter are widely used methods for estimating immune infiltration. We applied these algorithms to examine the relationship between CD24 expression and immune cell infiltration subtypes across various tumors in the TCGA database. The analysis revealed a strong positive association between CD24 and Neutrophils and Myeloid-derived suppressor cells (MDSC) in multiple tumors, including BLCA, HNSC-HPV, HNSC, KICH, KIRC, KIRP, TGCT, THCA, THYM, and UCEC (Fig. 1A, B; P < 0.001). Conversely, CD24 was negatively associated with NK cells and NKT cells in BRCA-Her2, ESCA, HNSC-HPV, KIRC, THCA, and THYM (Fig. 1C, D; P < 0.001). Furthermore, we investigated the proportions of immune and stromal cells across different tumor types using the R package "ESTIMATE." CD24 expression positively correlated with StromalScore in MESO, TGCT, and THYM. However, CD24 expression was negatively associated with ImmuneScore in BLCA, CESC, CHOL, COAD, GBM, KIRC, KICH, KIRP, LUAD, PCPG, READ, SARC, TGCT, and THCA. Similarly, CD24 expression was negatively related to ESTIMATEScore, the sum of ImmuneScore and StromalScore, in most cancer types (Fig. 2A, B, C). The top three tumors most significantly associated with CD24 expression were THCA, SKCM, and LGG (StromalScore); TGCT, THCA, and SKCM (ImmuneScore); and THCA, SKCM, and BLCA (ESTIMATE score) (Fig. 2D). These findings suggest that CD24 may contribute to tumor immune escape by creating an immunosuppressive microenvironment across various cancers.
3.2 Potential pathways of CD24 in pan-cancer
To further elucidate the pathways involved in CD24-mediated tumor immunosuppression, we conducted differential expression gene (DEG) analysis comparing high and low CD24 expression groups, followed by functional enrichment analysis of these differential genes. Our results showed that immune-related pathways, including TNFA-signaling-via-NFKB, IFN-α response, IFN-γ response, inflammatory response, IL6-JAK-STAT3 signaling, and IL2-STAT5 signaling, were negatively correlated with CD24 expression in a variety of tumors, particularly BLCA, CESC, CHOL, COAD, GBM, KIRP, OV, and THCA (Fig. 3A). Gene set enrichment analysis (GSEA) further assessed the functional enrichment of DEGs. KEGG enrichment analysis indicated that high CD24 expression was positively associated with GPI-anchor biosynthesis, peroxisome, and Vibrio cholerae infection while negatively associated with intestinal immune network for IgA production, graft versus host disease, allograft rejection, and ribosome. HALLMARK terms revealed that high CD24 expression was positively related to spermatogenesis, peroxisome, and bile acid metabolism, but negatively associated with TNFA-signaling-via-NFKB, IFN-γ response, inflammatory response, and allograft rejection (Fig. 3B). Together, these results indicate that CD24-mediated immunosuppression in pan-cancer is significantly guided by these pathways.
Gene set enrichment analysis (GSEA) was used to detect the potential functions of CD24 in pan-cancer. A The potential signaling pathways of CD24 in pan-cancer. The size of the circle was regarded as the false discovery rare (FDR) value, and the color meant the normalized enrichment score (NES). B The KEGG terms and Hallmark terms of high CD24 expression and low CD24 expression
3.3 Correlation between CD24 and immune checkpoints and chemokines
Spearman's correlation analysis was utilized to examine the relationships between CD24 expression and immune checkpoint genes across various cancers, including both immune inhibitory and stimulatory genes. CD24 expression was found to be negatively correlated with most stimulatory checkpoints in CESC, CHOL, COAD, ESCA, READ, TGCT, and THCA. In contrast, CD24 showed a positive correlation with the immune inhibitory gene VTCN1 in KIRP, LAML, LIHC, MESO, THYM, and UVM (Fig. 4A). Notably, in TGCT, CD24 expression was especially negatively correlated with TNFRSF14, LAIR1, CD244, LAG3, ICOS, CD40LG, and CTLA4. Further analysis of the correlations between CD24 expression and chemokines revealed that CD24 was negatively correlated with CXCL9, CXCL10, CXCL11, CCL2, CCL3, CCL4, CCL5, CCL16, CCL18, and CCL19. Conversely, CD24 was positively correlated with CCL28, CXCL1, CXCL2, CXCL3, and CXCL5 (Fig. 4B, C). These findings suggest that CD24 may influence immune cell infiltration by modulating chemokine secretion.
3.4 Correlations between CD24 and TMB, MSI, and MMR genes
Tumor mutation burden (TMB), microsatellite instability (MSI), and MMR genes are currently primary biomarkers used to predict the efficacy of immunotherapy. In this study, we examined the relationships between these biomarkers and CD24 expression. Positive correlations between CD24 expression and TMB were observed in BLCA, BRCA, ESCA, LAML, THYM, and UCEC. In contrast, negative correlations were found in COAD, HNSC, KICH, and THCA (Fig. 5A). CD24 expression showed positive correlations with MSI in TGCT and UCEC, while negative correlations were evident in COAD, KICH, PAAD, and SKCM (Fig. 5B). Additionally, our analysis of CD24 with MMR gene mutations revealed a positive association with MLH1, MSH2, MSH6, PMS2, and EPCAM across most cancer types, with the exception for ESCA (Fig. 5C). These findings suggest that CD24 could serve as a potential biomarker for immunotherapy, emphasizing its importance in assessing treatment efficacy in cancer patients.
3.5 Prognostic significance of CD24 in pan-cancer
To assess the prognostic impact of CD24 across various tumors, we utilized univariate Cox regression to evaluate its effect on OS, disease-specific survival (DSS), DFI, and PFI. The OS analysis indicated that CD24 acted as a risk factor in patients with BRCA, LIHC and CESC while serving as a protective factor in patients with GBMLGG, LGG, KIRC, KIPAN, and COADREAD (Fig. 6A). DSS analysis revealed that CD24 was positively associated with BRCA, LIHC, THYM, CESC and MESO but negatively associated with GBMLGG, KIRC, LGG, KIPAN, COADREAD, and KICH (Fig. 6B). Regarding DFI, CD24 was identified as a risk factor in BRCA, whereas it served as a protective factor in PCPG and ESCA (Fig. 6C). For PFI, CD24 expression was associated with a risk factor in BRCA, LIHC and STAD, and a protective factor in GBMLGG, LGG, KIRC, KIPAN, PCPG, and THCA (Fig. 6D). These findings suggest that CD24 primarily exhibits an oncogenic role in BRCA, LIHC, and CESC.
3.6 Expression profiling of CD24 in pan-cancer
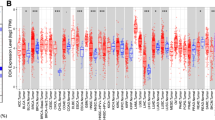
We analyzed the differential expression of CD24 between tumors and normal tissues using the TCGA database. The results indicated that CD24 was significantly overexpressed in various tumors, including BLCA, BRCA, CESC, CHOL, KIRP, LIHC, LUAD, LUSC, PCPG, STAD, UCEC, LGG, STES, and KIPAN. Conversely, CD24 expression was markedly lower in COAD, HNSC, KICH, READ, and THCA compared to control tissues (Fig. 7A). To address the limited availability of normal tissues in TCGA, we incorporated the GTEx (Genotype Tissue Expression) database for a more comprehensive analysis. As shown in Fig. 7B, CD24 was highly expressed in most tumor types, except for HNSC, WT, SKCM, LAML, ACC, and KICH. Thorsson et al. identified six immune subtypes—wound healing (C1), IFN-gamma dominant (C2), inflammatory (C3), lymphocyte depleted (C4), immunologically quiet (C5), and TGF-b dominant (C6)—by analyzing 1,000 tumors across 33 cancer types in TCGA [18]. We examined CD24 expression across these immune subtypes and found that the IFN-gamma dominant subtype (C2) exhibited lower CD24 expression in BLCA, BRCA, LIHC, TGCT, and THCA (Fig. 7C). These findings suggest that CD24 expression varies among different cancers and is elevated in most solid tumors, potentially contributing to tumor immune evasion.
The expression level of CD24 across pan-cancer. A The expression difference of CD24 in tumor tissues and normal tissues from TCGA database. B The expression profiles of CD24 in tumor tissues and normal tissues from TCGA and GTEx database. C The expression levels of CD24 in different immunophenotypes in TCGA database
3.7 CD24 expression and immunotherapy
Current immune checkpoint therapies primarily target PD1, PDL1 and CTLA4. Herein, we aimed to evaluate whether CD24 could serve as a predictive marker for immunotherapy efficacy. Peng Jiang et al. systematically assessed genes influencing cytotoxic T-cell function and patient outcomes using the TCGA database [19]. The TIDE algorithm was used to examine the interaction between candidate genes and cytotoxic T-cell function. Our analysis with the TIDE database revealed that higher cytotoxic T cell levels were generally associated with improved survival in patients with low CD24 expression across multiple tumor cohorts, including HNSC-HPV, PAAD, BLCA, OV, BRCA, and Melanoma, indicating that CD24 may facilitate tumor immune escape (Fig. 8A). Additionally, in cohorts treated with PD1 inhibitors, such as Melanoma and Glioblastoma, high CD24 expression was linked to poorer outcomes (Fig. 8B).
3.8 CD24 knockdown suppresses cell viability in Hepatocellular Carcinoma Cells
To elucidate the functional role of CD24 in tumor cells, we first assessed CD24 expression levels in the normal hepatocyte cell line L02 and four hepatocellular carcinoma cell lines: HCCLM3, Huh7, HepG2, and Hep3B, using qPCR and western blot (Fig. 9A, B). We then generated stable CD24 knockdown cell lines in HepG2 and Huh7 cells. The efficiency of CD24 knockdown was validated through qPCR and western blot (Fig. 9C, D). CCK8 assays demonstrated that CD24 knockdown significantly inhibited cell proliferation in Huh7 and HepG2 cells 24 h post-transfection (Fig. 9E). Moreover, colony formation assays confirmed that CD24 knockdown markedly reduced the colony-forming ability of these cells (Fig. 9F). To explore the mechanism by which CD24 influences proliferation, we conducted flow cytometry analyses to evaluate the effects of CD24 knockdown on the cell cycle. The results indicated that CD24 knockdown induced a notable cell cycle arrest in the Go/G1 phase and a substantial reduction in the proportion of cells in the S-phase (Fig. 9G). Collectively, these findings underscore the critical role of CD24 in tumorigenesis and cancer progression.
Knockdown of CD24 inhibits cell viability and proliferation of Huh7 and HepG2 cells. A Relative mRNA expression of CD24 in Hepatocellular Carcinoma cell lines. B Expression levels of CD24 protein in Hepatocellular Carcinoma cell lines. C, D RT-PCR and WB determine the efficiency of CD24 knockdown in Hepatocellular Carcinoma cell lines. E The cells growth rates were determined by CCK-8 proliferation assays at various time points. F Representative images of colony formation induced by si-NC, si-CD24. G Flow cytometry analyses of cell cycle in Huh7 and HepG2 cells after transfection with si-NC, si-CD24. (*p < 0.05, **P < 0.01, ***P < 0.001)
4 Discussion
ICIs have significantly advanced cancer treatment by providing long-lasting benefits, especially for patients with advanced metastatic cancers. Unlike traditional therapies, ICI can induce durable responses in a subset of patients. Nevertheless, only approximately one-third of patients respond to ICI [20]. The effectiveness of ICI can be influenced by several factors, including the presence of cytotoxic T cells, neoantigens, PD-L1 expression, interferon signaling, TMB, MSI, tumor aneuploidy, and intestinal microbiota [21,22,23,24,25,26,27,28]. However, these factors alone are insufficient for accurately predicting treatment outcomes. Therefore, identifying additional biomarkers and regulators of resistance is essential for improving the precision of ICI efficacy assessment. Cancer cells can evade macrophage-mediated phagocytosis by expressing high levels of anti-phagocytic signals on their surfaces. Monoclonal antibodies targeting these anti-phagocytic signals can effectively prevent tumor cells from avoiding phagocytosis. CD24, a well-documented anti-phagocytic signal, has been shown to directly protect cancer cells from being targeted by Siglec-10-expressing macrophages. The blockade of CD24-Siglec-10 signaling with monoclonal antibodies significantly enhances the clearance of CD24 + tumors, highlighting the potential of CD24 blockade as a promising approach in immunotherapy. In this present study, we investigated the role of CD24 across various cancers to elucidate its potential impact on immunotherapy and cancer progression.
Tumor-associated myeloid cells (TAMCs) are crucial in tumor development, metastasis, immune regulation, tumor angiogenesis, and cancer treatment response [29,30,31]. Current research indicates that TAMCs can suppress tumor immunity by secreting cytokines, regulating immune checkpoint expression, and modulating tumor metabolism, leading to the manifestation of a "cold tumor" microenvironment [32, 33]. Here, we first evaluated CD24 expression and immune cell infiltration. CD24 was primarily negatively correlated with NK and NKT cells, while it was positively correlated with tumor-infiltrating myeloid cells, including MDSCs and tumor-associated neutrophils (TANs). NK cells and NKT cells primarily possess anti-tumor activity. NK cells secrete perforin, which disrupts cancer cell membranes and induces cancer cell death. NKT cells, on the other hand, activate other immune cells by secreting cytokines, thereby regulating the anti-tumor response [34]. In contrast, TANs and MDSCs are key components of myeloid suppressor cells that contribute to tumor immunosuppression. TANs release particulate components, such as elastase, which promote cancer cell proliferation and invasion [35,36,37]. They also facilitate the spread of cancer cells by promoting their extravasation into pre-metastatic niches through the secretion of IL-1β and MPPs [36]. Additionally, TANs mediate tumor cell cytotoxicity by generating ROS and TRAIL [38, 39]. MDSCs, on the other hand, enhance cancer stem cell growth and provide pro-survival signals to tumor cells [40]. Accumulated MDSCs in the TME produce MMP9, which supports tumor growth and vasculature [41]. MDSCs also secrete TGF-β, EGF, and HGF, which induce epithelial-mesenchymal transition (EMT) and promote cancer cell proliferation [42]. Therefore, we hypothesize that CD24 may promote tumor proliferation, invasion, metastasis, drug resistance, and stemness formation by increasing the infiltration of MDSCs and TANs into the TME.
CD24 expression was negatively correlated with the ESTIMATE score across most cancer types. The ESTIMATE score reflects tumor purity, with lower purity generally indicating a poorer prognosis. Additionally, the immune score, which denotes the extent of immune cell infiltration, was also negatively correlated with CD24 expression and differential immune checkpoints, suggesting a strong immunosuppressive role for CD24 in various cancers. Chemokine secretion within the TME is frequently altered, and abnormal chemokine distribution can promote the differentiation and infiltration of immunosuppressive myeloid cells, cytotoxic T cells, NK cells, and NKT cells into tumors [43,44,45]. Our study found that CD24 was significantly negatively correlated with the chemokines CXCL9 and CXCL10, which are crucial for recruiting CD8 + T cells into the TME [46]. Furthermore, it has been reported that CXCL1 can be regulated by c-MYC and epigenetic mechanisms, leading to myeloid cell aggregation and inhibition of dendritic cell function, which results in an ineffective tumor immune response [47].
Moreover, we evaluated the expression level and prognostic value of CD24 across various cancers. The results indicated that CD24 was significantly overexpressed in 14 tumor types, including BLCA, BRCA, CESC, CHOL, KIRP, LIHC, LUAD, LUSC, PCPG, STAD, UCEC, LGG, STES, and KIPAN. Conversely, CD24 was expressed at low levels, primarily in COAD, HNSC, KICH, READ, and THCA. We examined the differences in CD24 expression across various immunophenotypes. The results revealed that CD24 expression was significantly lower in the IFN-gamma dominant (C2) subtype of several tumors, including BLCA, BRCA, LIHC, TGCT, and THCA. OS analysis showed that CD24 is a risk factor for patients with BRCA, LIHC, and CESC, while it is a protective factor for patients with GBMLGG, LGG, KIRC, KIPAN, and COADREAD. For DSS, CD24 was positively associated with BRCA, LIHC, THYM, CESC and MESO, but negatively associated with GBMLGG, KIRC, LGG, KIPAN, COADREAD, and KICH. These findings suggest that high CD24 expression predominantly acts as an oncogene in most tumor types.
Combined with GSEA, we found that CD24 was significantly negatively correlated with several immune-related pathways, including TNFA-signaling-via-NFKB, IFN-α response, IFN-γ response, inflammatory response, IL6-JAK-STAT3 signaling, and IL2-STAT5 signaling. Tumor immune evasion primarily involves two mechanisms: the infiltration of low levels of cytotoxic T cells and the infiltration of dysfunctional cytotoxic T cells. Using the TIDE algorithm, we analyzed the correlation between CD24 and cytotoxic T cells, as well as patient prognosis. Our analysis revealed that in patients with low CD24 expression, higher levels of cytotoxic T cells were associated with better survival across various tumors. Additionally, the TIDE database indicated that melanoma patients with high CD24 expression were less responsive to PD-1 therapy. These findings underscore the significant role of CD24 in promoting tumor immune escape and treatment resistance. Furthermore, we investigated the role of CD24 in LIHC using molecular biological methods. RT-PCR and Western blotting confirmed that CD24 expression was upregulated in LIHC cells. CCK-8 assays demonstrated that CD24 promoted CRC cell proliferation, and colony formation assays showed that CD24 knockdown significantly reduced the colony-forming ability of cells. Moreover, flow cytometry revealed that the proportion of cells in the G0/G1 phase was increased following CD24 knockdown, confirming its pro-proliferative effect. These results validate the findings from our pan-cancer bioinformatics analysis and suggest that similar molecular biological validations will be pursued in additional cancer types in the future.
This study has several limitations. First, our analysis was based solely on mRNA expression levels of CD24 from TCGA and GTE databases, without including data on CD24 protein levels in clinical tumor tissues. Second, a deeper understanding of how CD24 affects tumor proliferation, invasion, and metastasis across different cancers is needed. Third, the TIDE database predominantly focuses on melanoma, limiting the assessment of CD24 as an immune predictive marker in other solid tumors. Consequently, further research is required to elucidate the role of CD24 in cancer and to evaluate its potential as a target for anti-cancer therapies.
5 Conclusion
In this study, we conducted a comprehensive bioinformatics analysis of CD24, revealing its association with immune cell infiltration across various tumors and its potential impact on immunotherapy outcomes. We observed abnormal CD24 expression in multiple tumors, with significant correlations to prognosis, particularly in LIHC. These findings offer new insights into the role of CD24 in tumor development and progression, suggesting potential avenues for further research and therapeutic targeting.
Data availability
The original available statements demonstrated in the study are summarized in the article materials. Additional inquiries should be submitted to the corresponding authors.
Abbreviations
- ACC:
-
Adrenocortical carcinoma
- BLCA:
-
Bladder urothelial carcinoma
- BRCA:
-
Breast invasive carcinoma
- CESC:
-
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL:
-
Cholangiocarcinoma
- COAD:
-
Colon adenocarcinoma
- DLBC:
-
Lymphoid neoplasm diffuse large B-cell lymphoma
- ESCA:
-
Esophageal carcinoma
- GBM:
-
Glioblastoma multiforme
- HNSC:
-
Head and neck squamous cell carcinoma
- KICH:
-
Kidney chromophobe
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney papillary cell carcinoma
- LAML:
-
Acute myeloid leukemia
- LGG:
-
Brain lower grade glioma
- LIHC:
-
Liver hepatocellular carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- MESO:
-
Mesothelioma
- OV:
-
Ovarian serous cystadenocarcinoma
- PAAD:
-
Pancreatic adenocarcinoma
- PCPG:
-
Pheochromocytoma and paraganglioma
- PRAD:
-
Prostate adenocarcinoma
- READ:
-
Rectum adenocarcinoma
- SARC:
-
Sarcoma
- STAD:
-
Stomach adenocarcinoma
- SKCM:
-
Skin cutaneous melanoma
- TGCT:
-
Testicular germ cell tumors
- THCA:
-
Thyroid carcinoma
- UCEC:
-
Uterine corpus endometrial carcinoma
- UCS:
-
Uterine carcinosarcoma
- UVM:
-
Uveal melanoma
References
Paré L, et al. Association between PD1 mRNA and response to anti-PD1 monotherapy across multiple cancer types. Ann Oncol. 2018;29(10):2121–8.
Gordon S, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–9.
Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–99.
Barkal A, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19(1):76–84.
Advani R, et al. CD47 blockade by Hu5F9-G4 and rituximab in Non-Hodgkin’s lymphoma. N Engl J Med. 2018;379(18):1711–21.
Sharma P, Allison J. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
Pirruccello S, LeBien T. The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol. 1986;136(10):3779–84.
Tarhriz V, et al. Overview of CD24 as a new molecular marker in ovarian cancer. J Cell Physiol. 2019;234(3):2134–42.
Kristiansen G, et al. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9(13):4906–13.
Lim S. CD24 and human carcinoma: tumor biological aspects. Biomed Pharmacother. 2005;59:S351–54.
Weng C, et al. Mutant Kras-induced upregulation of CD24 enhances prostate cancer stemness and bone metastasis. Oncogene. 2019;38(12):2005–19.
Gao M, et al. Identification and characterization of tumor-initiating cells in multiple myeloma. J Natl Cancer Inst. 2020;112(5):507–15.
Sagiv E, Arber N. The novel oncogene CD24 and its arising role in the carcinogenesis of the GI tract: from research to therapy. Expert Rev Gastroenterol Hepatol. 2008;2(1):125–33.
Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35(3):255–62.
Chen G, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–5.
Barkal A, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6.
Shen W, et al. Sangerbox: a comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta. 2022;1(3):e36.
Jiang P, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–8.
Thorsson V, et al. The immune landscape of cancer. Immunity. 2018;48(4):812-830.e814.
Sharma P, Hu-Lieskovan S, Wargo J, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23.
Van Allen E, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–11.
Chan T, Wolchok J, Snyder A. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2015;373(20):1984.
Zaretsky J, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–29.
Nishino M, Ramaiya N, Hatabu H, Hodi F. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14(11):655–68.
Ayers M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Investig. 2017;127(8):2930–40.
Le D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Davoli T, Uno H, Wooten E, Elledge S. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017.
Kroemer G, Zitvogel L. Cancer immunotherapy in 2017: the breakthrough of the microbiota. Nat Rev Immunol. 2018;18(2):87–8.
Canli Ö, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32(6):869-883.e865.
Zeng X, et al. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol Ther. 2019;20(7):956–66.
Wang H, Joyce J. Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle. 2010;9(24):4824–35.
Mantovani A, Marchesi F, Jaillon S, Garlanda C, Allavena P. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cell Mol Immunol. 2021;18(3):566–78.
Nakamura K, Smyth M. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):1–12.
Mortezaee K, Majidpoor J. NK and cells with NK-like activities in cancer immunotherapy-clinical perspectives. Med Oncol. 2022;39(9):131.
Wada Y, et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol Rep. 2007;17(1):161–7.
Spiegel A, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630–49.
Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig. 2013.
Clark R, Klebanoff S. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975;141(6):1442–7.
Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Can Res. 2004;64(3):1037–43.
Cui T, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39(3):611–21.
Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–21.
Toh B, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9(9):e1001162.
Griffith J, Sokol C, Luster A. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702.
Schulz O, Hammerschmidt S, Moschovakis G, Förster R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol. 2016;34:203–42.
Kohli K, Pillarisetty V, Kim T. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2022;29(1):10–21.
Dangaj D, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885-900.e810.
Hu J, et al. Regulation of tumor immune suppression and cancer cell survival by CXCL1/2 elevation in glioblastoma multiforme. Sci Adv. 2021.
Funding
This study was supported by the Health Commission of Nantong (QA2021038).
Author information
Authors and Affiliations
Contributions
Chunmei Zhao was responsible for writing the manuscript. Ying Huang and Haotian Zhang performed data collection. Huimin Liu helped for the graphic processing and the revision of this paper. All authors contributed to this article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, C., Huang, Y., Zhang, H. et al. CD24 affects the immunosuppressive effect of tumor-infiltrating cells and tumor resistance in a variety of cancers. Discov Onc 15, 399 (2024). https://doi.org/10.1007/s12672-024-01284-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01284-7