Abstract
Background
Deoxycytidine kinase (DCK), an enzyme in the nucleoside biosynthetic pathway, can affect the development of immune cells. However, the relationships between the expression of DCK, patient prognosis, and tumor-infiltrating immune cells (TIICs) in hepatocellular carcinoma (HCC) are still unclear.
Methods
The expression of DCK in HCC was analyzed through the Oncomine and Tumor Immune Estimation Resource (TIMER) databases. The impact of DCK on clinical prognosis was investigated via the Kaplan-Meier plotter and verified in the Gene Expression Profiling Interactive Analysis (GEPIA) databases. The interrelationships between DCK expression and TIICs in HCC were analyzed by the TIMER database. Additionally, the relationship between DCK expression and immune cell gene markers was calculated through TIMER and GEPIA databases.
Results
Compared with the adjacent normal tissues, high expression of DCK was observed in HCC tissues. Also, the higher expression of DCK was correlated to poorer prognosis in HCC patients, and it was associated with decreased survival in those with early stage and grade. Moreover, DCK expression was positively correlated with TIICs, including CD4+ and CD8+ T cells, B cells, monocytes, tumor-associated macrophages (TAMs), M1 and M2 macrophages, neutrophils, natural killer cells, and dendritic cells. Specifically, DCK expression levels were significantly associated with diverse immune gene marker sets, including those of Tregs and exhausted T cells.
Conclusion
These findings suggest that DCK expression is correlated with patient outcomes and tumor infiltration cell levels in HCC patients. Additionally, the increased level of DCK was associated with marker genes of Tregs and exhaustion-related inhibitory receptors, suggesting the potential role of DCK in immunosuppression and immune escape. These findings suggest that DCK can function as a potential novel prognostic biomarker and reflect the immune infiltration status in HCC patients.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC), one of the major primary hepatic tumors, is the fourth most common cause of cancer-related death worldwide [1]. HCC often develops from chronic liver inflammation, especially liver cirrhosis. The common risk factors for liver cancer are hepatitis B/C virus infection, alcohol consumption, and non-alcoholic fatty liver disease. Although a remarkable advancement has been achieved in diagnosis, surgical treatment, adjuvant therapy, and immune therapy [2, 3], the prognosis after resection is unsatisfactory due to the high rate of recurrence [4, 5].
Deoxycytidine kinase (DCK) is one of the essential enzymes of the nucleoside salvage pathway, and its expression is associated with the resistance to antiviral and anticancer chemotherapeutic agents [6,7,8]. DCK is highly expressed in lymphoid cells or tissues, such as thymus, spleen, lymph nodes, peripheral blood mononuclear cell, and bone marrow cells. Interestingly, DCK was expressed differently in breast and pancreatic cancers, and the expression levels were associated with patient prognosis in both types [9, 10]. A previous study analyzing eight microarray datasets comprising 521 human HCC tissues found that DCK was upregulated in HCC tissues [11]. However, prognostic values and molecular mechanisms of DCK in HCC are still unclear.
Tumor microenvironment and tumor-infiltrating immune cells (TIICs) are topics of interest and have shown an important role in cancer studies [12, 13]. Immune cells of tumor microenvironment play an important role in tumor progression in HCC. The immunosuppressive microenvironment of HCC contributes to immune tolerance and immune escape through different mechanisms [14]. DCK can have an impact on peripheral T cell homeostatic proliferation and survival, and its deficiency can influence lymphocyte development [15,16,17], indicating its potential role in the immune microenvironment. However, whether DCK could influence immune cell and tumor microenvironment contributing to tumor progression still need investigation.
In this study, we investigated the expression level of DCK and determined its correlation with cancer patient prognosis based on the online public databases such as the Oncomine, Kaplan-Meier plotter, Tumor Immune Estimation Resource (TIMER), and Gene Expression Profiling Interactive Analysis (GEPIA). Specifically, we analyzed the correlation between DCK expression and TIICs in HCC through the TIMER database. The findings in this study showed the important role of DCK in HCC and provided an interrelationship and an underlying mechanism between DCK and TIIC interactions.
Materials and methods
The expression of DCK
The DCK expression levels were analyzed in different cancer types using the Oncomine database (https://www.oncomine.org/resource/login.html) [18]. The parameters about the threshold were as follows: p value of 0.05, fold change of 1.5, and gene ranking of all. The results are exhibited as p value, fold changes, and rank (%).
Prognosis analysis related to DCK expression in HCC patients
In order to determine the relationship between DCK expression and patient prognosis, Kaplan-Meier plots (http://kmplot.com/analysis/) were used in the HCC [19]. For the expression of the DCK, the expression between the lower and upper quartiles was analyzed and the best performing threshold was applied as the final cutoff value automatically in the Cox regression analysis. The results were presented with the hazard ratio (HR) and p values or Cox p values from a log-rank test.
Immune infiltration analysis related to the DCK expression
The TIMER database (https://cistrome.shinyapps.io/timer/) [20] includes gene expression profiles and immune infiltration cells in 32 cancer types based on RNA-Seq expression profiling data from The Cancer Genome Atlas (TCGA) database. It can detect the differential gene expression in tumor tissues, analyze the infiltration of immune cells, and find the correlation between two genes through these profiles [21]. The infiltration levels of immune cells were analyzed through estimation by the statistical method through gene expression data. Therefore, we investigated the relationship between DCK expression and TIICs, including CD4+ and CD8+ T cells, B cells, neutrophils, dendritic cells, and macrophages. Additionally, the correlations among DCK expression and different gene markers of TIICs, like T cells, B cells, tumor-associated macrophages (TAMs), monocytes, M1 and M2 macrophages, natural killer (NK) cells, neutrophils, dendritic cells (DCs), T helper (Th) cells, T helper 17 (Th17) cells, follicular helper T (Tfh) cells, Tregs, and exhausted T cells, were analyzed. The marker genes of TIICs were reported as previous study [22].
Gene correlation identification in GEPIA
The GEPIA database (http://gepia.cancer-pku.cn/index.html) contains the gene expression data from TCGA and the Genotype-Tissue Expression (GTEx) projects [23]. It can also analyze differential gene expression, patient prognosis, and relationship of two genes through online data. Therefore, gene expression levels related to patient prognosis were identified via the GEPIA database, and the interrelationships between the levels of gene expression and TIICs were established. A median value of the DCK expression was used as a cutoff to distinguish high expression from low expression of DCK.
Statistical analysis
All statistical analyses were performed in R (version 3.5.2). Survival curves were generated by the Kaplan-Meier plots and GEPIA database. Forest plots were constructed using R package “forestplot” (https://cran.r-project.org/web/packages/forestplot/index.html). Spearman’s correlation was used to evaluate the relation between gene expression and infiltrating immune cells. The strength of the correlation was defined as follows: 0.00–0.29 (weak), 0.30–0.59 (moderate), 0.60–0.79 (strong), 0.80–1.00 (very strong) [24]. p values < 0.05 were considered as statistically significant except for the correlation analysis.
Results
The expression levels of DCK in HCC
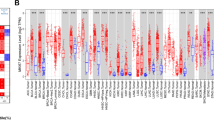
A previous study has reported that metabolic gene DCK was upregulated in the HCC tissues [11]. In order to validate the expression of DCK between HCC tissues and adjacent normal tissues, gene expression analysis was analyzed via the Oncomine database. We found that DCK expression was significantly higher in the HCC (Fig. 1a and Supplementary Table 1). Further evaluation of DCK expression in HCC was calculated using the TIMER database, and consistent results were found for HCC (Fig. 1b). These findings validated that DCK expression was highly expressed in HCC tissues.
Prognostic values of DCK expression in HCC
Next, we investigated the relationship between the DCK expression and prognosis in HCC using the Kaplan-Meier plotter database. Interestingly, a high expression of DCK was associated with poorer prognosis in HCC patients (overall survival (OS): HR = 1.90, 95% CI = 1.32–2.72, p = 0.00043, n = 364; relapse-free survival (RFS): HR = 1.53, 95% CI = 1.10–2.13, p = 0.011, n = 316; progression-free survival (PFS): HR = 1.70, 95% CI = 1.27–2.28, p = 0.00034, n = 370; disease-specific survival (DSS): HR = 2.02, 95% CI = 1.27–3.21, p = 0.0026, n = 362; Fig. 2a–d). These findings indicated the potential prognostic values of DCK in HCC. Also, we identified the relationship between the DCK expression and prognostic values in HCC using the GEPIA database. The results showed that high expression of DCK was associated with dismal prognosis in OS of HCC patients (Fig. 2e), but there was not statistical difference in PFS (Fig. 2f). These findings suggest that the expression of DCK influences the prognosis of HCC patients.
Prognostic values of DCK expression in HCC (a–g). a–d OS, RFS, PFS, and DSS in HCC cohorts through the Kaplan-Meier plots database (n = 364, n = 316, n = 370, n = 362, respectively). e–f OS and PFS in HCC cohorts through the GEPIA database (n = 364, n = 364, respectively). g Cox regression analysis of HCC patients in OS and PFS. OS overall survival, RFS relapse-free survival, PFS progression-free survival, DSS disease-specific survival
High expression of DCK related to clinical characteristics of HCC patients
Based on the differential expression of DCK and significant prognostic values related to its expression, which were observed in HCC, we investigated the relationship between the expression of DCK and different clinicopathological characteristics of HCC using the Kaplan-Meier plotter database. The high expression of DCK was related to worse OS and PFS rates in the females (OS: HR = 1.85, p = 0.042; PFS: HR = 1.70, p = 0.0013) and males (OS: HR = 2.37, p = 9.20 × 10− 5, PFS: HR = 1.80, p = 0.0012), Asians (OS: HR = 3.19, p = 5.10 × 10− 5; PFS: HR = 2.32, p = 0.00033), non-alcoholics (OS: HR = 2.08, p = 0.0026; PFS: HR = 1.60, p = 0.021), patients with hepatitis viral infection (OS: HR = 2.13, p = 0.019; PFS: HR = 1.98, p = 0.0031) and those without it (OS: HR = 1.71, p = 0.025; PFS: HR = 1.71, p = 0.041), and patients without vascular invasion (OS: HR = 2.23, p = 0.0019; PFS: HR = 2.50, p = 0.025) (Fig. 2g). Specifically, high expression of DCK was correlated with poorer OS and PFS rates in stages I, II, and I+II, grades II and III, and AJCC-T stage I patients, but was not associated with stage III and AJCC-T II and III stages (Fig. 2g). These findings indicate that the expression level of DCK can influence the prognosis in HCC patients with different clinicopathological factors, especially in these early-stage patients.
DCK expression levels are correlated with the immune infiltration in HCC
Tumor-infiltrating lymphocytes influence the survival of patients with cancer. Therefore, the interrelationship between the TIICs and the DCK expression was investigated by the TIMER database. The high expression of DCK was related to patient outcomes in HCC, and it was correlated with high infiltration levels of immune cells (Fig. 3). The DCK expression was associated with dismal outcomes and positively correlated with the infiltration levels of B cells (cor = 0.351, p = 1.97 × 10− 11), CD8+ T cells (cor = 0.310, p = 4.57 × 10− 9), CD4+ T cells (cor = 0.419, p = 4.61 × 10− 16), macrophages (cor = 0.484, p = 1.84 × 10− 21), neutrophils (cor = 0.556, p = 2.50 × 10− 29), and dendritic cells (cor = 0.485, p = 2.00 × 10− 21) in HCC (Fig. 3). These findings suggest immune infiltration may play a role in patient outcomes, and DCK could modulate immune infiltrating cells into HCC tissues. However, the expression of DCK was not correlated with tumor purity (cor = 0.024, p = 6.58 × 10− 1), suggesting the expression of DCK was from cells in the tumor microenvironment.
Correlation between DCK expression and different gene markers of immune cell subsets
We further investigated the relationships between DCK expression and multiple TIICs in HCC through TIMER and GEPIA databases based on the different immune cell gene markers. The correlation was adjusted for tumor purity due to its influences on the immune infiltration analysis. The immune cells included CD4+ T cells, CD8+ T cells, B cells, TAMs, monocytes, M1 and M2 macrophages, neutrophils, dendritic cells, and NK cells in HCC patients. Also, subsets of T cells, including Th1, Th2, Tfh, Th17, Tregs, and exhausted T cells, were investigated.
We found that the DCK expression was significantly correlated with the expression of gene markers in monocytes, TAM, and M2 macrophages in HCC patients after adjusting tumor purity (Fig. 4a, b). DCK expression was significantly associated with subsets of T cells, revealing its association with cytokine secretion (Fig. 4a, b). Tregs have an essential role in immune escape and angiogenesis, and immune checkpoint regulation is important for T cell-mediated cancer-killing effect. Interestingly, the expression of DCK was associated with the gene markers like CCR8, STAT5b, and TGFB1 of Tregs, and PD–1, CTLA–4, LAG3, TIM–3, and GZMB of exhausted T cells after adjusting tumor purity (Fig. 4a, b), revealing that increased expression of DCK was associated with immunosuppression in HCC. These findings suggest that immune infiltrating cells could influence patient prognosis in HCC. Similar expression results were observed in GEPIA (Supplementary Table 2). The results strongly indicated the potential functions of DCK in contributing to the angiogenesis and regulating immune escape in HCC.
Correlation between the expression of DCK and marker genes of infiltrating immune cells in HCC using the TIMER database. a Correlation between the expression of DCK and immune molecular genes. None, correlation without adjustment; Purity, correlation adjusted by purity; 0≤***<0.0001≤**<0.001≤*<0.01. b The scatter plots of correlation between DCK expression and the gene markers of monocytes, TAMs, M2 macrophages, Tregs and exhausted T cells in HCC. TAMs tumor-associated macrophage
Discussion
In this study, we demonstrate the DCK expression and corresponding patient prognosis in HCC. DCK expression was significantly higher in HCC patients, and its high expression was correlated with worse long-term outcomes. To the best of our knowledge, this is the first study that reported a prognostic impact of DCK in HCC. Moreover, high expression of DCK was correlated with unfavorable prognosis for HCC patients, especially for those with early stage. Additionally, the expression of DCK was correlated with TIICs and its marker genes in HCC. The increased level of DCK was correlated with marker genes of Tregs and exhaustion-related inhibitory receptors, suggesting potential mechanisms of DCK in immune escape. These findings demonstrate that DCK can be a promising prognostic biomarker and is correlated with immune infiltrates in HCC.
As an essential enzyme in the nucleoside biosynthetic pathway, DCK plays a role in metabolism and DNA synthesis during the embryogenesis, organogenesis, and essential cell developmental processes. The deficiency of DCK in mice blocks the lymphocyte development and shows a significant decrease in both T and B cell populations compared with wild-type mice [17]. Mutation of DCK impairs T and B cell function in mice, and mutant contains a CD44highCD62Llow memory phenotype lymphocytes in the peripheral, with high levels of proliferation and apoptosis [15]. However, the activation of DCK elevates the level of deoxyadenosine triphosphate (dATP) intracellularly, which suggests the link of DCK activation to the induction of apoptosis. Therefore, differentially expressed DCK in tumor tissues could influence the immune cell functions.
There is an urgent need for improving early diagnosis and treatment of HCC patients. The development of new biomarkers in detection, prognostic assessment, and treatment options should be a major research interest in the future [25]. The high expression of DCK was observed in liver tumors, and its low expression was found in normal liver. The high expression of DCK was correlated with dismal outcomes. Therefore, we have reason to believe that the DCK could be a prognostic biomarker in HCC. Tumor microenvironment are important for tumor progression. Recent studies demonstrated that the integration of TIICs and clinicopathologic characteristics can be a prognostic predictive model and predict the response of immune therapy [13, 26]. A positive correlation between the DCK expression and the TIICs indicates that DCK-based prediction of patient outcome in HCC may be associated with immune cell infiltration. Negative association between DCK and tumor purity suggested that DCK expression was from cells in the tumor environment. The expression of DCK was more likely from TIICs [27]. Moreover, the expression of DCK was associated with the expression of the immune cell markers, such as the markers of monocytes, TAM, M1, and M2 macrophages. These findings suggest the potential functions of DCK in regulating infiltration and activity of macrophages. Furthermore, we observed that DCK expression was relevant to the expression of markers of T cell subsets, like Th1, Th2, Tfh, and Th17, indicating its potential influence on the tumor progression in regulating the secretion of cytokines from the helper T cells.
Interestingly, the expression of DCK was correlated with the marker genes of Tregs and exhaustion-related inhibitory receptors. Tregs play an important part in immune escape and angiogenesis, which could influence patient outcomes. Overexpression of inhibitory receptors in HCC can inhibit immune response and dampen T cell functions, resulting in tumor progression [28,29,30,31]. In return, the anti-immune-checkpoint inhibitor can provide HCC patients with a survival benefit [32]. These findings suggest the role of DCK as an essential aspect in the immunosuppressive and immune escape and also indicate its potential function in regulating TIICs in HCC patients. Moreover, cytosolic Ca2+ ions are associated with the activity of DCK in cells [33]. Altered Ca2+ flux could promote T cell subsets, which in turn promote cytokine production and downregulate CTLA–4 and PD–1 expression [34]. In addition, altered Ca2+ influences Ca2+/cyclic AMP (cAMP) signaling pathway contributing to the regulation of cytokines [35]. Both the excretion of cytokines and change in cAMP pathway are associated with tumor progression [12, 36]. These findings could be the potential mechanism of DCK regulating the expression of inhibitor receptors and contributing to the progression of a tumor.
This study had several limitations. First, the role of DCK was investigated through various datasets. Some potential basis still exists because of the different methods in data collection and analysis. Second, the correlation coefficients were not very strong. In fact, when analyzing the relationship between gene levels and TIICs, the correlation coefficients were mostly uncorrelated or had a weak and moderate correlation, and strong correlations were rare [22, 24]. Third, we only used public datasets, further identified by our HCC patient profiles, and the detailed relationship should be confirmed by in vitro or animal experiments. Additionally, comparing the efficiency between DCK and traditional biomarkers in HCC should be further studied. The function of DCK in other cancer types should be investigated in the future.
Conclusions
In conclusion, our results suggested that elevated DCK expression levels were associated with dismal prognosis together with enhanced immune infiltration in HCC, and the increased level of DCK was correlated with marker genes of Tregs and exhaustion-related inhibitory receptors, which could be the potential mechanism of DCK affecting patient outcomes. Therefore, relatively high levels of DCK in HCC could indicate the increased immune infiltration status and reflect a higher risk of death.
Availability of data and materials
All datasets used and/or analyzed during the current study are available from the public database.
Abbreviations
- DCK:
-
Deoxycytidine kinase
- TIICs:
-
Tumor-infiltrating immune cells
- TIMER:
-
Tumor Immune Estimation Resource
- GEPIA:
-
The Gene Expression Profiling Interactive Analysis
- HCC:
-
Hepatocellular carcinoma
- TAMs:
-
Tumor-associated macrophages
- TCGA:
-
The Cancer Genome Atlas
- NK cells:
-
Natural killer cells
- DCs:
-
Dendritic cells
- Th cells:
-
T helper cells
- Th17 cells:
-
T helper 17 cells
- Tfh cells:
-
Follicular helper T cells
- GTEx:
-
The Genotype-Tissue Expression
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
- PFS:
-
Progression-free survival
- DSS:
-
Disease-specific survival
- cAMP:
-
Cyclic AMP
References
Villanueva A. Hepatocellular carcinoma. New England J Med. 2019;380:1450–62.
Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–16.
Zhou J, Sun H-C, Wang Z, Cong W-M, Wang J-H, Zeng M-S, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver cancer. 2018;7:235–60.
Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724–9.
Han H-S, Shehta A, Ahn S, Yoon Y-S, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63:643–50.
Qin T, Jelinek J, Si J, Shu J, Issa J-PJ. Mechanisms of resistance to 5-aza-2′-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–67.
Dahn ML, Cruickshank BM, Jackson AJ, Dean C, Holloway RW, Hall SR, et al. Decitabine response in breast cancer requires efficient drug processing and is not limited by multidrug resistance. Mol Cancer Ther. 2020;19(5):1110–22 molcanther.0745.2019.
Zhang Y, Lei Y, Xu J, Hua J, Zhang B, Liu J, et al. Role of Damage DNA-binding protein 1 in pancreatic cancer progression and chemoresistance. Cancers. 2019;11:1998.
Geutjes E-J, Tian S, Roepman P, Bernards R. Deoxycytidine kinase is overexpressed in poor outcome breast cancer and determines responsiveness to nucleoside analogs. Breast cancer research and treatment. 2012;131:809–18.
Sebastiani V, Ricci F, Rubio-Viqueira B, Kulesza P, Yeo CJ, Hidalgo M, et al. Immunohistochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin Cancer Res. 2006;12:2492–7.
Nwosu ZC, Megger DA, Hammad S, Sitek B, Roessler S, Ebert MP, et al. Identification of the consistently altered metabolic targets in human hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2017;4(2):303–323.e1.
Liu LZ, Zhang Z, Zheng BH, Shi Y, Duan M, Ma LJ, et al. CCL15 recruits suppressive monocytes to facilitate immune escape and disease progression in hepatocellular carcinoma. Hepatology. 2019;69:143–59.
Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–5.
Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:396.
Choi O, Heathcote DA, Ho K-K, Müller PJ, Ghani H, Lam EWF, et al. A deficiency in nucleoside salvage impairs murine lymphocyte development, homeostasis, and survival. J Immunol (Baltimore, Md : 1950). 2012;188:3920–7.
Dobrovolsky VN, Bucci T, Heflich RH, Desjardins J, Richardson FC. Mice deficient for cytosolic thymidine kinase gene develop fatal kidney disease. Mol Genet Metab. 2003;78:1–10.
Toy G, Austin WR, Liao H-I, Cheng D, Singh A, Campbell DO, et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc Natl Acad Sci U S A. 2010;107:5551–6.
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia (New York, NY). 2007;9:166–80.
Nagy Á, Lánczky A, Menyhárt O, Győrffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8:9227.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–10.
Li B, Severson E, Pignon J-C, Zhao H, Li T, Novak J, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174.
Wu X, Qu D, Weygant N, Peng J, Houchen CW. Cancer stem cell marker DCLK1 correlates with tumorigenic immune infiltrates in the colon and gastric adenocarcinoma microenvironments. Cancers. 2020;12:274.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102.
Chen B, Lai J, Dai D, Chen R, Li X, Liao N. JAK1 as a prognostic marker and its correlation with immune infiltrates in breast cancer. Aging. 2019;11:11124–35.
Zhou C, Liu C, Liu W, Chen W, Yin Y, Li C-W, et al. SLFN11 inhibits hepatocellular carcinoma tumorigenesis and metastasis by targeting RPS4X via mTOR pathway. Theranostics. 2020;10:4627–43.
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55.
Spasokoukotskaja T, Arnér ES, Brosjö O, Gunvén P, Juliusson G, Liliemark J, et al. Expression of deoxycytidine kinase and phosphorylation of 2-chlorodeoxyadenosine in human normal and tumour cells and tissues. Eur J Cancer. 1995;31a:202–8.
Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–75.
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37.
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–51.
Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153:1107–1119.e1110.
Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–80.
Keszler G, Spasokoukotskaja T, Csapo Z, Talianidis I, Eriksson S, Staub M, et al. Activation of deoxycytidine kinase in lymphocytes is calcium dependent and involves a conformational change detectable by native immunostaining. Biochem Pharmacol. 2004;67:947–55.
Rodríguez-Perea AL, Rojas M, Velilla-Hernández PA. High concentrations of atorvastatin reduce in-vitro function of conventional T and regulatory T cells. Clin Exp Immunol. 2019;196:237–48.
Ahuja M, Chung WY, Lin WY, McNally BA, Muallem S. Ca(2+) signaling in exocrine cells. Cold Spring Harb Perspect Biol. 2019;12(5):a035279.
Liu H, Kuang X, Zhang Y, Ye Y, Li J, Liang L, et al. ADORA1 inhibition promotes tumor immune evasion by regulating the ATF3-PD-L1 axis. Cancer Cell. 2020;37:324–339.e328.
Acknowledgments
Not applicable.
Funding
This work was supported by the Shanghai Municipal Key Clinical Specialty and the National Natural Science Foundation of China (grant 81572367 and 81772556).
Author information
Authors and Affiliations
Contributions
Conceptualization, W.X.Y.; data analysis, S.D.J., W.Y.N., and Z.K.; reference acquisition, S.D.J and T.L.Y.; comments and suggestions, G.Q; administrative support, Z.J. and F.J.; manuscript drafting or revision, S.D.J., W.Y.N., and Z.K.; funding acquisition, W.X.Y. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
The expression of DCK in hepatocellular carcinoma versus normal tissues in the Oncomine database. Table S2. Correlation analysis between DCK and related genes and markers of immunes cells in GEPIA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, D., Wang, Y., Zhu, K. et al. DCK is a promising prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. World J Surg Onc 18, 176 (2020). https://doi.org/10.1186/s12957-020-01953-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-020-01953-1