Abstract
Background
Everolimus plus exemestane is approved for the treatment of hormone receptor-positive metastatic breast cancer (MBC) after progression on nonsteroidal aromatase inhibitors. The role of everolimus is less well defined in other breast cancer phenotypes and in combination with other drugs.
Objectives
We conducted a systematic review and meta-analysis to assess the efficacy and safety of adding everolimus to standard of care (SoC) in MBC regardless of tumor phenotype and treatment type.
Methods
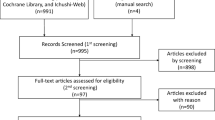
The electronic databases PubMed and EMBASE were searched for eligible randomized trials. Pooled hazard ratios (HRs) for progression-free survival (PFS) and overall survival (OS) and pooled risk ratios (RR) and odds ratios for objective response rates, disease control rate (DCR), and grade 3 or higher toxicity were meta-analyzed. Subgroup analyses compared survival outcomes by tumor phenotype.
Results
Data from 2826 patients from eight trials were analyzed. The addition of everolimus to SoC reduced the risk of disease progression by 29% (HR 0.71; 95% confidence interval [CI] 0.56–0.90). This did not translate into an OS benefit (HR 0.95; 95% CI 0.80–1.13). In addition, everolimus improved the DCR (RR 0.82; 95% CI 0.68–0.98), whereas it increased the risk of developing grade 3 or higher toxicity. The PFS benefit was more prominent for patients with hormone receptor-positive (+)/human epidermal growth factor receptor 2 (HER2)-negative (−) disease. For the HER2 (+) subgroup, the PFS benefit was restricted to patients with hormone receptor (−) disease.
Conclusions
Everolimus reduces the risk of disease progression in hormone receptor (+) MBC. In patients with HER2 (+) disease, the benefit is limited for those with hormone receptor (−) disease. Given the approval and use of newer drugs in MBC, clinical trials and real-world data are needed to confirm the benefit of everolimus and define the best treatment sequence strategy to adopt in that setting.




Similar content being viewed by others
References
Alqaisi A, Chen L, Romond E, Chambers M, Stevens M, Pasley G, et al. Impact of estrogen receptor (ER) and human epidermal growth factor receptor-2 (HER2) co-expression on breast cancer disease characteristics: implications for tumor biology and research. Breast Cancer Res Treat. 2014;148(2):437–44.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Miller TW, Rexer BN, Garrett JT, Arteaga C. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13(6):224.
Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–62.
She QB, Gruvberger-Saal SK, Maurer M, Chen Y, Jumppanen M, Su T, et al. Integrated molecular pathway analysis informs a synergistic combination therapy targeting PTEN/PI3K and EGFR pathways for basal-like breast cancer. BMC Cancer. 2016;16(1):587.
Li J, Zhang C, Jiang H, Cheng J. Andrographolide inhibits hypoxia-inducible factor-1 through phosphatidylinositol 3-kinase/AKT pathway and suppresses breast cancer growth. Onco Targets Ther. 2015;8:427–35.
Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16(8):797–803.
Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6(4):154–66.
Yardley DA, Noguchi S, Pritchard KI, Burris H, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013a;30(10):870–84.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al editors. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Eisenhauer EA, Therasse P, Bogaerts X, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Common Terminology Criteria for AdverseEvents version 3.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 9 Aug 2006.
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 9 Aug 2006.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane methods. Cochrane Database of Systematic Reviews 2016; no 10 (Suppl 1). https://doi.org/10.1002/14651858.CD201601.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490.
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. 2013. The GRADE Working Group; 2013. https://guidelinedevelopment.org/handbook.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177e88.
Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration. Wiley, New York; 2011.
Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2 positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16(7):816–29.
Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9.
Yardley DA, Noguchi S, Pritchard KI, Burris HA, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2final progression-free survival analysis. Adv Ther. 2013b;30(10):870–84.
Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factorreceptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014;25(12):2357–62.
Rugo HS, Pritchard KI, Gnant M, Noguchi S, Piccart M, Hortobagyi G, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25(4):808–15.
André F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(6):580–91.
Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancerwith prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718–24.
Kornblum N, Zhao F, Manola J, Klein P, Ramaswamy B, Brufsky A, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negativemetastatic breast cancer resistant to aromatase inhibitor therapy: results of PrE0102. J Clin Oncol. 2018;36(16):1556–63.
Yardley DA, Bosserman LD, O’Shaughnessy JA, Harwin WN, Morgan SK, Priego VM, et al. Paclitaxel, bevacizumab, and everolimus/placebo as first-line treatment for patients with metastatic HER2-negative breast cancer: a randomized placebo-controlled phase II trial of the Sarah Cannon Research Institute. Breast Cancer Res Treat. 2015;154(1):89–97.
Schmid P, Zaiss M, Harper-Wynne C, Ferreira M, Dubey S, Chan S, et al. Fulvestrant plus vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor-positive metastatic breast cancer: the manta phase 2 randomized clinical trial. JAMA Oncol. 2019;5(11):1556–63.
Decker T, Marschner N, Muendlein A, Welt A, Hagen V, Rauh J, et al. VicTORia: a randomised phase II study to compare vinorelbine in combination with the mTOR inhibitor everolimus versus vinorelbine monotherapy for second-line chemotherapy in advanced HER2-negative breast cancer. Breast Cancer Res Treat. 2019;176(3):637–47.
Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat. 2012;135:39–48.
Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–9.
Rugo HS, Seneviratne L, Beck JT, Glaspy JA, Peguero JA, Pluard TJ, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18(5):654–62.
Burris HA 3rd, Lebrun F, Rugo HS, Beck JT, Piccart M, Neven P, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119(10):1908–15.
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with Ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–16.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with Ribociclib plus Fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–24.
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, erbb2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–24.
Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–19.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36.
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40.
Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(7):904–16.
Di Leo A, Johnston S, Lee KS, Ciruelos E, Lonning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(1):87–100.
Jansen VM, Bhola NE, Bauer JA, Formisano L, Lee KM, Hutchinson KE, et al. Kinoma-wide RNA inferference screen reveals a role for PDK1K in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res. 2017;77:2488–99.
Wang N, Wang K, Liu YT, Song FX. Everolimus plus endocrine vs endocrine therapy in treatment advanced ER+, HER2− breast cancer patients: A meta-analysis. Curr Probl Cancer. 2019;43(2):106–14.
Loi S, Michiels S, Baselga J, Bartlett JMS, Singhal SK, Sabine VS, et al. PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS ONE. 2013;8:e53292.
Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJH, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol. 2014;32:2951–8.
Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA, Pritchard KI, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol. 2016;34(5):419–26.
Bachelot TD, Treilleux I, Schiffler C, Bieche I, Campone M, Patsouris A, et al. mTORC1 activation assessed in metastatic sample to predict outcome in patients with metastatic breast cancer treated with everolimus-exemestan: results from the SAFIRTOR study. J Clin Oncol. 2019;37(15 suppl):1024–1024.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Jacques Raphael has received honorarium (Hoffmann-La Roche) and participated on an advisory board for Lilly Oncology. Cory Lefebvre, Alison Allan, Joelle Helou, Gabriel Boldt, Theodore Vandenberg, and Phillip S. Blanchette have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
JR had the idea for the systemic review; JR, C.L, GB performed the literature search and data analysis, JR, CL, GB, JH, A.A, PB, and TV drafted and critically revised the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raphael, J., Lefebvre, C., Allan, A. et al. Everolimus in Advanced Breast Cancer: A Systematic Review and Meta-analysis. Targ Oncol 15, 723–732 (2020). https://doi.org/10.1007/s11523-020-00770-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00770-6