Abstract
Exosomes are small extracellular vesicles (30–150 nm) that are formed by endocytosis containing complex RNA as well as protein structures and are vital in intercellular communication and can be used in gene therapy and drug delivery. According to the cell sources of origin and the environmental conditions they are exposed to, these nanovesicles are very heterogeneous and dynamic in terms of content (cargo), size and membrane composition. Exosomes are released under physiological and pathological conditions and influence the pathogenesis of cancers through various mechanisms, including angiogenesis, metastasis, immune dysregulation, drug resistance, and tumor growth/development. Gastrointestinal cancer is one of the deadliest types of cancer in humans and can involve organs e.g., the esophagus and stomach, or others such as the liver, pancreas, small intestine, and colon. Early diagnosis is very important in this field because the overall survival of patients is low due to diagnosis in late stages and recurrence. Also, various therapeutic strategies have failed and there is an unmet need for the new therapeutic agents. Exosomes can become promising candidates in gastrointestinal cancers as biomarkers and therapeutic agents due to their lower immunity and passing the main physiological barriers. In this work, we provide a general overview of exosomes, their biogenesis and biological functions. In addition, we discuss the potential of exosomes to serve as biomarkers, agents in cancer treatment, drug delivery systems, and effective vaccines in immunotherapy, with an emphasis on gastrointestinal cancers.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Based on size, extracellular vesicles are classified into three groups: exosomes (30–150 nm), microvesicles (100–1000 nm), and apoptotic bodies (> 1000 nm) [1]. Exosomes are extracellular vesicles that were first described by Harding et al. in 1983, and confirmed by Johnstone et al. in 1987 [2]. Extracellular vesicles are surrounded by a lipid bilayer membrane and originate from multivesicular bodies secreted by various types of cells. Exosomes are formed via endocytosis and can be derived from most mammalian cells, including cytotoxic T cells, B lymphocytes, platelets, dendritic cells (DCs), mast cells, adipocytes, neurons, endothelial cells, and epithelial cells (Fig. 1) [3]. Depending on the cellular source and the environmental conditions to which exosomes are subjected, the membrane content and composition are highly heterogeneous and dynamic [4]. The release of exosomes occurs both under physiological and pathological conditions. In addition to cells, exosomes are also isolated from sources such as bovine milk [5, 6] and are present in almost all body fluids, including saliva, semen, plasma, human breast milk, amniotic fluid, bronchoalveolar lavage, cerebrospinal fluid bile, synovial fluid, urine, tears, nasal secretions, and pleural effusions [3]. Depending on cellular origin, exosomes can contain different components such as proteins, nucleic acids, and lipids; because of this diverse composition, they have the potential to regulate the expression of various genes [7]. Initially, exosome production was mainly considered as part of a process to dispose of cellular waste products; however, various other functions emerged over the years [8]. For example, exosomes play a critical role in intercellular communication (in addition to established mechanisms such as direct cell–cell contact and transfer of secreted molecule) [9], and can be used in gene therapy and drug delivery [10]. For example, exosomes derived from raw bovine milk were tested as carriers of extracellular RNAs aimed at delivering hsa-miR148a-3p to liver (HepG2) and intestinal (Caco-2) cell lines. The results showed that this cost-effective source can be used as a nanocarrier of functional microRNAs (miRNAs) in RNA-based therapy [6]. Compared to viral vectors/liposomes, exosomes are less immunogenic and have the ability to cross major physiological barriers such as the blood–brain barrier, making them an attractive option as biomarkers and therapeutic agents [11]. Furthermore, exosomes are vital in antigen presentation and immune system activation [7], properties that can be utilized in vaccination [8]. Effective application of exosomes has also been reported in the diagnosis and therapy of several diseases, particularly cancers [12]. Exosomes can affect cancer progression via various mechanisms, including angiogenesis, modulation of immune response, metastasis, drug resistance, and tumor growth or development [1, 3]. Gastrointestinal (GI) cancers are among the deadliest cancers and can develop in the upper parts of the GI tract, e.g., the esophagus and stomach, or in other organs such as the liver, pancreas, small intestine, and colon [13]. Gastric malignancy is the fourth most prevalent cancer and the most common cause of cancer-related deaths in recent years.. Despite significant advancements in various treatment strategies such as chemotherapy, radiotherapy, immunotherapy, and surgery, tumor metastasis and/or recurrence are still the most common causes of cancer death, which is due to poor prognosis in this field [14]. Based on the current literature, timelier (i.e., early) diagnosis and increased knowledge of risk factors would significantly benefit cancer survival. Thus, there is an urgent need for new non-invasive diagnostic methods to improve early cancer diagnosis and prognosis [15]. For example, the survival rate of patients with colorectal cancer (CRC), one of the most common cancers with high mortality rate, would be significantly increased by early diagnosis [16]. As indicated, exosomes have been reported as a novel approach in the diagnosis and treatment of cancers [17], especially in gastrointestinal cancers [16], and their role as diagnostic biomarkers or drug carriers is well studied [12]. Exosomes containing lncRNA RPPH1, derived from tumor cells, were shown to be important in early diagnosis of CRC [18]. In another study, the disbalance of exosomes containing miR-217 was considered a diagnostic biomarker in gastric cancer [19]. Recent work indicated that exosomes containing miR-9-3p or miR-21 can act as biomarkers for early detection of metastasis in liver cancer (HCC), a malignant cancer that has no specific symptoms in the early stages [20]. In this review, we describe the functional and mechanistic roles of exosomes in the development and progression of gastrointestinal cancers. In addition, we discuss the potential clinical and biomedical applications of exosomes in gastrointestinal cancers.
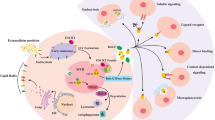
Schematic figure of the effect of exosomes on gastrointestinal cancer. Small extracellular vesicles are surrounded by a lipid bilayer membrane and are secreted by various types of cells, including cytotoxic T cells, B lymphocytes, platelets, dendritic cells (DCs), mast cells, adipocytes, neurons, endothelial and epithelial cells originate. Exosomes are isolated from sources such as saliva, plasma, milk, amniotic fluid, synovial fluid, urine, tears, nasal secretions and pleural effusion. Depending on the cell source and environmental conditions, the exosome content is different and may include DNA, RNA, polypeptide, CD molecules, etc., which have the potential to regulate the expression of various genes and interfere in cellular mechanisms. These characteristics can be used to carry drugs or therapeutic agents and also as biomarkers in gastrointestinal cancer
2 Exosome biogenesis, functions, mechanisms, and applications
2.1 The biogenesis of exosomes
Exosome biogenesis starts with double invasion of the plasma membrane by endocytosis, followed by formation of the primary endosome, which matures into a secondary endosome or multivesicular body (MVB) (Fig. 2), which itself contains intraluminal vesicles (ILVs) [21]. The formed MVB has two possible fates: it can combine with lysosomes and destroy its cargo, or merge with the cell membrane and release its ILVs into the extracellular environment [22]. The stages of exosome formation in MVBs include the entry of specific lipids and proteins into the endosomal membrane as well as the entry of molecules into primary ILVs and the subsequent separation of ILVs. The exact process of cargo sorting is still unknown; however, two main mechanisms have been identified for the entry of the cargo into the exosome. The first depends on ‘endosomal sorting complexes required for transport’ (ESCRT), which takes place on the cytosolic side of the MVB membrane and recognizes transport, trans-Golgi network, and cell surface proteins; these proteins are then ubiquitinated and directed into the exosome. The second mechanism is independent of ESCRT, and relies on the lipid content of the endosomal membrane [23, 24]. Studies have shown that ESCRT components play an important role in the formation of MVB and ILV. This complex contains thirty different types of proteins, including those classified into ESCRT-0, -I, II, and -III [8], VPS4 (vacuolar protein sorting-associated protein) [25], VTA1 (vesicle trafficking 1) [21], and Alix (apoptosis-linked gene 2-interacting protein X) [26]. The main function of ESCRT is to include and sort specific components in ILVs that are supposed to be converted into exosomes [8]. The reaction of ESCRT-0 with phosphatidyl inositol triphosphate, located on the endosomal membrane, activates ESCRT-0 and binding to ubiquitination proteins, leading to the recruitment of ESCRT-II components. The involvement of ESCRT-1 and -II signals the beginning of budding towards the inside of the MVB. Near the bend of the membrane of the forming ILVs, ESCRT-II activates the components of ESCRT-3, which with its ATPase enzymes causes the separation of ESCRT subsets and vesicles. Recent studies reported that inhibition of the expression of specific ESCRT components, such as tumor susceptibility gene 101 (TSG101) or hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), decreases the amount of exosome production and secretion [8, 20, 21, 27, 28]. As indicated, the second pathway of MVB formation is ESCRT-independent and relies on the lipid composition of the endosomal membrane. Thus, the formation, loading, and release of exosomes is highly dependent on ceramides produced as a result of sphingomyelinase activity [23, 29]. Other proteins involved in the process of exosome biogenesis include syndecan heparan sulfate proteoglycans and their cytoplasmic adaptor syntenin, sytenin1, syndecan-1, tetraspanins (CD9, CD63, and CD82), Ras-related protein GTPase Rab (Rab27a, Rab27b), and SNARE (soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor] [1, 23, 30].
(I) The biogenesis and composition of exosomes. Biogenesis is divided into three stages: (i) endocytosis, (ii) endosome formation, and (iii) exocytosis. The endosome is formed by endocytosis and then transforms into a late endosome that contains multivesicular bodies (MVBs). MVBs secrete small vesicles (exosomes) to the extracellular membrane. (II) The released exosomes can then target cells through three major pathways: endocytosis, fusion, and ligand-receptor interactions
2.2 Functions of exosomes
Exosomes were traditionally considered as cellular waste. Recently, however, exosomes have been demonstrated to play various biological and pathological roles. Depending on their cellular origin, they can have different compositions and, therefore, fulfil distinct functions [15, 30]. Exosomes are present in most body fluids and can transport various types of cargo affecting cellular activities [20]. Exosomes can be related to various types of diseases. The evidence shown an increase in exosome secretion in cancer patients. Cancer cell-derived exosomes can play an essential role in tumorigenesis and tumor growth. They can also integrate with cells at specific locations, creating a pre-metastatic niche facilitating metastasis and cancer progression. In addition, exosomes have been shown to be important in cancer drug resistance, angiogenesis, and immune escape. Furthermore, because they transport various molecules such as nucleic acids and proteins [8], exosomes have been linked to the development of cancers [23, 31]. Some exosomes contain non-coding RNAs (miRNA let-7) and can cause cancer progression; e.g., exosomes secreted from human gastric cancer cell line can activate the AKT signaling pathway and increase proliferation [32]. Moreover, exosomes derived from liver cancer cells cause tumor growth by establishing communication between cancer cells and activating the hedgehog signaling pathway [33]. Thus, based on the available literature, it appears that specific exosome cargo could be crucial in cancer formation, and particularly in tumorigenesis [23, 34]. Understanding the effects of exosomes on cancer development and progression would help us to develop therapeutic strategies specifically aimed at impacting exosome formation, release, and receptor cell uptake. Exosomes are considered as a suitable option for gene therapy due to their ability to transfer nucleic acids, without activating the host's immune system or causing (cellular) toxicity [35, 36]. Recently, the diagnostic aspects of exosomes have also been investigated. Thus, different patterns of exosomal microRNAs and RNAs in patients and healthy people would allow the use of exosomes as diagnostic biomarkers; for example, reduced expression of exosomal miR_92 can be indicative of hepatocellular carcinoma and leukemia [37]. Exosomes also play a role in the transfer of various substances or cell signals involved in cancer progression (e.g., KRAS mutation in pancreatic cancer). Furthermore, exosomes can serve as drug delivery systems, considering their high half-life, ability to target specific cells, biocompatibility, and non-toxicity [8, 38]. With regards to the role of dendritic cells (DCs) in antigen presentation, exosomes can act as antigens for DCs, which in turn can activate the immune system against cancers and regulate immune responses. The ability of exosomes to promote or suppress cancers, and their effective role in immunotherapy should qualify exosomes as targets/tools of interest in novel therapeutic strategies in the near future. Some studies have already shown that exosomes derived from NSCLC tumors can help DC maturation by increasing the expression of Rab27a and upregulation of MHC, thereby promoting the proliferation of CD4 + T cells [20].
2.3 Role of exosome in GI cancer progression
As indicated, exosomes can contribute to the development of cancer through various mechanisms, which we will discuss in the following sections.
2.3.1 Exosome and tumorigenesis
LncRNA HEIH, which is released by gastric cancer (GC) cells, can play a role in tumorigenesis. Exosomes secreted from a SGC-7901 cell line caused the propagation of BGC-823 and SGC-7901 cells via activation of the Akt signaling pathway [39]. Cancer exosome mirs have also been implicated in tumorigenesis. Importantly, exosomal miR biogenesis has been described, for example, this is the case for miR_Let7, which increases metastasis in GC [7, 14, 30, 40]. The role of exosomes in relation to cancer is summarized in Fig. 3.
The roles of exosomes in cancer. Exosomes can be used as agents against cancer. For example, they can carry and deliver theranostic agents (diagnostic and therapeutic agents simultaneously in one platform), cancer diagnostic agents (important biomarkers), and conventional chemotherapy drugs. Conversely, exosomes may also cause tumorigenesis or cancer progression as destructive agents, and certain exosomes have been found to play a role in drug resistance, immune escape, tumor progression, and development of pre-metastatic niches
2.3.2 Exosome and tumor growth
Both cancer- and microenvironment-derived exosomes can be involved in tumor growth. Cancer cells try to grow and survive through various mechanisms; the enhancing effects of exosomes on cancer cell growth have been widely reported in various cancers. For example, cancer cells can absorb exosomes that contain heat shock proteins (HSP), such as HSP90 and HSP70, which promotes their proliferation and inhibits apoptosis [41]. Colon cancer cell-derived exosomes containing the ΔNp73 gene are able to induce proliferation of recipient cells. Furthermore, tumor-derived exosomes are multiplied by mRNAs and eventually cause tumor growth in CRC. Other work showed that cancer-derived exosomes impact tumor growth of hepatocellular carcinoma (HCC) through the regulation of TAK1 expression. In addition, the loss of miR320a-containing exosomes derived from cancer-related fibroblasts (CAF) induced proliferation of HCC cells [23, 30, 42].
2.3.3 Exosomes and angiogenesis
Tumor growth and metastasis importantly rely on angiogenesis for supply of nutrients and oxygen. Exosomes act as mediators between tumor cells and vascular endothelial cells, and play a role in cancer progression as carriers of angiogenic factors. In addition, tumor-derived exosomes greatly affect metastasis through vascular remodeling [43]. They can also interfere with the integrity of endothelial cells; e.g., exosomal miR-105 increased vascular permeability and metastasis by reducing ZO-1 protein expression (44). It has been demonstrated that some exosome miRs are involved in the angiogenic process. For example, in CRC, miR-9 can promote angiogenesis and enhance endothelial cell migration by inhibiting the expression of suppressor of cytokine signaling 5 (SOCS5) [45]. Another study found that exosomes from colon cancers and pancreatic adenocarcinoma increase metastasis and angiogenesis by carrying tetraspanin 8 [46]. Thus, exosomes can affect angiogenesis in two ways, either by directly delivering angiogenic factors to endothelial cells or via exosomal miRNAs [7, 8, 20, 23, 30].
2.3.4 Exosomes and metastasis
Tumor metastasis involves cancer cells migrating to distant points and is one of the leading causes of cancer-related death. The higher the percentage of tumor malignancy, the more likely it is for cancer cells to invade and migrate to other organs and metastasize [14, 30]. Exosomes have been suggested to impact tumor metastasis in three main ways:
-
1.
Tumor-derived exosomes can increase cancer cell invasion and metastasis by acting on the extracellular matrix (ECM).
-
2.
Exosomes can loosen the tight connections between (endothelial) cells, which increases the penetration of tumor cells.
-
3.
Exosomes can increase the metastasis and invasion of cancer by enhancing epithelial-mesenchymal transition (EMT) [47,48,49,50].
Recent studies have shown that GC cells can induce the penetration of peritoneal mesostromal cells (PMCs) by releasing exosomes containing Wnt3a. PMCs then invade the stomach wall and provide the basis for metastasis [51]. In addition, it was demonstrated that GC cell-derived exosomes carrying EGFR are transferred to liver cells and increase hepatocyte growth factor (HGF) by inhibiting miR-26a, thereby favoring metastasis [30]. Other work showed that exosomes harboring miR-221/222 stimulate GC cells to migrate [52]. Further supporting a role for exosomes in metastasis, gastrointestinal stromal tumor cells release exosomes containing protein tyrosine kinases to convert smooth muscle progenitor cells into a premetastatic site [42].
2.3.5 Exosomes and drug resistance
One of the most critical obstacles in cancer treatment is drug resistance; recent studies have indicated that exosomes may play a role in this process through various mechanisms. Tumor cells can spread drug resistance by secreting exosomes that may transfer proteins, miRNAs, and/or long non-coding RNAs to other (recipient) cells [20, 30]. Tumor-derived exosomes can also transfer multidrug resistance (MDR) by impacting the expression of multidrug resistance proteins (MRP) as determinants of cancer drug resistance [20, 42]. Recent studies reported that miRNAs of exosomes derived from cancer stem cells (CSC), including the highly expressed miR-210 in pancreatic CSC exosomes, can participate in the transfer of resistance to sensitive cancer cells [20].
2.3.6 Exosomes and immune escape
Exosomes can affect the formation, maturation, and anticancer activity of immune cells by transferring suppressive proteins. In addition, they can transfer DNA, mRNA, and/or miRNA, and increase cancer progression via reprogramming the function of the response cells [30]. For example, tumor cells induce apoptosis in T lymphocytes by releasing exosomes containing cell death receptors [14]. In addition, exosomal miR-24-3p can inhibit the proliferation and differentiation of T cells by silencing FG11 expression (30). Exosomes can also suppress cell differentiation (e.g., from myeloid to DC), reduce immune system activation, and facilitate immune evasion. A recent study showed that exosomes derived from NPC cells interfere with T cell function by regulating miRNAs [42]. Moreover, GC-derived exosomes play a role in regulating the immune system to promote development of GC. In fact, this regulation is done through Noncoding RNAs (ncRNAs). ncRNAs represent a substantial portion of the content within exosomes, and certain ncRNAs with biological functions are specifically packaged into these extracellular vesicles. Recent studies have unveiled the critical roles played by exosome-derived ncRNAs in the tumorigenesis, progression, and drug resistance of gastric cancer (GC). Moreover, the regulation of exosomal ncRNA expression levels has the capacity to either promote or suppress the advancement of GC [14].
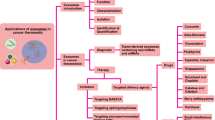
2.4 The application of exosomes
2.4.1 Exosomes as biomarkers
Exosomes have emerged as important diagnostic and prognostic biomarkers in several cancers (Table 1), and can be considered for many therapeutic purposes. In addition, tumor-derived exosomes can be utilized as vaccines in clinical and preclinical studies. Because exosomes are present in virtually all body fluids and may contain various bioactive molecules, it is much easier to detect cancer. Indeed, evaluating the expression of exosomal miRNAs has been used as a tool for diagnosing cancer progression in several cancers, including GC, in which lncRNA was identified as a new exosomal biomarker [42]. Several other exosomal miRNAs, i.e., miR 150-3p, miR-145-3p, miR-139-3p, and Let-7b-3p, have emerged as diagnostic biomarkers in colon cancer [53]. Urinary exosomal lncRNA, which includes PCAT-1, MALAT1 and SPRY4-IT1, serves as a biomarker for the detection and recurrence prediction of bladder cancer [12]. Exosomal miRNAs can also indicate metastasis in GC (e.g., miR-101-3p, miR-10b-5p, and miR-143-5p) [14]. In addition to miRNAs and lncRNAs, exosomes may contain other cargo helpful for cancer diagnosis; for example, the exosomal protein glypican-1 (GPC-1) is a biomarker for the diagnosis of pancreatic cancer [3].
2.4.2 Exosomes and drug delivery
Exosomes can potentially be used as drug carriers to treat various diseases. Due to their characteristics such as biocompatibility and biodistribution, they are suitable carriers for various substances. For example, the transfer of exosome-derived siRNA can cause specific gene silencing and induce cancer cell death [42]. Exosomes have several advantages over some other carriers (e.g., Liposomes and synthetic nanocarriers), including a higher drug delivery efficiency and the ability to prevent macrophage phagocytosis with limited immunogenicity. Thus, exosomes increase the half-life of the drug, do not cause toxicity, are accurate in cell targeting, and promote endocytosis, all of which facilitates the drug delivery process. Exosomes carrying nucleic acids, such as the highly expressed let-7 and several other miRs, can act as tumor suppressors. For example, exosomal miR-335-5p can reduce the size of liver tumors [54], and miR-145-5p (by activating the Smad3 pathway) inhibits pancreatic cancer cells multiplication [55]. In addition, chemical anticancer drugs, such as paclitaxel (PTX) and doxorubicin, can be carried by exosomes as well [12, 56]. Considering exosomes can identify specific cells, exosomal delivery of therapeutic compounds can be more effective and precise than with other biocarriers such as liposomes. Therefore, exosome therapy is not only suitable for diagnostic analysis but also promising in cancer treatment [12].
3 Exosomes as a biomarker for the diagnosis of gastrointestinal malignancies
3.1 Exosome proteins as biomarkers in GC
Some of the housekeeping protein markers used to distinguish exosomes from other extracellular vesicles include tumor susceptibility gene 101 (TSG101), ALG-2-interacting protein X (ALIX), CD63, CD81, and HSP70; these proteins are important in the biogenesis of exosomes [57]. In this section, however, we will discuss exosomal protein biomarkers that have been specifically linked to gastrointestinal cancers. Frizzled family proteins are important Wnt pathway receptors and can contribute to the development of cancer stem cells. In particular, Frizzled-10 (FZD-10) plays a role in gastrointestinal cancers and has been detected on the surface of exosomes derived from cells of these cancers [58]. Thus, a recent study utilizing Au nanoparticles (surface-functionalized with a FZD10 protein primary antibody) and a transmission electron microscopy (TEM) grid detected FZD10 protein on the surface of gastrointestinal cancer cell-derived exosomes [59]. Others studied exosomes isolated from the blood of cancer patients and healthy donors using atomic force microscopy (AFM) to detect exosomes positively expressing the surface marker CD41 [60]. The GPC-1 protein has been identified as an exosomal marker in pancreatic, breast, and colorectal cancer [58]. In addition, CD9 and CD147 have been found to be highly expressed in exosomes isolated from the serum of CRC patients. It should be noted, however, that exosomal surface expression of CD47 decreased after tumor surgery in these patients [61]. Costa Silva et al. demonstrated that pancreatic ductal adenocarcinoma (PDAC)-derived exosomes play a role in the development of hepatic pre-metastatic niches. Further investigations showed that these exosomes highly expressed macrophage migration inhibitory factor (MIF). Based on these results, the authors suggested that MIF positive exosomes could serve as biomarkers to indicate the development of PDAC liver metastasis [62].
3.2 Exosomal lipid profiles as biomarkers
Lipid metabolism is important in the carcinogenesis of many cancers, especially CRC [63,64,65]. For example, lysophosphatidylserine abundance is significantly increased in colon cancer tissues as compared to surrounding normal tissues [66]. Pro-inflammatory stimuli promote a microenvironment favoring cancer development [67]. Lipidomic evaluation of tissue inflammation revealed a significant relationship between the change in the structure of membrane lipids and the development of inflammation, implying some of these lipids associated with inflammation can serve as biomarkers [68, 69]. One study found that the lipid structure of the exosomal membrane is affected by the cells from which exosomes are extracted. In an in vitro study related to prostate cancer, it was demonstrated that the lipid composition of exosomes is similar to the membrane structure of cancer cells from which they were derived [58]. Recently, the lipid profile of exosomes extracted from human colon adenocarcinoma Caco-2 and human colon epithelial cells (HCEC-1CT) was evaluated. Exosomes derived from Caco-2 cells exhibited a distinct lipid profile, with high amounts of linoleic acid (LA), γ-linolenic acid (GLA), and arachidonic acid (AA). In addition, exosomes from HCEC-1CT cells showed higher amounts of omega-3 fatty acids, including eicosapentaenoic acid (EPA) and alpha-linolenic acid (ALA), as compared to those derived from Caco2 cells. The results indicated a proinflammatory role of omega-6 in CRC tumorigenesis, as evidenced by high n-6/n-3 and AA/EPA ratios in Caco2 cells [69, 70].
3.3 Exosomal RNAs as biomarkers
Several studies have investigated the effects of RNAs delivered by exosomes in tumorigenesis and cancer spread. For example, long non-coding (Lnc) RNAs such as Lnc-sox2ot, Lnc-h19, and LncRNA-ARSR, which have been identified in exosomes, are involved in the progression of tumors [71,72,73,74]. Dysregulation of miRNAs delivered by exosomes has been associated with gastrointestinal malignancies. MiR-10b-containing exosomes, which are secreted in the CRC tumor microenvironment by cells like cancer-associated fibroblasts (CAF), can increase the expression of transforming growth factor-beta (TGF-β) and smooth muscle (SM) α-actin, ultimately promoting the growth of CRC cells [75, 76]. Exosomal delivery of miR-21-3p and miR-769-3p has been shown to play a role in CRC metastasis to the lung through the activation of fibroblasts in the tumor microenvironment and lung tissue; this process occurs through the formation of premetastatic niches, and the secretion of these types of exosomes increases following p53 R273H mutation [77]. Cooks et al. reported that miR-1246-enriched exosomes are secreted from CRC cells that have mutations in p53, and contribute to CRC progression and metastasis. Therefore, these exosomes are important in the diagnosis of this type of CRC [78]. MiRNA-21-containing exosomes may be involved in the development of hepatocellular carcinoma through the conversion of normal hepatic stellate cells (HSCs) to CAFs. This cellular transformation (associated with tumorigenesis) is induced by exosomal targeting of phosphatase and tensin homologue (PTEN), and the subsequent secretion of factors such as TGF-β, fibroblast growth factor-2 (FGF-2), and endothelial growth factor (VEGF) [79]. Transcription of enzyme telomerase (hTERT mRNA) delivered by exosomes can cause the transformation of normal fibroblasts into telomerase-positive cells; this affects the microenvironment of pancreatic cancer, which can be important in terms of cancer metastasis [80]. MiR-21 has been identified in exosomes associated with GC cells. Considering it regulates the important PTEN/PI3K/AKT signaling pathways, this micronucleic acid can be effective in apoptosis inhibition and cisplatin resistance [81]. LncRNA ZFAS1 is another important RNA identified in GC-derived exosomes, and has been shown to impact MAPK signaling, EMT, cell cycle progression, as well as cancer growth and metastasis. Based on the results of this study, it is concluded that ZFAS1, plays a role in GC progression and metastasis. Therefore, it has been suggested that ZFAS1 can be considered as a diagnostic and prognostic biomarker in this cancer [82]. In addition to preclinical studies, several clinical trials evaluating exosomes as biomarkers and diagnostic factors for gastrointestinal cancers have been conducted (summarized in Table 2).
4 Utilizing exosomes as therapeutic agents in cancer treatment
The role of exosomes as therapeutic agents depends on their original parental cells [83]. The most commonly used exosomes in cancer treatment are those secreted by mesenchymal stem cells (MSCs), DCs (dexosomes), and cancer cells [84]. Several studies have focused on exosomes derived from these sources, revealing that exosomes are frequently involved in cancer progression [84]. Exosomes have been employed as carriers of therapeutic agents, especially for the targeted delivery of small molecules. Their small size allows exosomes to penetrate the tumor tissue through the enhanced permeability and retention (EPR) effect [85] (schematic representation in Fig. 4). In several recent studies, exosomes have been designed and evaluated as theranostic nanostructures [86, 87]. Due to favorable characteristics such as excellent biocompatibility, high effectiveness, and minimal immunogenicity, exosomes are considered suitable nanocarriers for drug delivery cancer-related studies [88]. For example, exosome-delivered drugs such as doxorubicin (Dox) reduce cytotoxicity in sensitive body organs [89]. In addition, several studies confirm the efficiency of exosomes in drug delivery against gastrointestinal cancers. Pascucci et al. designed a treatment method based on MSC-derived exosomes carrying paclitaxel (PAC) in the tumor microenvironment. They demonstrated that exosomal release increased the anti-proliferative activity of PAC, effectively reducing the proliferation of cancer cells in pancreatic adenocarcinoma [90]. Others investigated the effectiveness of exosomes carrying anti-miR-214 in reversing chemoresistance to cisplatin in GC. This in vitro, showed that the exosomes could sensitize GC cells to cisplatin [91].
Exosome scaffold proteins have also emerged as promising molecules; for instance, they can be applied to improve the recognition of tumor cells by the immune system. For example, an exosome scaffold protein characterized from cloned cancer exosomes contained SIRP α (signal regulatory protein α), an antagonist of CD47 on tumor cells that has been proposed as a therapeutic tool to increase tumor cell phagocytosis by bone marrow-derived macrophages. In vivo studies show that tumor growth is reduced following this phagocytosis. In addition, protein delivery through exosome scaffold proteins is more effective as compared to that via protein-scaffold-based nanocages such as ferritin-SIRP α [92]. Another study investigated exosomes isolated from A33-positive LIM1215 cells and, after Dox loading, surface-functionalized with superparamagnetic iron oxide nanoparticles (SPIONs) coated with A33 antibody to target CRC. The findings revealed that the A33Ab-superparamagnetic nanoparticle-Exo/Dox platform can effectively prevent the growth of colon cancer cells [93]. Several ongoing clinical trial studies evaluating exosomes for gastrointestinal cancer treatment are listed in Table 3.
5 Exosomes as biological drug carriers
In recent years, exosomes had a significant impact on the diagnosis and prognosis of numerous diseases, including diabetes, Parkinson’s, Alzheimer’s, cancer, and infectious diseases, as a result of the advancements made and the enormous increase in the number of various types of drug carrier systems available for use in clinical settings. The last several decades have seen increased interest in nanoparticles (e.g., polysomes, micelles, and liposomes) as drug carriers in clinical research. These nanoparticles are characterized by few undesirable side effects, a variety of medicinal substance delivery capabilities, large drug encapsulation, high efficacy, as well as low toxicity, and have the capacity to maintain drug concentrations, prevent drug degradation, interact with their biological environment, and increase drug absorption of the desired tissue. Exosomes are nanoparticles produced by cells that can outperform these ‘traditional’ nanocarriers. If these delivery systems are carefully developed in accordance with the target and route of administration, they could address some of the problems associated with the delivery of active molecules, such as peptides, proteins, genes, and oligonucleotides.
5.1 Targeted delivery
High toxicity, multiple drug resistance, non-specific targeting, and poor stability are typical examples of the drawbacks encountered in drug delivery research [94]. Using immature DCs, Alvarez et al. were the first to show that exosomes can deliver medicines in a targeted manner [95, 96]. Exosomes can protect drugs from breakdown by the extracellular environment and are crucial in both physiological and pathological processes. Compared to other pharmaceutical systems (e.g., liposomes, lipid nanoparticles, viral vectors), exosomes have benefits to ensure efficient delivery, including better biocompatibility, lower immunogenicity and cytotoxicity in normal tissues, increased stability due to surface expression of CD55 and CD59, small size, the ability to cross the blood–brain barrier, high specificity for binding to the target cell, and a longer half-life. Additionally, Additionally, exosomes rely on a natural mechanism for the transport and delivery of specific drugs [97,98,99]. Although inconsistent results can be obtained by variations in the cells of origin, separation techniques, or specific protein/lipid surface profiles, exosomes generally perform better than conventional synthetic drug administration techniques [100, 101].
The cellular resistance to therapeutics is one of the major problems in treating gastrointestinal (GI) cancer. Despite the fact that the precise mechanisms underlying drug resistance are still incompletely understood, a number of contributing factors have been identified. Exosomes have been linked to targeting GI cancer invasion, angiogenesis, and treatment resistance [102,103,104]. Thus, exosomes can mediate endocytosis of specific medications into cancer cells [58], and can be genetically modified to express peptides or ligands on their surface to facilitate this process. Such modifications improve targeting and specificity by transferring certain exosome receptors and shortening the time it takes for exosomes to reach the therapeutic concentration in the intended tissues, collectively resulting in enhanced drug performance and better therapeutic effects [105]. Exosomes released by MSCs from healthy tissue may slow the growth of tumors by obstructing signaling pathways involved in oncogenic reprogramming. On the other hand, exosomes generated from tumor cells can cause malignancy and subsequent cancer of recipient cells [106]. Other work further supported the notion that targeted exosomes can be employed as an effective drug delivery method, demonstrating HER2 + cells take them up more readily than HER2- cells [107]. One of the main treatments for advanced stomach cancer is chemotherapy, Of course, it can also face challenges. The requirement for high dosages, low therapeutic indices, and maximum therapeutic concentrations, in addition to side effects, sensitive immunological responses, and the presence of mucosal physiological barriers represent some of the therapeutic challenges. It has been well documented that the necessary high medicine dosages can lead to drug resistance [99]. Exosomes fulfil a vital function in the detection and treatment of stomach cancer [108]. Exosomal circRNAs are highly promising therapeutic agents with anticancer effects, as indicated by their roles in controlling tumor growth, metastasis, angiogenesis, metabolism, and dissemination of GI cancers [109]. The development of targeted therapy has been considered for the treatment of gastric cancer; thus, gastric cancer exosomes have been reported to have high target efficacy, albeit with relatively low efficiency. Tian et al. developed a straightforward technique to produce high-performance gastric cancer hybrid exosomes as a possible drug carrier for targeted therapy of gastric cancer (HGCE). In vitro and in vivo studies demonstrated that Dox-loaded HGCE (Dox/HGCE) exhibited good anticancer efficacy as well as high and specific activity for gastric cancer cells (SGC 7901), indicating the therapeutic potential of this delivery system [110].
The importance of exosomes in GC suggests they could represent therapeutic targets. Proton pump inhibitors (PPIs) regulate the HIF-1-FOXO1 axis to stop stomach cancer from spreading. A high dose of PPI can inhibit GC malignancy and control the microenvironment surrounding the tumor. PPIs improve the effects of anticancer medications in GC cells by reducing stomach acid production. PPIs may be helpful as a therapeutic strategy for the treatment of GC considering they inhibit GC cells from releasing exosomes and prevent them from producing CAFs [111, 112]. Another study used exosomes as nanocarriers to transport circDIDO1 to GC cells; results showed that circDIDO1 can counterbalance the effects of miR-1307-3p overexpression in GC by serving as a miRNA sponge to stimulate SOCS2 expression, which in turn prevents the proliferation of GC cells [113]. Hosseini et al. looked at the targeted administration of Dox-loaded HEK293-derived exosomes functionalized with an anti-nucleolin (AS1411) aptamer for the treatment of CRC. The results showed that this functionalization markedly enhanced the binding affinity and uptake rate in nucleolin-positive cancer cells, suggesting Dox loading of AS1411-functionalized exosomes could serve as a potential cancer treatment approach in clinical settings [114]. Bagheri et al. looked at the potential of Dox-loaded exosomes produced by MSCs as a versatile tool for treating CRC and platform for therapeutic use. In vitro data demonstrated that DOX@exosome-apt delivers Dox to MUC1-positive cancer cells in a highly effective manner. Additionally, a single intravenous dose of DOX@exosome-apt significantly suppressed tumor growth as compared to free Dox in an in vivo study utilizing BALB/c mice and the C26 ectopic model (mouse colon cancer). Ex vivo fluorescence imaging further validated the beneficial biodistribution of DOX@exosome-apt by showing higher tumor accumulation and quicker liver clearance when compared to DOX@exosome and free Dox. Thus, MUC1 aptamer-functionalized exosomes can be used therapeutically to deliver Dox to colon cancer in a flexible and safe manner [102].
5.2 Co-delivery therapy
According to recent studies, gene therapy or a cocktail of medications can be used to more effectively target pathways associated with cancer [115, 116]. Natural therapies can improve sensitivity to chemotherapy and strengthen immunity because they are risk-free and rarely cause harm. Widely biodistributed medications show both beneficial off-target effects and anticancer consequences. Combination drug therapy has been shown to be effective because the suppression of numerous mechanisms or junctions leads to the activation of a single mechanism. The benefits of combination therapy are supported by clinical trials showing synergistic effects [117,118,119]. Accumulating evidence suggests that combining (natural) chemotherapy sensitizers with chemotherapeutic agents can reduce drug-associated side effects and battle multidrug resistance (MDR) [120]. Co-delivery of various medications via a drug carrier may improve treatment for malignancies by synchronizing medication exposure and promoting synergistic pharmacological activity in tumor cells [121]. In addition, this approach allows for optimal loading capacity, stability, release kinetics, biocompatibility, and tumor targeting [122], and may effectively reduce toxicity, minimize adverse drug reactions, and overcome MDR, which is a significant obstacle to the long-term efficacy of chemotherapeutic agents. Furthermore, co-delivery systems can benefit controlled release in cancer treatment protocols, decreasing side effects of prescribed medications, and improving treatment effectiveness [123]. Selecting appropriate nanocarriers for efficient encapsulation of natural active substances and chemotherapy drugs is a significant issue [124]. Liposomes, micelles, nanoparticles, and inorganic nanoparticles are just a few of the co-delivery systems that have become available as a result of the advent of nanotechnology to battle tumor MDR. To prevent MDR, these nanocarriers are co-loaded with chemotherapeutics and natural products, limiting drug efflux and/or enhancing intracellular drug accumulation in either an active or passive manner. Understanding the features of the individual carriers, which all have distinct nanostructures, materials, and preparation procedures, will help with the design of co-delivery nanocarriers [125]. Ideally, these co-delivery systems should be able to encapsulate hydrophobic as well as hydrophilic medications, and transport both conventional chemotherapies and cellular regulatory molecules like nucleic acids [126]. Despite the significant advancements in nanotechnology, there are still a number of issues that need to be resolved in order to create the ideal drug delivery system. These include those related to encapsulating drugs with a variety of solubilities and physicochemical properties, increasing drug concentration in tumor tissues, and controlling their sequential drug release [127]. Theoretically, considering multiple cargo delivery capabilities of natural intercellular delivery systems would qualify exosomes as ideal nanoplatforms for the development of novel integration techniques [128]. Surprisingly, only a relatively small number of papers have reported on exosomes as co-delivery systems. Qi Zhan et al. described a novel combination gene/chemistry anticancer method in which blood exosomes were developed as a nanoplatform for the targeted and effective delivery of hydrophobic medicines and nucleic acids to tumor cells. This study demonstrated that these vesicles could efficiently and flexibly transport hydrophobic drugs like Dox and cholesterol-modified miR-21i by fully utilizing their original lipid bilayer structure. The addition of L17E peptides maximized the effectiveness of cargo delivery by hastening the endocytic absorption and endosomal egress of exosome-encapsulated payloads. This co-delivery nanosystem was able to preferentially accumulate in tumors because of the clusters of superparamagnetic nanoparticles and nanoscale size. In mice carrying the U87 gene that were systemically fed D-Exos/miR21i-L17E, tumor suppression was significantly enhanced with only moderate side effects. This is the first example of the use of blood exosomes in combined cancer chemo- and gene-therapy; by substituting a therapeutic medication combination and considering a range of tumor suppressor pathways, this constructed exosome-based nanosystem could be considered for a wide range of medicinal applications [129]. Recent work proposed a viable way to overcome drug resistance in CRC and boost the effectiveness of cancer treatment by simultaneously delivering functional miR-21 inhibitory oligonucleotide (miR-21i) and 5-fluorouracil (5-FU) using exosomes. This exosome-based 5-FU and miR-21i co-delivery system promoted cellular uptake and reduced the expression of miR-21 in 5-FU resistant HCT-1165FR cell lines expressing the Her2 gene. Downregulation of miR-21induced cell cycle arrest, lowered tumor growth, increased apoptosis, and rescued the expression of PTEN and hMSH2. Importantly, as compared to either mono-therapy, combined delivery of miR-21i and 5-FU effectively reversed drug resistance and increased the cytotoxicity in 5-FU-resistant colon cancer cells [130]. Exosomes containing oxaliplatin and PGM5-AS1 can also reverse drug resistance, offering another method for treating CRC [131]. Others developed tumor-derived exosomes for co-delivering aggregation-induced emission luminogens (AIEgens) and PPIs. This combined therapy was designed to promote AIEgens-based photodynamic therapy (PDT) via PPI-mediated inhibition of cell glutamine metabolism. Evaluation in a MGC803 gastric cancer subcutaneous model revealed that this exosome-based co-therapy can effectively prevent tumor growth and promote tumor immunogenic death [132].
6 Exosomes as vaccines against cancer
Over the past half-century, therapeutic cancer vaccines (TCVs) have been investigated as a potential immunotherapeutic approach to treat cancer by stimulating CD8 + cytotoxic T cells to generate tumor-specific responses. TCVs have gained renewed enthusiasm due to their potential to improve the efficacy of checkpoint inhibition [133]. They target antigens specifically associated with malignant cells, resulting in fewer side effects and increased safety compared to existing cancer treatments. TCVs can be administered through various techniques using different antigens, adjuvants, and delivery vectors [134]. These techniques include peptide-, DNA/RNA-, and cell transfer-based cancer vaccines, each with its own advantages and disadvantages. Additionally, adoptive cell immunotherapies (ACTs) such as CAR-T and TIL have shown to be excellent antitumor therapies with strong and highly personalized immunogenic profiles. However, they are costly, time-consuming, and labor-intensive. [135,136,137,138,139]. Exosome vaccines have been introduced as a new platform for more efficient delivery of tumor-associated antigens, showing better efficacy than ACTs in eradicating tumors in a T cell-dependent and MHC-restricted manner. Exosomes combine processed peptides derived from antigenic material expressing surface MHC I/II and deliver functional peptide-MHC complexes to naive target cells, stimulating the expansion of peptide-specific clonal T cells and promoting tumor cytotoxicity through MHC-I and MHC-II antigen processing. Exosomal vaccines have shown superior efficacy compared to ACTs in eradicating tumors in a T cell-dependent and MHC-restricted manner [140, 141]. Understanding how exosomes activate antitumor immunity is crucial for advancing this promising immunotherapy. Exosomes deliver processed peptides expressing surface MHC I/II, stimulating target cells and promoting T cell activation [142, 143]. Several studies demonstrated that the best model of using exosomes is to load DCs with tumor antigens and subsequently extract the produced exosomes [144]. Loading DCs with tumor antigens and extracting the produced exosomes has been identified as an effective model. Exosomes stimulate the expansion of peptide-specific clonal T cells, promote CD8 + T cell maturation, and activate NF-κB in macrophages for tumor cytotoxicity through MHC-I. Additionally, antigen processing through MHC-II leads to more efficient activation of CTLs. Studies have demonstrated that different exosomes can effectively stimulate naive T cell proliferation and differentiation into cytotoxic T lymphocytes, leading to stronger killing activities against tumor cells [141, 144, 145]. A study examining DC-OVA-derived exosomes (EXODC) showed that EXODC can more effectively stimulate naive OVA-specific CD8 + T cell proliferation and differentiation into cytotoxic T lymphocytes in vivo as compared to EG7 tumor cell line-derived exosomes (TEXEG7); the stronger killing activities by EXODC against lung tumor cells were attributed to the expression of co-stimulatory molecules such as CD40 and CD80 [146]. Other work demonstrated that heat shock protein-70 (Hsp70)-enriched tumor exosomes increased the expression of MHCII and induced strong Th1 immune responses, eliminating CT26 (mouse colon carcinoma cells) cancer cells in allogeneic hosts [147]. These findings suggest that Hsp70 exosomes can be used as an innovative vaccination for the management of CRC [148, 149]. In a phase I clinical trial, ascites-derived exosomes (Aex) were used in combination with granulocyte–macrophage colony-stimulating factor (GM-CSF) in 40 patients with CRC. The results showed that treatment with the combined vaccine, but not Aex alone, triggered a specific anti-tumor CTL response, and was safe and well tolerated. Thus, immunotherapy using Aex in combination with GM-CSF could be considered as an effective vaccine in the treatment of patients with metastatic CRC [150]. The exosomes in addition of induction of strong immune responses, eliminate cancer cells, and modulate tumor progression. Furthermore, exosomes interact with immune cells to induce anti-metastatic effects and trigger specific anti-tumor CTL responses. In preclinical and clinical trials, exosomes have shown promise as cell-free anti-cancer vaccines. The potential of exosome-based cancer immunotherapy is highly promising and warrants further clinical validation [151].
7 Conclusion
Recognition of the utilization of small extracellular vesicles for diagnostic and therapeutic purposes is fast growing. We will have an opportunity to advance the therapeutic use of exosomes as we learn more about their biogenesis and function. Furthermore, recent research has unequivocally established the value of exosomes as both natural drug delivery vehicles and biomarkers for disease diagnosis. Further advancement in the applicability of exosomes depends on the development of more precise and trustworthy separation techniques. In this regard, it is essential to combine basic science research with cutting-edge technology. For example, the use of exosomes as natural nanocarriers can have high potential in personalized treatments. To achieve this attractive goal, future research is required to fully understand the nature of exosomes in terms of their membrane composition and cargo. After identification of the desired properties of the exosome, it can be engineered in accordance with the therapeutic goals for treatment of various cancers, including gastrointestinal cancers. However, many challenges remain for the widespread use of exosomes in the clinic. Indeed, it is still incompletely understood how exosomes interact with the TME. In addition, it is unclear which cellular source of exosomes is safest and most efficient for the delivery of therapeutic agents, and techniques for targeting exosomes for clinical applications have not yet been optimally developed (there is a need for sensitive and accurate platforms). There are a multiple exosomal nucleic acids and proteins that can be used as biomarkers to detect cancers, selecting the best one is difficult. To facilitate the widespread clinical use of exosomes, it is necessary to be able to isolate and purify exosomes in a fast and cost-effective way. Furthermore, the clinical applicability of exosomes strongly relies on whether or not the many preclinical in vitro and in vivo findings can be successfully translated into clinical trial outcomes. Conceivably, in anticipation of clinical trial results and development of high-throughput technologies, exosomes have the potential to greatly improve and revolutionize cancer diagnosis as well as treatment strategies in the not-so-distant future.
Data availability
No datasets were generated or analysed during the current study.
References
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478): eaau6977.
Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–51.
Tschuschke M, Kocherova I, Bryja A, Mozdziak P, Angelova Volponi A, Janowicz K, et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med. 2020;9(2):436.
Mahmoudvand S, Shokri S, Nakhaie M, Jalilian FA, Mehri-Ghahfarrokhi A, Yarani R, Shojaeian A. Small extracellular vesicles as key players in cancer development caused by human oncogenic viruses. Infect Agents Cancer. 2022;17(1):1–16.
Kumar DN, Chaudhuri A, Dehari D, Shekher A, Gupta SC, Majumdar S, et al. Combination therapy comprising paclitaxel and 5-fluorouracil by using folic acid functionalized bovine milk exosomes improves the therapeutic efficacy against breast cancer. Life. 2022;12(8):1143.
del Pozo-Acebo L, López de las Hazas M, Tomé-Carneiro J, Gil-Cabrerizo P, San-Cristobal R, Busto R, et al. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int J Mol Sci. 2021;22(3):1105.
Kalluri R. The biology and function of exosomes in cancer. J Clin Investig. 2016;126(4):1208–15.
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharmaceutica Sinica B. 2021;11(9):2783–97.
Nam GH, Choi Y, Kim GB, Kim S, Kim SA, Kim IS. Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv Mater. 2020;32(51):2002440.
Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65(3):357–67.
Whitford W, Guterstam P. Exosome manufacturing status. Future Med Chem. 2019;11(10):1225–36.
Hou H, Tian Z, Zhang W. Application of exosomes as markers and drug carriers in tumors.
Wang D-K, Zuo Q, He Q-Y, Li B. Targeted immunotherapies in gastrointestinal cancer: from molecular mechanisms to implications. Front Immunol. 2021;12: 705999.
Tang X-H, Guo T, Gao X-Y, Wu X-L, Xing X-F, Ji J-F, Li Z-Y. Exosome-derived noncoding RNAs in gastric cancer: functions and clinical applications. Mol Cancer. 2021;20(1):1–15.
Li W, Li C, Zhou T, Liu X, Liu X, Li X, Chen D. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16(1):1–12.
Rahbari M, Rahbari N, Reissfelder C, Weitz J, Kahlert C. Exosomes: novel implications in diagnosis and treatment of gastrointestinal cancer. Langenbecks Arch Surg. 2016;401(8):1097–110.
Munson P, Shukla A. Exosomes: potential in cancer diagnosis and therapy. Medicines. 2015;2(4):310–27.
Liang Z-X, Liu H-S, Wang F-W, Xiong L, Zhou C, Hu T, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Disease. 2019;10(11):1–17.
Liu H, Yang Z, Zhang J, Zhu X. MicroRNA-217 in plasma: a potential biomarker in gastric cancer. Int J Clin Exp Med. 2017;10(2):3313–20.
Wang Y, Zhao R, Jiao X, Wu L, Wei Y, Shi F, et al. Small extracellular vesicles: functions and potential clinical applications as cancer biomarkers. Life. 2021;11(10):1044.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208.
Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):1–16.
Guo W, Gao Y, Li N, Shao F, Wang C, Wang P, et al. Exosomes: new players in cancer. Oncol Rep. 2017;38(2):665–75.
Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–37.
Jackson CE, Scruggs BS, Schaffer JE, Hanson PI. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys J. 2017;113(6):1342–52.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):1–19.
Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131–9.
Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–62.
Edgar JR, Eden ER, Futter CE. Hrs-and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic. 2014;15(2):197–211.
Li X, Wang Y, Wang Q, Liu Y, Bao W, Wu S. Exosomes in cancer: small transporters with big functions. Cancer Lett. 2018;435:55–65.
Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharmacol. 2017;7:533.
Tang XH, Guo T, Gao XY, Wu XL, Xing XF, Ji JF, Li ZY. Exosome-derived noncoding RNAs in gastric cancer: functions and clinical applications. Mol Cancer. 2021;20(1):99.
Li L, Zhao J, Zhang Q, Tao Y, Shen C, Li R, et al. Cancer cell-derived exosomes promote HCC tumorigenesis through hedgehog pathway. Front Oncol. 2021;11: 756205.
Li Y, Meng L, Li B, Li Y, Shen T, Zhao B. The exosome journey: from biogenesis to regulation and function in cancers. J Oncol. 2022;2022:1–13.
Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387–97.
Zhou Y, Zhou G, Tian C, Jiang W, Jin L, Zhang C, Chen X. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip Rev RNA. 2016;7(6):758–71.
Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71(4):537–43.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6(4):287–96.
Ma M, Chen S, Liu Z, Xie H, Deng H, Shang S, et al. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. OncoTargets Thera. 2017;10:4161–71.
Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–41.
Wang L, Zhang J. Exosomal lncRNA AK139128 derived from hypoxic cardiomyocytes promotes apoptosis and inhibits cell proliferation in cardiac fibroblasts. Int J Nanomed. 2020;15:3363–76.
Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8(1):1–13.
Mahmoudvand S, Shokri S, Nakhaie M, Jalilian FA, Mehri-Ghahfarrokhi A, Yarani R, Shojaeian A. Small extracellular vesicles as key players in cancer development caused by human oncogenic viruses. Infect Agents Cancer. 2022;17(1):58.
Lin Y, Zhang C, Xiang P, Shen J, Sun W, Yu H. Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. J Extracell Vesicles. 2020;9(1):1722385.
Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31(17):3513–23.
Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta. 2019;1871(2):455–68.
Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875.
Lokody I. Exosomally derived miR-105 destroys tight junctions. Nat Rev Cancer. 2014;14(6):386–7.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15.
Vella LJ. The emerging role of exosomes in epithelial–mesenchymal-transition in cancer. Front Oncol. 2014;4:361.
Tanaka M, Kuriyama S, Itoh G, Maeda D, Goto A, Tamiya Y, et al. Mesothelial cells create a novel tissue niche that facilitates gastric cancer invasion. Can Res. 2017;77(3):684–95.
Liu W, Song N, Yao H, Zhao L, Liu H, Li G. miR-221 and miR-222 simultaneously target RECK and regulate growth and invasion of gastric cancer Cells. Med Sci Monit Int Med J Exp Clin Res. 2015;21:2718–25.
Danac JMC, Uy AGG, Garcia RL. Exosomal microRNAs in colorectal cancer: overcoming barriers of the metastatic cascade (Review). Int J Mol Med. 2021;47(6):1–16.
Thapa N, Chwae YJ, Yoo KH, Won T-B, Kang D, Choi D, Kim J. Exosomal delivery of TRAIL and miR-335 for the treatment of hepatocellular carcinoma (review). Int J Mol Med. 2023;51(1):3.
Chu X, Yang Y, Tian X. Crosstalk between pancreatic cancer cells and cancer-associated fibroblasts in the tumor microenvironment mediated by exosomal MicroRNAs. Int J Mol Sci. 2022;23(17):9512.
Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–68.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
Scavo MP, Depalo N, Tutino V, De Nunzio V, Ingrosso C, Rizzi F, et al. Exosomes for diagnosis and therapy in gastrointestinal cancers. Int J Mol Sci. 2020;21(1):367.
Lane RE, Korbie D, Hill MM, Trau M. Extracellular vesicles as circulating cancer biomarkers: opportunities and challenges. Clin Transl Med. 2018;7(1): e14.
Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemostasis JTH. 2010;8(2):315–23.
Li A, Zhang T, Zheng M, Liu Y, Chen Z. Exosomal proteins as potential markers of tumor diagnosis. J Hematol Oncol. 2017;10(1):175.
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur Basant K, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–26.
Notarnicola M, Altomare DF, Calvani M, Orlando A, Bifulco M, D’Attoma B, Caruso MG. Fatty acid synthase hyperactivation in human colorectal cancer: relationship with tumor side and sex. Oncology. 2006;71(5–6):327–32.
Notarnicola M, Messa C, Caruso MG. A significant role of lipogenic enzymes in colorectal cancer. Anticancer Res. 2012;32(7):2585.
Van de Sande T, Roskams T, Lerut E, Joniau S, Van Poppel H, Verhoeven G, Swinnen JV. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol. 2005;206(2):214–9.
Kitamura C, Sonoda H, Nozawa H, Kano K, Emoto S, Murono K, et al. The component changes of lysophospholipid mediators in colorectal cancer. Tumor Biology. 2019;41(5):1010428319848616.
Janakiram NB, Rao CV. The role of inflammation in colon cancer. Adv Exp Med Biol. 2014;816:25–52.
Coviello G, Tutino V, Notarnicola M, Caruso MG. Erythrocyte membrane fatty acids profile in colorectal cancer patients: a preliminary study. Anticancer Res. 2014;34(9):4775–9.
Notarnicola M, Lorusso D, Tutino V, De Nunzio V, De Leonardis G, Marangelli G, et al. Differential tissue fatty acids profiling between colorectal cancer patients with and without synchronous metastasis. Int J Mol Sci. 2018;19(4):962.
Haraszti RA, Didiot M-C, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5(1):32570.
Huang T, Deng C-X. Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int J Biol Sci. 2019;15(1):1–11.
Kosaka N, Kogure A, Yamamoto T, Urabe F, Usuba W, Prieto-Vila M, Ochiya T. Exploiting the message from cancer: the diagnostic value of extracellular vesicles for clinical applications. Exp Mol Med. 2019;51(3):1–9.
Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41.
Tomasetti M, Lee W, Santarelli L, Neuzil J. Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med. 2017;49(1): e285.
Dai G, Yao X, Zhang Y, Gu J, Geng Y, Xue F, Zhang J. Colorectal cancer cell–derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bull Cancer. 2018;105(4):336–49.
Herrera M, Llorens C, Rodríguez M, Herrera A, Ramos R, Gil B, et al. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and cancer-associated fibroblasts in colorectal cancer. Mol Cancer. 2018;17(1):114.
Ju QZL, Gao J, Zhou L, Xu Y, Sun Y, Zhao X. Mutant p53 increases exosome-mediated transfer of miR-21-3p and miR-769-3p to promote pulmonary metastasis. Chin J Cancer Res. 2019;31(3):533–46.
Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9(1):771.
Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37(1):324.
Gutkin A, Uziel O, Beery E, Nordenberg J, Pinchasi M, Goldvaser H, et al. Tumor cells derived exosomes contain hTERT mRNA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget. 2016;7(37):59173.
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53.
Pan L, Liang W, Fu M, Huang Z-H, Li X, Zhang W, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143(6):991–1004.
Melzer CRV, Yang Y, Bähre H, von der Ohe J, Hass R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers. 2019;11(6):798.
Zhou Y, Zhang Y, Gong H, Luo S, Cui Y. The role of exosomes and their applications in cancer. Int J Mol Sci. 2021;22(22):12204.
Syn NL, Wang L, Chow EK-H, Lim CT, Goh B-C. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665–76.
Palazzolo S, Bayda S, Hadla M, Caligiuri I, Corona G, Toffoli G, Rizzolio F. The clinical translation of organic nanomaterials for cancer therapy: a focus on polymeric nanoparticles, micelles, liposomes and exosomes. Curr Med Chem. 2018;25(34):4224–68.
Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24(6):1159–66.
Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang W, et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–82.
Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, Rizzolio F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (Lond). 2016;11(18):2431–41.
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Controlled Release. 2014;192:262–70.
Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26(3):774–83.
Cho E, Nam GH, Hong Y, Kim YK, Kim DH, Yang Y, Kim IS. Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J Controlled Release. 2018;279:326–35.
Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomed Nanotechnol Biol Med. 2018;14(7):1973–85.
Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomed. 2011;6:765.
Jiang X-C, Gao J-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521(1–2):167–75.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917.
He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237.
Zhang Y, Liu Q, Zhang X, Huang H, Tang S, Chai Y, et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J Nanobiotechnol. 2022;20(1):1–29.
Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–43.
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5(1):1–10.
Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261: 118369.
Nedaeinia R, Manian M, Jazayeri M, Ranjbar M, Salehi R, Sharifi M, et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24(2):48–56.
Salehi M, Vafadar A, Khatami SH, Taheri-Anganeh M, Vakili O, Savardashtaki A, et al. Gastrointestinal cancer drug resistance: the role of exosomal miRNAs. Mol Biol Rep. 2022;49(3):2421–32.
Xitong D, Xiaorong Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene. 2016;575(2):377–84.
Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72(4):659–71.
Gomari H, Forouzandeh Moghadam M, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. OncoTargets Thera. 2018;11:5753–62.
Jiang L, Gong X, Liao W, Lv N, Yan R. Molecular targeted treatment and drug delivery system for gastric cancer. J Cancer Res Clin Oncol. 2021;147(4):973–86.
Wang D, Li R, Jiang J, Qian H, Xu W. Exosomal circRNAs: novel biomarkers and therapeutic targets for gastrointestinal tumors. Biomed Pharmacother. 2023;157: 114053.
Tian Q, Guo Y, Li D, Dong L. Hybrid gastric cancer exosome as potential drug carrier for targeted gastric cancer therapy. J Biomater Tissue Eng. 2022;12(11):2228–32.
Guan X-W, Zhao F, Wang J-Y, Wang H-Y, Ge S-H, Wang X, et al. Tumor microenvironment interruption: a novel anti-cancer mechanism of proton-pump inhibitor in gastric cancer by suppressing the release of microRNA-carrying exosomes. Am J Cancer Res. 2017;7(9):1913.
Fu M, Gu J, Jiang P, Qian H, Xu W, Zhang X. Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer. 2019;18(1):1–12.
Guo Z, Zhang Y, Xu W, Zhang X, Jiang J. Engineered exosome-mediated delivery of circDIDO1 inhibits gastric cancer progression via regulation of MiR-1307-3p/SOCS2 Axis. J Transl Med. 2022;20(1):1–15.
Hosseini NF, Amini R, Ramezani M, Saidijam M, Hashemi SM, Najafi R. AS1411 aptamer-functionalized exosomes in the targeted delivery of doxorubicin in fighting colorectal cancer. Biomed Pharmacother. 2022;155: 113690.
Zeinali M, Abbaspour-Ravasjani S, Ghorbani M, Babazadeh A, Soltanfam T, Santos AC, et al. Nanovehicles for co-delivery of anticancer agents. Drug Discov Today. 2020;25(8):1416–30.
Wang Y, Gao S, Ye W-H, Yoon HS, Yang Y-Y. Co-delivery of drugs and DNA from cationic core–shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–6.
Alexis F. Nano-polypharmacy to treat tumors: coencapsulation of drug combinations using nanoparticle technology. Mol Ther. 2014;22(7):1239–40.
He C, Tang Z, Tian H, Chen X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv Drug Deliv Rev. 2016;98:64–76.
Vahed SZ, Salehi R, Davaran S, Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng, C. 2017;71:1327–41.
Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist (Alhambra, Calif). 2019;2(2):141–60.
Wan X, Beaudoin JJ, Vinod N, Min Y, Makita N, Bludau H, et al. Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials. 2019;192:1–14.
Yang Z, Gao D, Cao Z, Zhang C, Cheng D, Liu J, Shuai X. Drug and gene co-delivery systems for cancer treatment. Biomater Sci. 2015;3(7):1035–49.
Nezhadi S, Dorkoosh FA. Co-delivery systems: hope for clinical application? Drug Deliv Transl Res. 2022;12(6):1339–54.
Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–61.
Liang Y, Liu ZY, Wang PY, Li YJ, Wang RR, Xie SY. Nanoplatform-based natural products co-delivery system to surmount cancer multidrug-resistant. J Controlled Release. 2021;336:396–409.
Mahira S, Kommineni N, Husain GM, Khan W. Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: a new insight into nanomedicine based combinational chemotherapy for prostate cancer. Biomed Pharmacother. 2019;110:803–17.
Pan J, Rostamizadeh K, Filipczak N, Torchilin VP. Polymeric co-delivery systems in cancer treatment: an overview on component drugs’ dosage ratio effect. Molecules. 2019;24(6):1035.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q, et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. 2020;10(17):7889.
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18(1):1–15.
Hui B, Lu C, Wang J, Xu Y, Yang Y, Ji H, et al. Engineered exosomes for co-delivery of PGM5-AS1 and oxaliplatin to reverse drug resistance in colon cancer. J Cell Physiol. 2022;237(1):911–33.
Zhu D, Zhang T, Li Y, Huang C, Suo M, Xia L, et al. Tumor-derived exosomes co-delivering aggregation-induced emission luminogens and proton pump inhibitors for tumor glutamine starvation therapy and enhanced type-I photodynamic therapy. Biomaterials. 2022;283: 121462.
Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18(3):183–94.
Lopes A, Vandermeulen G, Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38(1):1–24.
Walters JN, Ferraro B, Duperret EK, Kraynyak KA, Chu J, Saint-Fleur A, et al. A novel DNA vaccine platform enhances neo-antigen-like T cell responses against WT1 to break tolerance and induce anti-tumor immunity. Mol Ther. 2017;25(4):976–88.
Hobernik D, Bros M. DNA vaccines—how far from clinical use? Int J Mol Sci. 2018;19(11):3605.
Kumai T, Kobayashi H, Harabuchi Y, Celis E. Peptide vaccines in cancer—old concept revisited. Curr Opin Immunol. 2017;45:1–7.
Hay AE, Cheung MC. CAR T-cells: costs, comparisons, and commentary. J Med Econ. 2019;22(7):613–5.
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–8.
Pitt JM, André F, Amigorena S, Soria J-C, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Investig. 2016;126(4):1224–32.
Gu X, Erb U, Büchler MW, Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136(4):E74–84.
Krogsgaard M, Davis MM. How T cells “see” antigen. Nat Immunol. 2005;6(3):239–45.
Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–62.
Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy K, et al. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett. 2012;148(1):34–8.
Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8+ CTL-and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010;14(11):2655–66.
Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3(3):205–11.
Cho J-A, Lee Y-S, Kim S-H, Ko J-K, Kim C-W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275(2):256–65.
Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–83.
Wang J, Wang Z, Mo Y, Zeng Z, Wei P, Li T. Effect of hyperthermic CO2-treated dendritic cell-derived exosomes on the human gastric cancer AGS cell line. Oncol Lett. 2015;10(1):71–6.
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16(4):782–90.
Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67(4):739–48.
Leah K Miller Mea. https://classic.clinicaltrials.gov/ct2/show/NCT03874559.
Jarnagin W. https://classic.clinicaltrials.gov/ct2/show/NCT02393703.
Hinestrosa JP, Kurzrock R, Lewis JM, Schork NJ, Schroeder G, Kamat AM, et al. Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun Med. 2022;2:29.
Gerald W. Dryden J. https://classic.clinicaltrials.gov/ct2/show/NCT01294072.
Tang M, Chen Y, Li B, Sugimoto H, Yang S, Yang C, et al. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. FASEB J. 2021;35(5): e21557.
Acknowledgements
Faye Yun was supported by the Cal State Fullerton U-RISE at Cal State Fullerton grant 5T34 GM149493-02 from the National Institutes of Health. The authors would like to acknowledge Science Impact (Winnipeg, Canada) for post-editing the final manuscript.
Author information
Authors and Affiliations
Contributions
A.S., M.S.P., SR.N.K., Z.S.A., A.K., A.A., F.Y. and K.B. wrote the main manuscript text. A.S. and SR.N.K. prepared the figures. S.P. and R.A. supervised and wrote the main manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shojaeian, A., Naeimi Torshizi, S.R., Parsapasand, M.S. et al. Harnessing exosomes in theranostic applications: advancements and insights in gastrointestinal cancer research. Discov Onc 15, 162 (2024). https://doi.org/10.1007/s12672-024-01024-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01024-x