Abstract
Background
The global BOLERO-2 trial established the efficacy and safety of combination everolimus (EVE) and exemestane (EXE) in the treatment of estrogen receptor positive (ER +), HER2-, advanced breast cancer (ABC). BOLERO-5 investigated this combination in a Chinese population (NCT03312738).
Methods
BOLERO-5 is a randomized, double-blind, multicenter, placebo controlled, phase II trial comparing EVE (10 mg/day) or placebo (PBO) in combination with EXE (25 mg/day). The primary endpoint was progression-free survival (PFS) per investigator assessment. Secondary endpoints included PFS per blinded independent review committee (BIRC), overall survival (OS), overall response rate (ORR), clinical benefit rate (CBR), pharmacokinetics, and safety.
Results
A total of 159 patients were randomized to EVE + EXE (n = 80) or PBO + EXE (n = 79). By investigator assessment, treatment with EVE + EXE prolonged median PFS by 5.4 months (HR 0.52; 90% CI 0.38, 0.71), from 2.0 months (PBO + EXE; 90% CI 1.9, 3.6) to 7.4 months (EVE + EXE; 90% CI 5.5, 9.0). Similar results were observed following assessment by BIRC, with median PFS prolonged by 4.3 months. Treatment with EVE + EXE was also associated with improvements in ORR and CBR. No new safety signals were identified in BOLERO-5, with the incidence of adverse events in Chinese patients consistent with the safety profile of both drugs.
Conclusion
The efficacy and safety results of BOLERO-5 validate the findings from BOLERO-2, and further support the use of EVE + EXE in Chinese post-menopausal women with ER + , HER2- ABC. NCT03312738, registered 18 October 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer is the most common malignancy in women and one of the leading causes of cancer deaths worldwide [1]. The incidence of breast cancer in China has increased rapidly over the last number of decades, and currently increases by 3–5% annually [2, 3]. Despite evidence suggesting that China demonstrates a lower mortality rate compared to other countries, the size of the population and the current increase in breast cancer incidence reinforce the need for safe and effective treatment options [2].
Approximately 70% of breast cancers are estrogen receptor positive (ER +) and are treated with endocrine therapy (such as nonsteroidal aromatase inhibitors [NSAIs, anastrozole or letrozole] or steroidal aromatase inhibitors [exemestane, EXE]) in combination with CDK4/6 inhibitors in the first-line setting [4,5,6]. Although these treatments are effective, both de novo and acquired resistance are common, with the majority of patients receiving endocrine therapy developing progressive disease [7, 8]. Many patients retain sensitivity to hormonal agents, even upon disease progression, but sequential endocrine monotherapy achieves only moderate clinical benefits [8, 9]. As such, combination approaches with mammalian target of rapamycin (mTOR) inhibitors have been developed as a second-line treatment option [8, 10]. The PI3K/Akt/mTOR pathway plays a central role in breast cancer cell proliferation and progression [11, 12], providing a strong rationale for combining endocrine therapy with mTOR inhibition [13]. The PI3K/Akt/mTOR pathway is also implicated in the development of resistance to endocrine therapy, further supporting the rationale for mTOR inhibition in the treatment of ER + breast cancer [14, 15]. Everolimus (EVE), an mTOR inhibitor, has demonstrated efficacy in enhancing the effects of endocrine therapy in both pre-clinical models and clinical trials in breast cancer [12, 16, 17].
The global, pivotal, phase III Breast Cancer Trials of OraL EveROlimus-2 (BOLERO-2) trial investigated a combination strategy of EVE and EXE for the treatment of postmenopausal women with locally advanced/metastatic, hormone receptor positive (HR +) disease progressing after anastrozole or letrozole [18]. This study established the efficacy and safety of the EVE and EXE treatment combination, with significantly improved progression-free survival (PFS) observed in patients treated with EVE + EXE versus placebo (PBO) + EXE, and no serious toxicity reported [14, 18]. By investigator assessment, treatment with EVE + EXE prolonged PFS from 3.2 months to 7.8 months (HR, 0.45; 95% CI 0.38, 0.54; p < 0.0001) [18]. Similar results were observed following central assessment, with PFS prolonged from 4.1 months to 11.0 months (HR, 0.38; 95% CI 0.31, 0.48; p < 0.0001) [18].
The results of the BOLERO-2 trial led the FDA and EMA to approve combination EVE + EXE for the treatment of HR + , HER-2- advanced breast cancer (ABC) in 2012. This treatment combination is also recommended by both the National Comprehensive Cancer Network (NCCN) and the ESO-ESMO (European School of Oncology—European Society for Medical Oncology) guidelines for postmenopausal women with HR + , HER2- ABC with progressive disease on NSAIs [4, 5].
Ethnicity is well-known to impact treatment efficacy and safety, supporting the opinion that potential inter-ethnic differences in anti-cancer drug effect should be considered [19, 20]. A post-hoc analysis of the BOLERO-2 study was therefore conducted to assess the combination of EVE + EXE in Asian versus non-Asian patients [21]. The results of these analyses were consistent with the primary results of BOLERO-2, which demonstrated improved PFS with EVE + EXE in both Asian and non-Asian patients, with no significant differences in the incidence of most adverse events (AEs) observed between Asian and non-Asian patients. However, some AEs such as stomatitis, nasopharyngitis, pneumonitis, and interstitial lung disease were reported more frequently in Asian patients [21]. Combination EVE + EXE provided substantial clinical benefit in both an Asian and non-Asian patient population, representing an improvement in the management of postmenopausal women with HR + , HER2- ABC progressing on NSAIs, regardless of ethnicity [18, 21].
The China-specific BOLERO-5 study aimed to confirm the efficacy and safety of combination treatment with EVE + EXE (seen in BOLERO-2) in postmenopausal Chinese women with locally advanced/metastatic, ER + , HER2- disease following recurrence or progression on anastrozole or letrozole.
2 Methods
2.1 Study design and participants
BOLERO-5 is a double-blind, randomized, phase II study evaluating combination EVE + EXE in a Chinese population of postmenopausal women, and was conducted at 15 clinical sites across China. Patients were randomized 1:1 to receive either EVE + EXE (10 mg/day and 25 mg/day, respectively) or PBO + EXE (25 mg/day) as oral tablets (Fig. 1). EVE and placebo were identical in packaging, labeling, schedule of administration and in appearance. Patients were registered into Interactive Response Technology (IRT), which assigned a randomization number to the patient used to link the patient to a treatment arm. Randomization was stratified by the presence of visceral disease (lung, liver, brain, pleural and peritoneal involvement) and sensitivity to prior hormonal therapy. Sensitivity to prior hormonal therapy was defined as either A) documented clinical benefit (complete response, partial response, stable disease ≥ 24 weeks) to at least one prior hormonal therapy in the advanced setting, or B) ≥ 24 months of adjuvant hormonal therapy prior to recurrence.
This study (ClinicalTrials.gov: NCT03312738) was conducted in accordance with the Declaration of Helsinki and the ICH E6 Guidelines for Good Clinical Practice. The study protocol and all amendments were approved by the Independent Ethics Committee or Institutional Review Board of each investigative site. Written informed consent was obtained from each patient before any study-specific procedure was performed. The study was designed and sponsored by Novartis Pharmaceuticals, who collected and analyzed the data in conjunction with the authors.
Postmenopausal women (≥18 years) with histological/cytological confirmation of ER + , HER2- breast cancer and recurrence/progression on prior NSAI (letrozole or anastrozole) were included is this study. These were defined as recurrence while on or within one year of end of adjuvant NSAI treatment, or progression while on or within one month of end of prior NSAI treatment in the advanced or metastatic setting. Key exclusion criteria included HER2-overexpression, previous treatment with more than one line of chemotherapy for ABC, prior treatment with EXE, mTOR inhibitors, PI3K inhibitors, or AKT inhibitors, and known hypersensitivity to mTOR inhibitors.
Treatment continued until disease progression (assessed by Response Evaluation Criteria In Solid Tumors [RECIST] 1.1), unacceptable toxicity, death or treatment discontinuation for any other reason. Patients were permitted to withdraw from the study at any time. Everolimus dose adjustments were also permitted to allow patients to continue study treatment. Only two dose reductions were permitted per patient. Patients with therapy interruptions for more than four weeks were permanently discontinued from the study. To mitigate the increased risk of stomatitis associated with EVE, all patients were advised to practice good oral hygiene and were recommended to use a preventive dexamethasone mouthwash.
2.2 Endpoints
The primary endpoint of the study was PFS by local assessment (per RECIST 1.1 criteria), with PFS per blinded independent review committee (BIRC) as a secondary endpoint. PFS was defined as time from the date of randomization to the date of first documented progression or death due to any cause, whichever occurred first. Overall survival (OS), overall response rate (ORR), clinical benefit rate (CBR), time to response, and duration of response (DOR) were all secondary endpoints, and were assessed both locally and by BIRC. Secondary endpoints also included time to deterioration in Eastern Cooperative Oncology Group (ECOG) performance status, AEs, and laboratory abnormalities. The pharmacokinetics of combination EVE + EXE were also assessed.
2.3 Study assessments
Tumor evaluation (based on computed tomography or magnetic resonance imaging) was carried out at baseline (within 21 days of treatment initiation) and every 8 weeks (± 7 days) post-randomization until radiological progression, per local assessment. Objective tumor response and disease progression were assessed per RECIST version 1.1. All patients were followed for survival at least every three months post-randomized treatment or post-treatment follow-up, unless they discontinued due to death, withdrawal of consent or were lost to follow-up.
Safety assessments included AEs, serious AEs (SAEs), laboratory analyses (hematology, serum chemistry, coagulation), vital signs and physical assessments, and were carried out within 7 days of tumor assessment. Information on patient deaths was also collected. All patients were followed-up for safety up to 30 days following the last dose of treatment. AEs were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v4.03. An AE was defined as the appearance of (or worsening of any pre-existing) undesirable sign(s), symptom(s), or medical condition(s) that occur after patient’s signed informed consent has been obtained.
2.4 Statistical analysis
The primary analysis was planned when approximately 110 PFS events were documented, based on local assessment. As this is a bridging study, an estimation strategy was used over formal hypothesis testing to estimate sample size. Approximately 160 patients were estimated as being needed for randomization to the two treatment arms to observe the 110 PFS events at approximately 22 months post-randomization of the first study patient.
PFS was estimated by Kaplan–Meier analysis using the intention-to-treat (ITT) principle, with median PFS and 90% confidence interval (CI) presented by treatment arm. A Cox regression model (stratified by randomization stratification factors) was used to estimate the hazard ratio (HR) and associated 90% CI for PFS, OS, ORR, and CBR. No hypothesis testing was performed as this was an estimation-based approach. Baseline demographic and disease characteristics data were listed and summarized descriptively by treatment arm. Duration of study treatment exposure, dose intensity, and safety were also summarized by treatment.
2.5 Role of the funding source
Novartis Pharmaceuticals Corporation sponsored the study, designed the study and analyzed the data. All authors had full access to the study data and had the final responsibility for the decision to submit this manuscript for publication. The corresponding author had access to all study data and had final responsibility to submit this manuscript for publication.
3 Results
3.1 Patients
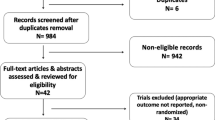
A total of 159 postmenopausal women were enrolled across multiple centers in China between 15 September 2017 and 31 March 2020 and were randomized to receive either EVE + EXE (n = 80) or PBO + EXE (n = 79) (Fig. 2). Baseline demographic characteristics were well matched between treatment arms (Table 1); median age (range) at baseline was 56.0 years (36–81) and most patients had an ECOG performance score of 1 (EVE + EXE, 61.3%; PBO + EXE, 68.4%). Baseline disease characteristics were also similar between arms, with most patients initiating study treatment within 3 months of last disease progression (EVE + EXE, 91.3%; PBO + EXE, 97.5%). All patients had stage IV disease at study entry and baseline tumor burden was similar between treatment arms. Overall, 71.1% of patients had visceral involvement and 44.7% of patients had bone metastases; most patients had metastases in other sites besides the central nervous system (CNS), bone, lung, liver, and visceral sites (EVE + EXE, 75.0%; PBO + EXE, 81.0%) (Table 1).
All patients were heavily pre-treated, with most patients receiving > 3 therapies prior to enrollment (EVE + EXE, 91.3%; PBO + EXE, 91.1%). As required by the study protocol, all patients were treated with at least one NSAI regimen prior to study entry. Other common prior therapies included tamoxifen (EVE + EXE, 32.5%; PBO + EXE, 20.3%) and fulvestrant (EVE + EXE, 15.0%; PBO + EXE, 16.5%). For the majority of patients, the most recent prior therapy was received in the metastatic setting (EVE + EXE, 57.5%; PBO + EXE, 46.8%) and 30.2% of patients had received chemotherapy in a metastatic setting (EVE + EXE, 36.3%; PBO + EXE, 24.1%).
A total of 116 PFS events occurred by the data cutoff of 19 May 2020, with a median follow-up of 14.5 months. Most patients (n = 138; 86.8%) had discontinued randomized treatment by data cutoff, with disease progression the primary reason for discontinuation (EVE + EXE, 55.0%; PBO + EXE, 79.7%). At data cutoff, median duration (range) of EVE therapy in the EVE + EXE arm was 16.1 weeks (2.4‒95.0), while median duration (range) of EXE therapy was 17.1 weeks (range: 2.4–95.1). For the PBO + EXE arm, median duration of EXE therapy was 8.4 weeks (range: 2.1–96.1); the same median duration and range were observed for PBO therapy. Both treatment arms achieved a median relative dose intensity of 100%, with a median dose intensity of 10 mg/day for PBO and EVE, and 25 mg/day for EXE. Mean (SD) dose intensity in the EVE + EXE arm was 9.1 mg/day (1.45) for EXE and 25.0 mg/day (0.00) for EVE, whereas in the PBO + EXE arm it was 10.0 mg/day (0.14) for PBO and 25.0 mg/day (0.00) for EXE. In the EVE + EXE arm, 23 patients (28.8%) were exposed to EVE and 28 patients (35.0%) were exposed to EXE for a period of ≥ 32 weeks; in the PBO + EXE arm, just 13 patients (16.5%) received treatment for ≥ 32 weeks.
Most patients (62.5%) in the EXE + EVE arm had at least one dose adjustment (reduction and/or temporary interruption) of EVE, while 17.7% of patients in the PBO + EXE arm had at least one dose adjustment of PBO. At least one EVE dose reduction was required in 32.5% of patients in the EVE + EXE arm compared to 2.5% of patients with at least one reduction of PBO in the PBO + EXE arm. At least one dose interruption in EVE was required in 56.3% of patients in the EVE + EXE arm versus 17.7% of patients with at least one interruption in PBO in the PBO + EXE arm. In the EVE + EXE arm, the primary reason for EVE dose adjustments or interruptions was AEs (responsible for 21.3% of reductions and 47.5% of interruptions). More patients had at least one dose interruption of EXE in the EVE + EXE arm (40.0%) vs the PBO + EXE arm (16.5%); no patient in either arm required an EXE dose reduction.
3.2 Progression-free survival
By investigator assessment, treatment with EVE + EXE provided a clinically meaningful PFS benefit. Treatment with EVE + EXE reduced the risk of disease progression or death by 48% (HR 0.52; 90% CI, 0.38, 0.71), and prolonged median PFS by 5.4 months (from 2.0 months with PBO + EXE [90% CI 1.9, 3.6] to 7.4 months with EVE + EXE [90% CI 5.5, 9.0]) (Fig. 3A). Similar results were observed following BIRC assessment; treatment with EVE + EXE reduced the risk of disease progression or death by 54% (HR 0.46; 90% CI 0.32, 0.67) and prolonged median PFS by 4.3 months (from 3.1 months with PBO + EXE [90% CI 1.9, 3.7] to 7.4 months with EVE + EXE [90% CI 5.5, 9.3]) (Fig. 3B). The PFS rate at 12 months was 25.5% in the EVE + EXE arm versus 7.6% in the PBO + EXE arm per investigator assessment.
3.3 Overall survival
The OS analysis was considered immature at data cutoff due to the small number of deaths reported (EVE + EXE, n = 22, 27.5%; PBO + EXE, n = 21, 26, 6%).
3.4 Overall response rate and clinical benefit rate
ORR (complete response + partial response) as assessed by the investigator and based on RECIST 1.1 criteria was 8.8% (90% CI 4.2, 15.8) in patients receiving EVE + EXE versus 1.3% (90% CI 0.1, 5.9) in those receiving PBO + EXE (Table 2). CBR (complete response + partial response + stable disease ≥ 24 weeks + non-complete response/non-progressive disease) per investigator assessment was higher in patients treated with EVE + EXE (35.0%; 90% CI 26.1, 44.7) than in those treated with PBO + EXE (16.5%, 90% CI 10.0, 24.9) (Table 2). Similar results were observed following BIRC assessment of ORR and CBR (Table 2). Progressive disease was the best overall response for 16.3% of patients receiving EVE + EXE and 49.4% of patients receiving PBO + EXE.
3.5 Time to response, duration of response and ECOG performance status
Analyses of the Kaplan–Meier estimate of the time to response were not performed due to the low number of responses in both arms (8 partial responses per investigator assessment; one complete response and 8 partial responses per BIRC assessment). Time to response and duration of response data are shown in Table S1. Deterioration in ECOG performance status by ≥ 1 point was noted in few patients (8/80) in the EVE + EXE arm and in no patients in the PBO + EXE arm (Figure S1).
3.6 Safety
The majority of patients reported at least one AE during the study (EVE + EXE, 98.8%; PBO + EXE, 84.8%), many of which were Grade 1 or 2 in severity (EVE + EXE, 46.2%; PBO + EXE, 70.9%). The most common AEs associated with EVE + EXE included increased aspartate aminotransferase (45%), hyperglycemia (43.8%), stomatitis (33.8%), increased alanine aminotransferase (31.3%) and anemia (31.3%). Reported rates of grade ≥ 3 AEs were higher overall in the EVE + EXE arm versus the PBO + EXE arm (53.8% versus 29.1%). The incidence of treatment-related adverse events (TRAEs) in the BOLERO-5 study was consistent with the known safety profile of both drugs, with no new safety signals identified in Chinese patients (Table 3). Most TRAEs were Grade 1 or Grade 2, but the incidence of Grade 3 TRAEs was higher in the EVE + EXE arm (45.0%) compared to the PBO + EXE arm (11.4%). The most common Grade ≥ 3 TRAEs in the EVE + EXE arm versus the PBO + EXE arm were hyperglycemia (10.0% versus 0%), hypokalemia (6.3% versus 0%), hypophosphatemia (6.3% versus 0%), stomatitis (7.5% versus 1.3%) and pneumonia (5% versus 0).
The most common adverse events of special interest (AESIs) all occurred more frequently in the EVE + EXE arm (Table S2). These included stomatitis (reported in 73.8% of patients in the EVE + EXE arm versus 11.4% of patients in the PBO + EXE arm), cytopenia (53.8% versus 24.1%), hyperglycemia/new onset diabetes mellitus (51.3% versus 8.9%), dyslipidemia (47.5% versus 7.6%), and severe infections (45.0% versus 17.7%). Non-infectious pneumonitis, frequently associated with mTOR inhibitors, was reported in 19 patients (23.8%) randomized to EVE + EXE and all cases were considered to be treatment-related. The majority of cases were mild in nature, with only 1 case (1.3%) of Grade ≥ 3 non-infectious pneumonitis reported. No patients in the PBO + EXE arm experienced non-infectious pneumonitis.
No unexpected findings were reported in relation to clinical laboratory or vital signs. Hematological abnormalities were more commonly observed in the EVE + EXE arm than in the PBO + EXE arm. Decreased hemoglobin was the most common abnormality (EVE + EXE, 57.5%; PBO + EXE, 24.1%), followed by decreased absolute lymphocyte count (EVE + EXE, 45.0%; PBO + EXE, 22.8%). Clinical abnormalities were also more frequent in patients treated with EVE + EXE versus PBO + EXE, and included increased triglycerides (63.8% versus 25.3%) and increased cholesterol (83.8% versus 29.1%).
The incidence of AEs leading to permanent study discontinuation was low in both treatment arms (EVE + EXE, 11.3%; PBO + EXE, 3.8%). The most frequent AEs resulting in discontinuation were pneumonia (n = 3 in the EVE + EXE arm) and bilirubin increase (n = 2 in the EVE + EXE arm). Serious adverse events (SAEs) were reported more frequently in the EVE + EXE arm vs the PBO + EXE arm (20% versus 12.7% reporting ≥ one SAE). The most common SAEs in the EVE + EXE arm were pneumonia (7.5%) and interstitial lung disease (2.5%). A total of five deaths occurred during the study treatment (EVE + EXE, n = 3; PBO + EXE, n = 2), all of which were attributed to the underlying malignancy.
4 Discussion
The BOLERO-5 study provides strong evidence for the efficacy and safety of EVE + EXE in Chinese post-menopausal women with ER + , HER2-, locally advanced, recurrent, or metastatic breast cancer following recurrence or progression on prior letrozole or anastrozole. The global BOLERO-2 study established the efficacy and safety of combination of EVE + EXE [14, 18] and led to the approval of EVE for the treatment of post-menopausal women with HR + , HER2- ABC. The Asian sub-population analysis of BOLERO-2 (N = 143), as well as the recent Phase IIIB EVEREXES trial of this treatment combination in Asian patients (N = 199), support the use of combination treatment with EXE + EVE to improve PFS without compromising quality of life (QoL) [18, 21,22,23], representing a significant advancement in the management of ABC. Following the publication of the BOLERO-2 results, several international guidelines (including the NCCN and ESO-ESMO ABC5 treatment guidelines) recommended combination treatment with EVE + EXE for this population [4, 5].
In BOLERO-5, combination EVE + EXE achieved remarkably similar PFS to that observed in the global BOLERO-2 trial (median PFS per investigator assessment: 7.4 months versus 7.8 months, respectively) and provided clinically meaningful benefits for the Chinese patients enrolled [14, 18, 21]. Treatment with EVE + EXE yielded a 48% risk reduction in PFS (per investigator assessment) and prolonged median PFS by 5.4 months, from 2.0 months (PBO + EXE) to 7.4 months (EVE + EXE). The PFS rate at 12 months was also numerically greater in the EVE + EXE arm compared to the PBO + EXE arm, indicating a more sustained treatment benefit with this combination. The magnitude of this PFS benefit compares favorably with other studies of everolimus and anti-estrogen therapy in this setting [16, 17], and also with the alternative treatment options available for these patients [8, 9, 24]. Patients receiving combination treatment with EVE + EXE also achieved greater ORR and CBR, further supporting the primary PFS outcome. Similar to PFS data, the observed ORR for EVE + EXE in BOLERO-5 was very similar to that reported in the BOLERO-2 global trial (8.8% versus 9.5%, respectively). The similarity of results between investigator and BIRC assessment of primary and secondary outcomes lends further credence to the robust nature of these data. While the OS data were immature at the primary cutoff, long-term follow up will enable further evaluation of the benefits of EVE + EXE combination treatment. The publication of the pharmacokinetic results of this trial will also provide an opportunity to evaluate the impact of combination EVE + EXE on estrogen concentration.
The combination of EVE + EXE was well tolerated in this Chinese patient population, with the incidence of AEs consistent with that previously reported with EVE and other rapamycin analogues [25], as well as with the safety profile of this treatment combination in HR + , HER2- ABC [14, 18, 21]. While the incidence of AEs was increased in the EVE + EXE group versus PBO + EXE, analysis of patient-reported outcomes in a similar patient population in the BOLERO-2 study revealed that QoL was maintained with this treatment combination, despite the increase in AEs [22]. Thus, the clinical efficacy of combination EVE + EXE may outweigh the potential toxicity, particularly when the impact on patient QoL is negligible. Most AEs reported in this study were Grade 1 or 2 in severity and could be managed with appropriate dose adjustments (reduction and/or interruption) and supportive therapies. The low discontinuation rate due to AEs in the EVE + EXE arm indicates that most AEs were well managed, allowing patients to continue the treatment as long as it provided clinical benefit.
Some differences in AESIs were reported between BOLERO-5 and BOLERO-2, although these differences were < 15%. These were for stomatitis (73.8% versus 68.0%), hemorrhages (16.3% versus 30.3%), and non-infectious pneumonitis (23.8% versus 21.8%) [26]. These events can all be effectively managed in this setting using clinically-defined management strategies [27,28,29]. A higher incidence of hyperglycemia and lower incidence of stomatitis were observed in BOLERO-5 compared with the BOLERO-2 Asian population. Stomatitis was reported in 73.8% of patients administered EVE + EXE in BOLERO-5 compared to 84.7% of patients in the Asian sub-population of BOLERO-2 [26]. It is reassuring that the incidence of stomatitis was lower in BOLERO-5, which can likely be attributed to the implementation of prophylactic treatment with corticosteroid-based mouthwash and to the improved management of this event over time [30]. However, no data on patient adherence to mouthwash usage were collected. The higher incidence of hyperglycemia in BOLERO-5 versus the Asian patients of BOLERO-2 (51.3% versus 11.2%) can possibly be explained by the evolution in case retrieval strategies used for mapping preferred terms to AESI [26]. The BOLERO-2 Asian subgroup analysis case retrieval strategy included hyperglycemia, diabetes mellitus, blood glucose increased, glycosuria, type 2 diabetes mellitus, and glucose urine present under the AESI of hyperglycemia/new onset diabetes mellitus, while the BOLERO-5 case retrieval strategy included hyperglycemia, diabetes mellitus, blood glucose increased, diabetic ketosis, glucose tolerance impaired, and glycosylated hemoglobin increased [26]. Of note, starting treatment with EVE at 5 mg/day rather than 10 mg/day may increase treatment compliance due to good tolerability and similar effectiveness [31].
The efficacy of combination EVE + EXE observed in BOLERO-5 was remarkably similar to the global BOLERO-2 trial, particularly the results of the BOLERO-2 Asian sub-population analysis [18, 21]. The safety profile of this treatment combination in a Chinese population was also largely consistent with the safety profile observed in the global BOLERO-2 trial [18, 21]. Similar efficacy results were also observed in the EVEREXES trial of combination EVE + EXE conducted in Asia, providing further support for this treatment combination in Chinese patients [23].
Treatment guidelines currently recommend the use of endocrine therapy in combination with CDK4/6 inhibitors in the first-line setting [4,5,6]. This study was carried out before this recommendation was implemented, and as such—as in the BOLERO-2 study [18]–no enrolled patients had received CDK4/6 inhibitors prior to the study start. No clinical trials have been carried out to date assessing the efficacy of EVE + EXE in patients pretreated with CDK4/6 inhibitors; however, a retrospective analysis of 622 patients with HR +, HER2- metastatic breast cancer showed that EVE + EXE was effective in patients who had received endocrine therapy alone and in combination with CDK4/6 inhibitors [32]. Similar results showing no impact of previous treatment with CDK4/6 inhibitors in OS were obtained in two smaller retrospective studies [33, 34].
This study is not without its limitations, and these results should be interpreted with a degree of caution. The small sample sizes in each group, in particular the small number of patients classed as responders, limit the interpretation of these data. Secondly, no QoL data were collected during this study, so the impact of AEs on the QoL of the Chinese postmenopausal women enrolled in this trial is unknown.
In conclusion, treatment with EVE + EXE provided a clinically meaningful PFS benefit in this heavily pre-treated population of Chinese patients with ER + , HER2- breast cancer, further validating the clinical benefit reported in the global BOLERO-2 trial. Combination treatment with EVE + EXE thus offers a significant improvement in the management of Chinese patients with ER + , HER2- breast cancer.
Data availability
The dataset supporting the conclusions of this article is included within the article (and its additional file(s). Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. Trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Code availability
Not applicable.
References
Sung H, et al. “Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries.” CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660. (in eng).
Li T, Mello-Thoms C, Brennan PC. “Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence.” Breast Cancer Res Treat. 2016;159(3):395–406. https://doi.org/10.1007/s10549-016-3947-0. (in eng).
Huang Z, et al. “Breast cancer incidence and mortality: trends over 40 years among women in Shanghai, China.” Ann Oncol. 2016;27(6):1129–34. https://doi.org/10.1093/annonc/mdw069. (in eng).
Cardoso F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49. https://doi.org/10.1016/j.annonc.2020.09.010.
Gradishar WJ, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–78. https://doi.org/10.6004/jnccn.2020.0016. (in eng).
Howlader N, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014. https://doi.org/10.1093/jnci/dju055. (in eng).
Ring A, Dowsett M. “Mechanisms of tamoxifen resistance.” Endocr Relat Cancer. 2004;11(4):643–58. https://doi.org/10.1677/erc.1.00776. (in eng).
Chia S, et al. “Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT.” J Clin Oncol. 2008;26(10):1664–70. https://doi.org/10.1200/jco.2007.13.5822. (in eng).
Di Leo A, et al. “Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer.” J Clin Oncol. 2010;28(30):4594–600. https://doi.org/10.1200/jco.2010.28.8415. (in eng).
Klein P, Mculloch W, Ordentlich P, Cruickshank S, Rees M, Yardley DA. Characterization of the overall survival benefit in ENCORE 301, a randomized, placebo-controlled phase II study of exemestane with and without entinostat in ER+ postmenopausal women with metastatic breast cancer. Am Soc Clin Oncol. 2012. https://doi.org/10.1200/jco.2012.30.27_suppl.128.
Miller TW, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120(7):2406–13. https://doi.org/10.1172/JCI41680.
Boulay A, et al. “Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer.” Clin Cancer Res. 2005;11(14):5319–28. https://doi.org/10.1158/1078-0432.Ccr-04-2402. (in eng).
Villarreal-Garza C, Cortes J, Andre F, Verma S. mTOR inhibitors in the management of hormone receptor-positive breast cancer: the latest evidence and future directions. Ann Oncol. 2012;23(10):2526–35. https://doi.org/10.1093/annonc/mds075.
Baselga J, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9. https://doi.org/10.1056/NEJMoa1109653.
Gnant M. “Overcoming endocrine resistance in breast cancer: importance of mTOR inhibition.” Expert Rev Anticancer Ther. 2012;12(12):1579–89. https://doi.org/10.1586/era.12.138. (in eng).
Baselga J, et al. “Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer.” J Clin Oncol. 2009;27(16):2630–7. https://doi.org/10.1200/jco.2008.18.8391. (in eng).
Bachelot T, et al. “Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study.” J Clin Oncol. 2012;30(22):2718–24. https://doi.org/10.1200/jco.2011.39.0708. (in eng).
Yardley DA, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–84. https://doi.org/10.1007/s12325-013-0060-1.
Ling WH, Lee SC. Inter-ethnic differences–how important is it in cancer treatment? Ann Acad Med Singap. 2011;40(8):356–61.
Gao JJ, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US food and drug administration pooled analysis. Lancet Oncol. 2020;21(2):250–60. https://doi.org/10.1016/S1470-2045(19)30804-6.
Noguchi S, et al. Efficacy of everolimus with exemestane versus exemestane alone in Asian patients with HER2-negative, hormone-receptor-positive breast cancer in BOLERO-2. Breast Cancer. 2014;21(6):703–14. https://doi.org/10.1007/s12282-013-0444-8.
Burris HA 3rd, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119(10):1908–15. https://doi.org/10.1002/cncr.28010.
Im YH, et al. “Safety and efficacy of everolimus (EVE) plus exemestane (EXE) in postmenopausal women with locally advanced or metastatic breast cancer: final results from EVEREXES.” Breast Cancer Res Treat. 2021;188(1):77–89. https://doi.org/10.1007/s10549-021-06173-z. (in eng).
Robert NJ, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–60. https://doi.org/10.1200/JCO.2010.28.0982.
Motzer RJ, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65. https://doi.org/10.1002/cncr.25219.
Novartis data on file, "CRAD001Y2301 clinical study report: A Randomized double-blind, placebo-controlled study of everolimus in combination with exemestane in the treatment of postmenopausal women with estrogen receptor positive locally advanced or metastatic breast cancer who are refractory to letrozole or anastrozole—Asian subgroup analysis." 2018.
Porta C, et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47(9):1287–98. https://doi.org/10.1016/j.ejca.2011.02.014.
White DA, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182(3):396–403. https://doi.org/10.1164/rccm.200911-1720OC.
EMA. "Afinitor summary of product characteristics." https://www.ema.europa.eu/en/documents/product-information/afinitor-epar-product-information_en.pdf. Accessed 22 Sep 2021.
Rugo HS, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18(5):654–62. https://doi.org/10.1016/s1470-2045(17)30109-2.
Zhang HQ, et al. Efficacy and safety of low-dose everolimus combined with endocrine drugs for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer. Ann Transl Med. 2021;9(19):1493. https://doi.org/10.21037/atm-21-4273.
Rozenblit M, et al. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14. https://doi.org/10.1186/s13058-021-01394-y.
Cook MM, et al. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021;26m(2):101–6. https://doi.org/10.1002/onco.13609.
Mo H, et al. Real-world outcomes of everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer in patients previously treated with CDK4/6 inhibitors. Clin Breast Cancer. 2022;22(2):143–8. https://doi.org/10.1016/j.clbc.2021.10.002.
Acknowledgements
We would like to thank all the patients who participated in this study and their caregivers. We acknowledge Haifu Li from Novartis Global Drug Development for their contribution to the study protocol and study management. We would also like to acknowledge Miguel Izquierdo, Woody Tang, Yi Qin, and Yanqing Bai from Novartis for their support throughout the study. Medical writing assistance was provided by Mary-Clare Cathcart, PhD, and Declan McCarthy, BSc, of Novartis Ireland Ltd., and was funded by Novartis Pharmaceuticals Corporation in accordance with Good Publication Practice 2022 (GPP2022) guidelines (https://www.ismpp.org/gpp-2022). We are also grateful for the support of all those involved in the execution of this study. This study was funded by Novartis Pharmaceuticals.
Funding
The study was funded by Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
All authors (Z-M.S, L.C., S.W., X.H., K.Shen, H.W., H.L., J.F., Q.L., J.C., X.Wu, X.Wang, H.L., T.L., J.L., K.A., K. Slimane, Y.Q., Y.L., Z.T.) participated in data collection and interpretation, and reviewed, read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study (ClinicalTrials.gov: NCT03312738) was conducted in accordance with the Declaration of Helsinki and the ICH E6 Guidelines for Good Clinical Practice. The study protocol and all amendments were approved by the Independent Ethics Committee or Institutional Review Board of each investigative site (Fudan University Cancer Hospital, Shanghai; Ruijin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai; Beijing Cancer Hospital Medical Ethics Committee/Beijing Cancer Hospital Clinical Trial Institution, Beijing; Tianjin Cancer Hospital Medical EC, Tianjin; The 1st Affiliated Hospital of Chongqing Medical University Clinical Trial Ethics Committee, Chongqing; West China Hospital of Sichuan University Clinical Trial Ethics Committee, Chengdu; Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology, Wuhan; Ethics Committee of Hubei Cancer Hospital, Wuhan; Zhejiang Cancer Hospital, Hangzhou; Jiangsu Tumor Hospital, Nanjing; Ethics Committee, Sun Yat-Sen University Cancer Center, Guangzhou; Medical Ethics Committee, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou; Tumor Hospital of Harbin Medical University EC, Harbin; Sichuan Academy of Medical Science/Sichuan Province People’s Hospital Ethics Committee for Science Research and Clinical Trial of Medicines, Chengdu; Affiliated Hospital of Medical College Qingdao University Ethics Committee/Affiliated Hospital of Medical College Qingdao University Clinical Trial Institution). Written informed consent was obtained from each patient before any study-specific procedure was performed. The study was designed and sponsored by Novartis Pharmaceuticals, who collected and analyzed the data in conjunction with the author. Informed consent was obtained from all individual participants included in the study.
Competing interests
K. A., K. Slimane, Y. Q., and Y. L. are employees of Novartis. All other authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shao, ZM., Cai, L., Wang, S. et al. BOLERO-5: a phase II study of everolimus and exemestane combination in Chinese post-menopausal women with ER + /HER2- advanced breast cancer. Discov Onc 15, 237 (2024). https://doi.org/10.1007/s12672-024-01027-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01027-8