Abstract
Background
The role of mast cells in malignancies remains unclear, and there is no clear correlation between mast cells and tumor microvessels, tumor growth, or lung adenocarcinoma (LUAD) prognosis. This study aims to explore the association between mast cell density (MCD) and intratumoral microvessel density (MVD), clinicopathological parameters, and prognosis in LUAD, by evaluating mast cell infiltration characteristics and their prognostic significance.
Methods
This retrospective investigation involved 238 patients with LUAD undergoing complete resection. Tumor and normal lung tissue sections outside the tumor were immunohistochemically stained for MCD in the intratumoral and outside regions, respectively. CD34 polyclonal antibody was used to measure intratumoral MVD.
Results
Intratumoral regions of LUAD had a higher MCD (P < 0.001) than normal lung tissue. In the intratumoral region, MCD and CD34-MVD were positively correlated (r = 0.411, P < 0.001). Intratumoral MCD correlated with sex, smoking history, tumor differentiation, pathological subtype, and tumor size. Female sex (P = 0.012), no smoking history (P = 0.002), acinar predominant type (P = 0.012), and tumor size ≤ 3 cm (P = 0.009) were associated with a higher MCD, whereas poorly differentiated (P = 0.039) and solid/micropapillary predominant types (P = 0.001) were associated with a lower MCD. Higher intratumoral MCD exhibited a marginally improved overall survival, and individuals with higher MCD infiltration ratios (intratumoral MCD/outside the MCD) had higher disease-free and overall survival rates (log-rank P < 0.001). A high MCD infiltration ratio was associated with decreased risk of tumor progression and death following complete resection.
Conclusion
The tumor microenvironment controls mast cell infiltration in LUAD, and patients with increased intratumoral mast cell infiltration have better prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lung cancer is a type of malignant tumor that has the highest rate of morbidity and mortality worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of cases of lung cancer [2]. Lung adenocarcinoma (LUAD) is the most common type of NCSLC and is the main pathological type responsible for lung cancer-related morbidity and mortality. Surgical resection is the best treatment for patients with early-stage LUAD. The longevity in patients with LUAD has improved, evidenced by the declining death rate in recent years. However, despite complete resection, many patients still have significant risks of death and recurrence [3].
Currently, TNM staging is useful for assessing patient prognosis and indicating the extent of tumor progression [4]. Additionally, the prognosis in patients is significantly affected by the various pathological subtypes of LUAD [5, 6]. Tumor stage and histological classification are used to determine the prognosis in patients with LUAD. Tumor growth, metastasis, and treatment are closely correlated with the number of stromal cells in the tumor microenvironment, including tumor-associated fibroblasts, immune cells, and endothelial cells [7], in particular, the considerable abundance of tumor-infiltrating immune cells [8]. Thus, investigating the efficient and targeted immune cells that infiltrate tumors is clinically valuable for assessing the prognosis of LUAD.
Although mast cells are vital components of the immune cell family, they are also involved in the onset and progression of malignancies [9]. An increased number of mast cells in lung cancer has been linked to tumor growth and poor patient prognosis [10,11,12,13]. Although the number of mast cells in the intratumoral zone is not correlated with the prognosis of NSCLC patients [14, 15], patients with a high number of mast cells in the peritumoral area have a lower probability of death by stage I NSCLC [14]. However, other studies have reported that a higher quantity of mast cells in NSCLC tumor tissues improves patient prognosis [16, 17]. Therefore, this disagreement between studies underscores the complex association between mast cells and NSCLC prognosis. Recently, mast cells have been shown to create angiogenic and lymphangiogenic chemicals, which may play a role in inflammation and tumor angiogenesis [18]. However, extensive research on this subject and conflicting findings regarding the association between mast cells and angiogenesis in NSCLC are lacking [19].
Both mast cell chymase and tryptase enzymes are considered as prominent markers of mast cells [20]. The classification of mast cells is based on the specific enzymes they possess: mast cells with tryptase exclusively, mast cells with both chymase and tryptase, and those with chymase exclusively [21]. Mast cells contain the highest concentration of tryptase [22], and their chymase content could be 10 times lower than their tryptase levels [23].
Only a few researchers have employed anti-chymase antibodies in their studies on mast cells and lung cancer. For example, Carlini et al. [14] and Shikotra et al. [17] reported that of 65 and 49 patients with NSCLC, those with LUAD were even fewer; neither of these studies included patients with LUAD examined in isolation. Nagata et al. [12] included patients only with small (tumor diameter ≤ 2.0 cm) LUAD. No studies have used anti-mast cell chymase antibody to stain LUAD specimens isolated from patients following full resection of stage I–III disease. Consequently, it is currently unclear how many chymase-labeled mast cells infiltrate patient tumor tissues after LUAD surgery, and what is the relationship between tumor microvessels, tumor development, and patient prognosis.
In this study, the anti-mast cell chymase antibody CC1 was utilized as a mast cell marker to evaluate mast cell density (MCD), and CD34 as a pan-vascular endothelial cell marker was used to analyze and measure microvessel density (MVD). Our findings may be useful for determining the features of mast cell infiltration and their prognostic value in LUAD.
2 Methods
2.1 Study subjects and enrollment conditions
We utilized data of patients with LUAD who underwent full resection at the Second Affiliated Hospital, Zhejiang University School of Medicine, between January 1, 2011, and December 30, 2015. Requirements for case admission eligibility were as follows: (1) a clear pathological diagnosis of LUAD and no history of other cancers post surgery; (2) stage I–III; (3) chemotherapy and radiation were not used as preoperative adjuvant therapy in any of the patients; (4) no history of allergic reactions, including asthma; and (5) the patient’s lung cancer tissue samples were formally collected by the pathology department and the tumor tissue was sectioned in paraffin blocks to produce tissue sections that were well preserved and had a thickness of 3–5 μm. Patients with distant metastases were excluded from the study.
A total of 238 patients (133 female and 105 male) aged 26–87 years (mean age, 60.48 years) were enrolled. Table 1 provides further information on clinicopathological features.
2.2 Obtaining clinicopathological data
Through the hospital information system, all patient clinicopathological data, including name, age, gender, smoking history, tumor location, size, degree of differentiation, pathological subtype, lymph node status, and TNM staging, were meticulously documented. A combination of phone consultations and hospital information system gathering was used to carry out the follow-up. Tumor progression (recurrence or metastasis), progression period, survival status, and time of death were also monitored. Disease-free survival (DFS) was calculated as the interval between the start of the resection procedure and tumor progression. Overall survival (OS) was calculated as the interval between LUAD diagnosis and death. Lung cancer staging was performed according to The Union for International Cancer Control (UICC), standard version 8. This study was approved by the Ethics Committee of the Second Affiliated Hospital of the Zhejiang University School of Medicine (Document batch number 2019-308). Informed consent was waived by our Ethics Committee because of the retrospective nature of our study. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
2.3 Immunohistochemical staining
Prior to antigen repair, paraffin slices were deparaffinized in xylene and 100%, 85%, and 75% ethanol. Citric acid antigen repair solution was then applied. The sections were additionally exposed to endogenous peroxidase blocking using a 3% hydrogen peroxide solution. Subsequently, the histochemical ring was blocked with 3% bovine serum albumin for 30 min at room temperature. The sections were incubated for 1 h at room temperature following the addition of the primary antibodies. Antibodies used were anti-mast cell chymase antibody CC1 (Abcam, ab2377, Cambridge, United Kingdom) prepared at a ratio of 1:200 with phosphate-buffered saline (PBS) and polyclonal antibody CD34 (Proteintech, 14486-1-ap, Wuhan, China) prepared at a ratio of 1:800 with PBS. Hypersensitive mouse and rabbit universal secondary antibodies (RecordBio, RC0080RM, Shanghai, China) were applied to the tissues and incubated for 30 min at room temperature. Freshly synthesized diaminobenzidine (diluted and concentrated solutions prepared at a ratio of 1000:50) was added to create the hue. The color development period was monitored under a microscope, and the resultant positive color was brown-yellow. The nuclei were counterstained with hematoxylin. The final sections were then dehydrated and sealed with neutral gum. A digital pathological slice scanner (KFBIO, KF-PRO-120, Ningbo, China) was used to obtain slice images for microscopic examination.
2.4 Interpretation of results
K-Viewer V1 (KFBIO, code 1.5.5.10, Ningbo, China) was used to read the slides. On hematoxylin staining, blue indicated the nucleus, and brown-yellow indicated the positive expression of diamine benzidine.
2.5 Calculating the MCD and MVD
The Weider correction method [24] was applied, and three “hot spots” containing the highest number of microvessels and mast cells were identified for each specimen at low magnification (× 10). The number of microvessels and mast cells in each location were counted at high magnification (× 40), and the average value was determined using the MVD and MCD values.
2.6 Statistic analysis
The statistical package SPSS 25.0 (IBM corporation, New York, USA) and R version 4.2.1 were used for data analysis. Rank correlation analysis was conducted to determine the relationship between MCD and MVD. A paired design t-test was used to compare the difference in MCD between intratumoral and normal areas. A t-test or F-test was used to analyze the correlation between MCD and clinicopathological characteristics. The Kaplan–Meier method was used to calculate survival rates, and the log-rank test was used to compare survival differences. Univariate and multivariate analyses were performed with the Cox hazards regression model to assess the prognostic factors. A P value of less than 0.05 was considered statistically significant.
3 Results
3.1 Infiltration of mast cells in LUAD
Both mast cells and CD34 microvessels were detected in LUAD (Fig. 1). In the intratumoral area, MCD ranged from 2 to 37 per high magnification (× 40) field of view, with an average of 13.29 ± 7.571. The MVD ranged from 8 to 38 per high magnification (× 40) field of view, with an average of 20.29 ± 6.352. A significant positive association was observed between MCD and MVD (Pearson's correlation coefficient r = 0.411, P < 0.001) (Fig. 2).
Expression of chymase-positive mast cells and CD34 microvessels in LUAD tissues. A The intratumoral mast cells are detected at low magnification of × 10. B and C The intratumoral mast cells are detected at high magnification of × 40, B shows a Low MCD group and C a High MCD group. D The intratumoral CD34-microvessels are detected at a high magnification of × 40. E The mast cells are detected in the normal lung tissue outside the tumor at high magnification of × 40. LUAD: lung adenocarcinoma; MCD: mast cell density; MVD: microvessel density
In the normal lung tissue outside the tumor, the MCD ranged from 1 to 6 per high-magnification (× 40) field of view, with an average of 2.49 ± 1.046. The MCD in the intratumoral area was significantly higher than that in the normal areas (difference value 10.807, 95%CI, 9.910–11.703, P < 0.001). The median infiltration ratio was 5.37 (4.00), which was determined by dividing intratumoral MCD by outside MCD.
3.2 Relevance of MCD to clinicopathological features
Age, sex, tumor location, smoking history, pathological subtype, degree of differentiation, tumor size, tumor stage, and other clinicopathological features were not associated with MCD in normal lung tissue that was outside the tumor (P ≥ 0.05).
Intratumoral MCD was higher in female patients (T = 2.536, P = 0.012), non-smokers (T = 3.210, P = 0.002), those with acinar predominant type (T = 2.546, P = 0.012), and those with tumor size ≤ 3 cm (T = 2.622, P = 0.009), but lower in those with solid/micropapillary predominant type (T = − 3.614, P = 0.001) and poorly differentiated tumors (F = 2.932, P = 0.039). Age, tumor site, and TNM stage did not significantly alter MCD (P < 0.05) (Table 1).
3.3 Survival analysis
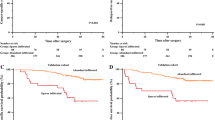
The mean value of intratumoral MCD was used as the boundary, we classified those with MCD ≤ 13 into the low MCD group and those with MCD > 13 into the high MCD group. Patients in the low MCD group had a 5-year DFS rate of 45.5%, whereas those in the high MCD group had a rate of 47.2%. Figure 3A shows no significant difference in DFS between the two groups (log-rank P = 0.726).
Survival analysis. A DFS curves in patients with different intratumoral MCD levels (Log rank P = 0.726). B OS curves in patients with different intratumoral MCD levels (Log rank P = 0.115). C DFS curves in patients with different MCD infiltration ratios (Log rank P < 0.001). D OS curves in patients with different MCD infiltration ratios (Log rank P < 0.001). DFS, disease-free survival; MCD: mast cell density; OS, overall survival
The low- and high-MCD groups had 5-year OS rates of 69.1% and 77.5%, respectively. Patients in the high MCD group had a higher OS rate than those in the low MCD group (Fig. 3B), although the difference between the two groups was not statistically significant (log-rank P = 0.115).
The median infiltration ratio served as the boundary for grouping the patients into high- and low-infiltration categories. Patients in the high infiltration group had higher disease-free and overall survival rates. Figure 3C and D show significant differences between the two groups (log-rank P < 0.001).
3.4 Univariate and multivariate analysis of DFS and OS
We ran a univariate analysis of DFS and OS for every variable related to clinicopathological characteristics. Survival was correlated with tumor differentiation, TNM stage, lymph node metastasis, MCD invasion rate, and disease progression (P < 0.05). The above variables were included to construct a multivariate Cox proportional hazards model was constructed for disease progression and death in patients with LUAD following complete resection. A high infiltration ratio of MCD decreased the risk of tumor progression and death in patients with LUAD after complete resection (P < 0.001). The details are shown in Table 2.
4 Discussion
Because tumors have mast cell infiltration, the connection between mast cells and cancers has received considerable attention. In this study, we evaluated mast cell and CD34 microvessel densities in tumor samples from 238 patients with stage I–III LUAD following complete surgical resection, using an immunohistochemical approach. We concentrated on the two protagonists, LUAD and chymase-positive mast cells, as they had not been the subject of previous studies.
Mast cells have been identified in LUAD tumor tissues, with MCD being used as a discerning metric for mast cell infiltration. The fact that mast cells may promote tumor angiogenesis is supported by the positive correlation between MCD and CD34-MVD, which aligns with findings in other studies on mast cells and angiogenesis in lung cancer [10, 12,13,14, 25]. Angiotensin I can be activated and converted to angiotensin II, which in turn has the capacity to activate vascular growth factor-1 in malignant tumors by chymase. Additionally, chymase can break down matrix metalloproteinase-9 and indirectly promote angiogenesis [23, 26]. Nevertheless, lung cancer tumor tissues possess both differentiated (CD34 +) and undifferentiated (CD31 + /CD34-) microvessels [27, 28]. Furthermore, MVD, as indicated by CD34, is linked to necrosis and hypoxia [29], suggesting that CD34 is a more suitable marker for mature microvessels. Our results indicated that mature microvessels control mast cell infiltration into tumors via hypoxia in LUAD.
Mast cell chymase is more destructive than tryptase and induces the activation of matrix metalloproteinases, which in turn stimulates the breakdown of the extracellular matrix and aids in tumor invasion and metastasis [21, 23]. This biological action of chymase also adequately explains one of the results of our study: intratumoral MCD was higher in the tumor size ≤ 3 cm group than in the > 3 cm group. In the early stages of tumor formation, mast cells accumulate in the tumor microenvironment. As the tumor grows, mast cells degranulate and release chymase granules into the extracellular space, decreasing the number of mast cells. Chymase plays a role in extracellular matrix degradation to facilitate its penetration by the tumor cells during invasion [23, 30].
Furthermore, we discovered that sex, smoking history, and pathological subtype of LUAD were associated with the level of intratumoral MCD. Estrogen can stimulate an increase in mast cells in the endometrium and cause endometriosis [31]. The higher amount of mast cell infiltration observed in female patients with LUAD may be related to the higher level of estrogen. Patients with a smoking history had reduced intratumoral MCD, suggesting that smoking may diminish the degree of mast cell infiltration in LUAD tumors, possibly because cigarette smoke promotes mast cell degranulation [32]. Different pathological subtypes of LUAD have different intratumoral MCDs. Compared to patients with acinar- and lepidic-predominant adenocarcinomas, those with solid- and micropapillary-predominant adenocarcinomas had a lower degree of mast cell infiltration. Numerous LUAD pathological subtypes correspond to distinct tumor growth patterns. These findings imply that the degree of mast cell infiltration within the tumor may be influenced by variations in the LUAD tumor microenvironment. This distinction encompasses both the internal environmental aspects of the tumor (MVD, tumor size, growth pattern, etc.) and the external environmental factors of the tumor (hormone levels, smoking, etc.).
The results of previous studies evaluating MCD and lung cancer prognosis have differed. Those who used tryptase to identify intratumoral mast cells in lung cancer revealed that patients with high MCD in LUAD had a shorter OS [10]. Imada et al. [13] also revealed that the prognosis of the high mast cell count group was worse in stage I LUAD but not in lung squamous cell carcinoma. Nagata et al. [12] demonstrated the number of tryptase-positive mast cells did not significantly affect postoperative recurrence or survival rates in small-sized LUAD. However, the group with a high number of chymase-positive mast cells experienced worse outcomes than that with a low number of chymase-positive mast cells. In contrast, some studies have found that the prognosis in patients with NSCLC is unrelated to the level of MCD [14, 15], but only in stage I patients; those with a high chymase-positive MCD in the peritumoral area have a decreased mortality risk [14]. Mast cell counts in tumor islets are higher in long-lived patients with NSCLC than in short-lived patients [17]. However, Welsh et al. [16] did not find a connection between mast cell counts in the tumor stroma and survival; however, they showed that the presence of tryptase-positive mast cells in the tumor islets was an independent indication of a favorable prognosis.
Our study observed no correlation between the level of intratumoral MCD in LUAD and patient prognosis, probably because we only counted chymase-positive MCD and did not count MCD in the tumor islets and stroma separately. However, we found that patients with high intratumoral MCD tended to have a better OS rate than those with low intratumoral MCD. Consequently, the different roles played by mast cells in lung cancer may be related to the mast cell phenotype, mast cell micro-position, tumor type, and tumor stage.
Furthermore, we first assessed the prognosis in each patient using the intratumoral/outside MCD infiltration ratio. The findings indicated that patients with a high infiltration ratio had better DFS and OS and that a high infiltration ratio decreased the risk of tumor progression and death in patients with LUAD following complete resection. In addition to mast cells, Evidence suggests that other immune cells infiltrate at different sites in the tumor microenvironment. For example,the distribution distance of T cells close to tumor cells in esophageal cancer [33], the accumulation of intratumoral T cells in clear cell renal-cell carcinoma [34],the high degree of B cell infiltration around the gastric carcinoma [35],the distribution of T cells, B cells and macrophages in different regions of the tumor in triple-negative breast cancer [36],all of them are related to the therapeutic effect and prognosis of tumors. This novel finding of our study regarding differences in spatial distribution may shed new light on the controversial topic of the relationship between mast cells and the prognosis of cancer patients.
Targeting mast cells or their mediators is a potential strategy in cancer therapy. For example, cromolyn sodium, a mast cell stabilizer, can dramatically reduce the growth of thyroid cancer [37]. Medications that target tryptase function, such as tranilast, namostat mesylate, and gabexate mesylate, have antitumor benefits [38]. Mast cell chymase may be a good option for treating lung cancer because low doses of chymase can stimulate the growth of lung cancer cells, whereas high doses have the opposite effect [39].
Our study has some limitations. First, because this was a retrospective study, recollection bias may have affected the data. Second, the “hot spot” approach we used does not accurately represent the overall state of the tumor because it only counts the MCD and MVD in the regions of the tumor with the highest level of expression of the respective markers. Third, our study utilized a small sample size. If more patients were included to increase the sample size and assess whether intratumoral MCD could be utilized as an indicator of patient survival, the results may have differed statistically. Fourth, our findings offer a starting point for the identification of prognostic markers in patients in clinical practice. Multi-center studies with larger sample sizes are required if more promotion is required, and procedures such as counting, interpreting, and slide-reading software should be standardized.
In conclusion, the current study demonstrates that MCD is a quantitative measure of mast cell infiltration, and that intratumoral mast cell infiltration is significantly influenced by variations in the tumor microenvironment of LUADs. Patient prognosis was improved with increasing intratumoral mast cell infiltration levels. In patients with LUAD, the intratumoral or external MCD infiltration ratio can be used as an independent risk factor for postoperative recurrence and death.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to some reasons but are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377(9):849–61.
Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: a clinicopathologic study based on the new international association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6(9):1496–504.
Ujiie H, Kadota K, Chaft JE, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015;33(26):2877–84.
Li Z, Zheng Z, Li C, et al. Therapeutic drugs and drug delivery systems targeting stromal cells for cancer therapy: a review. J Drug Target. 2020;28(7–8):714–26.
Pan Y, Sha Y, Wang H, et al. Comprehensive analysis of the association between tumor-infiltrating immune cells and the prognosis of lung adenocarcinoma. J Cancer Res Ther. 2020;16(2):320–6.
Varricchi G, Rossi FW, Galdiero MR, et al. Physiological roles of mast cells: collegium internationale allergologicum update 2019. Int Arch Allergy Immunol. 2019;179(4):247–61.
Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88(12):2686–92.
Ullah E, Nagi AH, Lail RA. Angiogenesis and mast cell density in invasive pulmonary adenocarcinoma. J Cancer Res Ther. 2012;8(4):537–41.
Nagata M, Shijubo N, Walls AF, et al. Chymase-positive mast cells in small sized adenocarcinoma of the lung. Virchows Arch. 2003;443(4):565–73.
Imada A, Shijubo N, Kojima H, et al. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir J. 2000;15(6):1087–93.
Carlini MJ, Dalurzo MC, Lastiri JM, et al. Mast cell phenotypes and microvessels in non-small cell lung cancer and its prognostic significance. Hum Pathol. 2010;41(5):697–705.
Niczyporuk M, Hermanowicz A, Matuszczak E, et al. A lack of correlation between mast cells, angiogenesis, and outcome in non-small cell lung cancer. Exp Lung Res. 2012;38(6):281–5.
Welsh TJ, Green RH, Richardson D, et al. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23(35):8959–67.
Shikotra A, Ohri CM, Green RH, et al. Mast cell phenotype, TNFα expression and degranulation status in non-small cell lung cancer. Sci Rep. 2016;6:38352.
Marone G, Varricchi G, Loffredo S, et al. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol. 2016;778:146–51.
Longo V, Catino A, Montrone M, et al. Controversial role of mast cells in NSCLC tumor progression and angiogenesis. Thorac Cancer. 2022;13(21):2929–34.
Elieh Ali Komi D, Wöhrl S, Bielory L. Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol. 2020;58(3):342–65.
Saxena S, Singh A, Singh P. Tumor associated mast cells: biological roles and therapeutic applications. Anat Cell Biol. 2020;53(3):245–51.
Hallgren J, Pejler G. Biology of mast cell tryptase. An inflammatory mediator Febs J. 2006;273(9):1871–95.
Atiakshin D, Buchwalow I, Tiemann M. Mast cell chymase: morphofunctional characteristics[J]. Histochem Cell Biol. 2019;152(4):253–69.
Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84(24):1875–87.
Ibaraki T, Muramatsu M, Takai S, et al. The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2005;28(4):617–21.
De Souza JDA, Santana AC, Da Silva EZ, et al. The Role of Mast Cell Specific Chymases and Tryptases in Tumor Angiogenesis[J]. Biomed Res Int. 2015;2015:142359.
Zhao YY, Xue C, Jiang W, et al. Predictive value of intratumoral microvascular density in patients with advanced non-small cell lung cancer receiving chemotherapy plus bevacizumab[J]. J Thorac Oncol. 2012;7(1):71–5.
Liu D, Ding G. Predictive value of microvascular density for response to anlotinib in advanced NSCLC. Medicine (Baltimore). 2022;101(3):e28647.
Pomme G, Augustin F, Fiegl M, et al. Detailed assessment of microvasculature markers in non-small cell lung cancer reveals potentially clinically relevant characteristics[J]. Virchows Arch. 2015;467(1):55–66.
Dalton DK, Noelle RJ. The roles of mast cells in anticancer immunity. Cancer Immunol Immunother. 2012;61(9):1511–20.
Mccallion A, Nasirzadeh Y, Lingegowda H, et al. Estrogen mediates inflammatory role of mast cells in endometriosis pathophysiology. Front Immunol. 2022;13:961599.
Vasconcelos TB, Araújo FY, Pinho JP, et al. Effects of passive inhalation of cigarette smoke on structural and functional parameters in the respiratory system of guinea pigs. J Bras Pneumol. 2016;42(5):333–40.
Yan C, Huang H, Zheng Z, et al. Spatial distribution of tumor-infiltrating T cells indicated immune response status under chemoradiotherapy plus PD-1 blockade in esophageal cancer. Front Immunol. 2023;14:1138054.
Qi Y, Xia Y, Lin Z, et al. Tumor-infiltrating CD39(+)CD8(+) T cells determine poor prognosis and immune evasion in clear cell renal cell carcinoma patients. Cancer Immunol Immunother. 2020;69(8):1565–76.
Liu H, Wu J, Xu X, et al. Peritumoral TIGIT(+)CD20(+) B cell infiltration indicates poor prognosis but favorable adjuvant chemotherapeutic response in gastric cancer. Int Immunopharmacol. 2022;108:108735.
Ren X, Song Y, Pang J, et al. Prognostic value of various immune cells and Immunoscore in triple-negative breast cancer. Front Immunol. 2023;14:1137561.
Melillo RM, Guarino V, Avilla E, et al. Mast cells have a protumorigenic role in human thyroid cancer[J]. Oncogene. 2010;29(47):6203–15.
Ammendola M, Leporini C, Marech I, et al. Targeting mast cells tryptase in tumor microenvironment: a potential antiangiogenetic strategy. Biomed Res Int. 2014;2014:154702.
Jiang Y, Wu Y, Hardie WJ, et al. Mast cell chymase affects the proliferation and metastasis of lung carcinoma cells in vitro. Oncol Lett. 2017;14(3):3193–8.
Acknowledgements
We acknowledge and express our deepest gratitude to all participants of this study. We also thank Editage (www.editage.cn) for English language editing.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Study design: Pingli Wang. Data collection: Zijian Qiu, Guanchao Pang, Xia XU and Jun Lin. Data analysis: Zijian Qiu and Guanchao Pang. Draft writing: Zijian Qiu. Final revision: Pingli Wang. All the authors have read and approved the final version of the manuscript and agreed with the order of presentation of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, Z., Pang, G., Xu, X. et al. Characteristics of mast cell infiltration in lung adenocarcinoma and its impact on prognosis. Discov Onc 15, 208 (2024). https://doi.org/10.1007/s12672-024-01062-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01062-5