Abstract
Previous clinic models for patients with hepatocellular carcinoma (HCC) receiving transarterial chemoembolization (TACE) mainly focused on the overall survival, whereas a simple-to-use tool for predicting the response to the first TACE and the management of risk classification before TACE are lacking. Our aim was to develop a scoring system calculated manually for these patients. A total of 437 patients with hepatocellular carcinoma (HCC) who underwent TACE treatment were carefully selected for analysis. They were then randomly divided into two groups: a training group comprising 350 patients and a validation group comprising 77 patients. Furthermore, 45 HCC patients who had recently undergone TACE treatment been included in the study to validate the model’s efficacy and applicability. The factors selected for the predictive model were comprehensively based on the results of the LASSO, univariate and multivariate logistic regression analyses. The discrimination, calibration ability and clinic utility of models were evaluated in both the training and validation groups. A prediction model incorporated 3 objective imaging characteristics and 2 indicators of liver function. The model showed good discrimination, with AUROCs of 0.735, 0.706 and 0.884 and in the training group and validation groups, and good calibration. The model classified the patients into three groups based on the calculated score, including low risk, median risk and high-risk groups, with rates of no response to TACE of 26.3%, 40.2% and 76.8%, respectively. We derived and validated a model for predicting the response of patients with HCC before receiving the first TACE that had adequate performance and utility. This model may be a useful and layered management tool for patients with HCC undergoing TACE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transarterial chemoembolization (TACE) is universally acknowledged as an effective treatment for patients with intermediate and advanced hepatocellular carcinoma (HCC) [1,2,3] and definitely prolongs the survival of those patients [1, 4]. However, due to the heterogeneity of patients with HCC, which contributes to a heterogeneous response to TACE [5,6,7], approximately half of the patients receiving TACE did not obtain an objective response [8]. The accurate identification of patients with a high probability of nonresponse to the first TACE is crucial for treatment strategy decisions [9]. Therefore, a convenient and effective way to screen out those patients before TACE might light on decision making.
Based on the different tumor characteristics and statuses of individual patients with HCC, selecting the most appropriate treatment for every patient with HCC is the “ideal” treatment procedure. The establishment of clinical prediction models has been widely used to customize personalized treatment [10, 11]. Some individualized prediction models for patients receiving TACE have been developed to predict the prognosis of those patients and include the six-and-twelve score [12], HAP score [13] and SNACOR model [14]. However, those models did not focus on the treatment response of the first TACE, but the prediction of overall survival (OS). Recently, an increasing number of predictive models have focused on the first response to TACE. Fundamental prognostic models of radiomics based on ultrasound [15], computed tomography (CT) [16, 17], magnetic resonance imaging (MRI) [18, 19] and digital subtraction angiography (DSA) [20] for predicting the response to the first TACE showed adequate performance. However, only the texture of the tumor was included in those models, and personal liver function was excluded, which might overlook some vital clinical information [3]. A machine-learning model that combined imaging features and liver function, provided an accurate prediction of the first response to TACE, but the complicated computational formula limited its practicability in the clinic [21]. Thus, the development of a simple-to-use, accurate and customized model for predicting the initial response to TACE among patients with HCC is desired.
In this study, we aimed to drive and validate the personalized model, which incorporated both objective imaging characteristics and serological testing, for predicting the probability of a response to the first TACE, and it can be simply and accurately used to guide clinical decisions.
2 Patients and methods
2.1 Study design and patient eligibility
This study was designed according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) [22] to establish a reliable and feasible prediction model for calculating the nonresponse probability of TACE [23]. The checklist of items is shown in Supplemental Table 1.
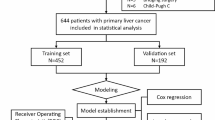
This retrospective study consisted of consecutive adult patients who underwent DSA between January 2012 and January 2022 at the Department of Gastroenterology or Interventional Surgery, the First Affiliated Hospital of Nanchang University. A total of 4482 consecutive patients receiving DSA were retrospectively screened. Our target patients are HCC patients who have not received any before TACE. Patients were excluded if (1) the diagnosis was not HCC, (2) patients were received TACE, surgery, targeted drug therapy or radiotherapy and chemotherapy before hospitalization, (3) CT or MRI was not performed at the 4–8-week follow-up after TACE, or (4) a serious lack of clinical data existed. The flow chart of the selection process is shown in Fig. 1. Finally, 437 patients with unresectable who underwent a first TACE were recruited for analysis. Besides, for enhanced model validation and to ascertain its stability, we gathered data from patients who underwent first TACE between February 2022 and July 2023.
Patients with BCLC B and C stage undergo conventional TACE treatment, including those with multiple lesions, portal vein invasion, or extrahepatic spread, which based on the Barcelona Clinic Liver Cancer (BCLC) stage [24], strictly.
To ensure an ample sample size for rigorous model validation, effectively achieving our goal, the total patients were randomly divided into two groups, including training (70%) and validation groups (30%), which is supported by TRPOD standards [22] and seven steps for development and an ABCD for validation [25].
The clinical baseline characteristics were collected before the first TACE, including age, sex, HBV infection status, the status of cirrhosis, diabetes, rupture and bleeding of HCC, hemoglobin (Hb) level, red blood count (RBC), white blood cell count (WBC), neutrophil count, lymphocyte count, platelet (PLT) count, albumin (ALB), globulin (GLB), total bilirubin (TBIL), direct bilirubin (DBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), potassium (K+), prothrombin time (PT), international normalized ratio (INR) and a-fetoprotein (AFP) levels. The objective imaging characteristics of the tumor, including the maximum diameter of the lesion, hepatic arteriovenous fistula (HAVF), portal vein tumor thrombus (PVTT) and the integrality of the tumor capsule, were independently screened before the first TACE by two radiologists. When the radiologists disagreed on the results of objective imaging characteristics, the two radiologists discussed them together until they reached an agreement. The independent assessment of these objective radiological biomarkers by the two radiologists showed good consistency. However, there was inconsistency in HAVF, PVTT, and the integrality of the tumor capsule in fewer than 10 patients. All clinical data were obtained within a week before TACE. MRI or CT was performed within 4–6 weeks after TACE to obtain the first treatment response. The response to TACE was strictly evaluated by one gastroenterologist experienced with TACE and two radiologists independently, which was based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [26]. According to the response to TACE, progressive disease (PD) and stable disease (SD) were considered nonresponses to TACE, and a complete response (CR) and partial response (PR) were considered responses to TACE.
2.2 Diagnosis and staging of HCC
The diagnosis of HCC was based on the guideline proposed by the National Health Commission of the People’s Republic of China [27]. According to the guideline, HCC was diagnosed if patients had one or more risk factors: (1) lesions less than 2 cm in diameter and two typical imaging manifestations, including ultrasound, enhanced CT, MRI and DSA; (2) lesions more than 2 cm in diameter with one typical imaging manifestation; and (3) one typical imaging manifestation, and an AFP level ≥ 400 μg/L.
The BCLC staging [24], albumin–bilirubin (ALBI) scoring [28] and Child–Pugh (C–P) grade [29] were also evaluated. The classification of the ALBI score was as follows: ALBI grade 1 ≤ − 2.60, ALBI grade 2 between − 2.6 and − 1.39, ALBI grade 3 > − 1.39.
2.3 Treatment procedures
The femoral artery approach was routinely chosen. The patient was placed in a supine position, disinfected, covered with a towel, and subjected to local infiltration anesthesia. The Seldinger technique was used to percutaneously puncture the femoral artery, insert the catheter sheath, and insert the catheter into the celiac trunk artery or common hepatic artery for angiography. If vascular scarcity or absence is identified in a specific liver region, it suggests the presence of collateral circulation that supports tumor growth. Therefore, further investigation of corresponding arteries is necessary, including the superior mesenteric artery, intercostal arteries below the ribs, subdiaphragmatic artery, adrenal inferior artery branching from the renal artery, adrenal middle artery, left gastric artery, lumbar artery, internal mammary artery, among others. Conventional catheters or microcatheters were used to superselect the tumor feeding artery, and cisplatin, oxaliplatin, lobaplatin, and fluorouracil were diluted 0.9% saline solution or 5% glucose solution and slowly injected into the target artery. After arteriovenous fistula was excluded by angiography, an iodized oil emulsion was injected. The ratio of iodized oil (1–20 mL) and aqueous pirarubicin solution was 2:1, and the iodized oil emulsion was prepared after mixing. The iodized oil emulsion was slowly injected into the target artery. If arteriovenous fistulas were present, the fistulas were embolized with coils or gelatin sponges before the iodized oil emulsion was injected. After the injection of the iodized oil emulsion, the blood flow was slowed, and the injection embolization was performed with microspheres or gelatin sponge particles. Further angiography confirmed that the end point of embolism had been reached. If the patient was considered for emergency embolization treatment of rupture and hemorrhage of liver cancer, the infusion of chemotherapy drugs was not administered. The embolization endpoint is achieved when the supplying artery appears as a “dry twig”, indicating successful embolization of small tumor-feeding arteries while preserving the patency of liver segment or lobar arteries. This approach facilitates subsequent embolization treatments. Abdominal CT or MRI was performed 4–8 weeks after TACE to evaluate the treatment effect, and the next TACE was performed if necessary.
2.4 Statistical analysis
Based on whether the data fit a normal distribution, quantitative variables for patients at baseline are presented as the medians with interquartile ranges [IQRs] or the means with standard deviations. Categorical variables for patients at baseline and tumor characteristics measured using CT are presented as counts and percentages. The T test or nonparametric Mann‒Whitney U test and Chi square test or Fisher’s exact test were used to compare quantitative or categorical variables between the two groups, respectively. The information of missing data was showed in Supplemental Figure 2, including overall cohorts, development and training data. In the development data, a total of 337 patients with complete serum and imaging data were available for lasso regression. Among them, only 13 patients had missing data, with ALB, one of the predictive indicators, being missing in only 2 cases. Given the limited number of missing values, we opted to exclude these instances while selecting the predictive factors (Supplemental Figure 4).
Candidate risk factors for a nonresponse to TACE in patients with HCC were evaluated using a univariate logistic regression analysis and least absolute shrinkage and selection operator (LASSO) logistic regression analysis [30]. Potential variables with P < 0.05 in the univariate logistic regression analysis or a coefficient > 0 were selected for the multivariate logistic regression analysis to identify the variables to construct the models. In the development group, there were a total of 337 patients with complete serum and imaging data available for lasso regression. Only 13 patients had missing data, and among them, only 2 patients had missing values for ALB, one of the predictive indicators. Generalized variance inflation factor (GVIF) and correlation analyses were performed to avoid multicollinearity in the construction of models. The GVIF and correlation coefficient values were lower than 5 [31] and 0.25 respectively, indicating the probability of multicollinearity among the variables was low. The candidate models of the discrimination and calibration abilities were generated by constructing receiver operating characteristic curves (ROCs) and calibration plots, respectively. The best model was selected using the Hosmer‒Lemeshow test, Akaike information criterion (AIC), area under the receiver operating characteristic (AUROC) curve, calibration plot slope and decision curve analysis (DCA) [32].
We evaluated approximately 10 variables in the final model. Based on the rule of 10 events per variable fitting the logistic regression model [33], the probability of objective response to TACE was approximately 52.5% [8]. Thus, at least 200 eligible patients were included in the training group. In fact, 154 patients in the training group did not respond to TACE, which exceeded the expected number. Therefore, this study had an adequate sample size.
Patients with missing values were included in the baseline analysis but excluded from the correlation analysis, LASSO and logistic regression analyses. A P value less than 0.05 was considered significant. An analysis of trends was performed using the Cochran–Armitage trend test. All statistical analyses were performed using R software (version 4.2.1). The packages dplyr, tableone, rms, PredictABEL, nomogramFormula, corrplot, DescTools, ggpolt2, VIM and ggDCA were used to clear, analyze, and visualize the data.
3 Results
3.1 Baseline characteristics
A total of 427 eligible patients were selected, of which 350 patients were assigned to the training group. The demographics and clinical characteristics are shown in Table 1. The demographics, objective imaging characteristics and HCC staging were not different between the training and validation groups, except for K+. The nonresponse rates to the first TACE in the two groups were 44% (154 events) and 51.9% (40 events). The demographics and clinical characteristics of 45 HCC patients who had recently undergone TACE treatment are shown in Supplemental Table 6.
3.2 Candidate predictive factors for a nonresponse to the first TACE
Based on the TRIPOD guideline, a comprehensive literature review of TACE was performed. The five objective imaging characteristics, including the maximum tumor size, numbers of tumors, HAVF, PVTT, and integrality of the tumor capsule, were potential predictive factors of nonresponse to the first TACE. We also identified that ALB, TBIL, DBIL, AST and ALT levels were potentially associated with nonresponse to the first TACE. In addition, other serum indices and clinical characteristics were also selected for LASSO and univariate logistic regression analyses.
In the LASSO logistic regression analysis, 28 features were reduced to 11 candidate predictors with nonzero coefficients, including numbers of tumors, integrality of the tumor capsule, HAVF, diabetes, cirrhosis, ALB, GLB, DBIL, AST and PVTT (Fig. 2A, B). The results from the univariate logistic regression analysis are shown in Table 2. In the univariate logistic regression analysis, cirrhosis, ALB, AST, max tumor size, numbers of tumors, HAVF, PVTT and integrality of the tumor capsule were potential predictive factors of nonresponse to the first TACE, whereas the DBIL level showed borderline statistical significance (P value = 0.06). The status of cirrhosis might be associated with ALB, AST and DBIL levels, which might contribute to multicollinearity. Finally, AST, ALB, DBIL, maximum tumor size, numbers of tumors, HAVF, PVTT and integrality of the tumor capsule were selected for the multivariate logistic regression analysis (Table 3).
Texture feature selection using LASSO regression. A, B Showed the shrinkage process of coefficients. LASSO least absolute shrinkage and selection operator, RBC red blood count, WBC white blood cell count, PLT lymphocyte count, platelet; ALB albumin, GLB globulin, TBIL total bilirubin, DBIL direct bilirubin, AST aspartate aminotransferase, ALT alanine aminotransferase, K+ potassium, PT prothrombin time, L lymphocyte count, N neutrophil count, INR international normalized ratio, AFP a-fetoprotein, HAVF hepatic arteriovenous fistula, PVTT portal vein tumor thrombus, Number numbers of tumor, Size maximum diameter of tumor
3.3 Construction of the candidate predictive models
Based on the results from the multivariate logistic regression analysis, the ALB level, maximum tumor size, HAVF and integrality of the tumor capsule were used to develop the predictive model. The DBIL level is associated with liver function, which might affect the response to TACE. Therefore, the TBIL level was selected as a candidate predictive factor to construct the models. The candidate models are listed in Table 4. The GVIF of candidate predictive models and correlation analyses of predictors were performed to evaluate multicollinearity, and the results are shown in Supplemental Table 2 and Supplemental Figure 1. The value of GVIF was less than 1.5 in every model, and the correlation analysis indicated a weak correlation among those predictors.
3.4 Evaluation and comparison of the performance of those candidate models and other staging systems
ROC curves of the candidate models, BCLC staging system, ALBI scoring system and C–P class are shown in Fig. 3A, B. The discrimination and calibration abilities of those models are shown in Table 5 and Fig. 4. The AUROCs of those candidate models were all greater than 0.7, indicating that those models had adequate discrimination ability in both the training and validation groups. Although the BCLC stage, C–P class and ALBI grade had modest discrimination and good calibration abilities for predicting the response to TACE in the training group, those staging systems showed poor calibration in the validation group. Continuous variables were converted to dichotomous variables, which might result in the loss of data, but the discrimination and calibration abilities of Model 3 was not worse than those of Model 1 and Model 2. Model 3 contained the variables of liver function (ALB and DBIL) and objective imaging characteristics (tumor size, HAVF, integrality of the tumor capsule) of the tumor. Therefore, Model 3 was named the T (Tumor size) A (HAVF) C (integrality of the tumor Capsule) F (liver Function) model. The AIC and the p value of the Hosmer‒Lemeshow test are shown in Supplemental Table 3 and Supplemental Table 4. Because the TACF model was more feasible than Model 1 and Model 2, we selected this model as the final model.
3.5 Visualization of the model and clinical use
The nomogram of the final model is shown in Fig. 5A, and the exact scoring of the nomogram is shown in Supplemental Table 5. The results from the DCA of the candidate models and other staging systems are presented in Fig. 5B, C. In the training dataset, the DCA showed that for a risk threshold probability between 0.25 and 0.75, the final model obtained a greater net benefit than either the treat-all-patients or the treat-none scheme. However, in the validation group, the range of the risk threshold probability of the final model was narrower than that in the training group. However, the TACF model obtained a greater net benefit than the CLC stage, C–P class and ALBI grade.
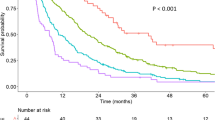
According to the TACF model, the score for predicting nonresponse to TACE was categorized as low risk (less than 145), median risk (between 145 and 194) and high risk (greater than 194). An increasing trend for the risk was observed from the low-risk group to the high-risk group in both the training and validation sets (p value < 0.001 and p value = 0.001, respectively) (Table 6).
In different time periods, the TACF model demonstrates excellent discrimination ability among HCC patients receiving TACE. The TACF model exhibits an AUROC value of 0.884 (Supplemental Figure 2), which consistently surpasses the BCLC staging (AUROC: 0.750), CTP staging (AUROC: 0.514), and ALBI staging (AUROC: 0.514). Furthermore, the TACF model also displays superior calibration ability compared to the BCLC staging, CTP staging, and ALBI staging (Supplemental Figure 3).
4 Discussion
In the present study, we developed and validated simple and evidence-based TACF models incorporating objective imaging characteristics and individual liver function to stratify candidate patients with HCC into three risk categories before TACE. The TACF model showed an adequate discrimination ability, validation ability and clinical utility in both the training and validation groups. The features and novelties of this study are described below. (1) We designed and implemented the current study strictly based on the TRIPOD guidelines, and the sample size was adequate according to rigorous calculations. (2) We developed the first prognostic model, the results of which were obtained using manual calculation. (3) The three risk categories, which were divided by the TACF model, screened potential patients with HCC who would present a noneffective response to TACE.
The TACF model incorporated three objective imaging characteristics, including tumor size, HAVF, and integrality of the tumor capsule. The maximum diameter of the tumor size was proved that which were associated with OS and recurrence after surgical resection [3, 34,35,36]. In addition, it was also a risk factor for TACE refractoriness [37]. In our study, we divided the tumor size into three classifications, and it was associated with the first response to TACE, consistent with a previous study [21]. Numerous prior studies have identified the prognostic value of the number of tumors in patients with HCC receiving TACE [12, 14, 38]. However, the primary end outcome of those studies was OS or progression-free survival (PFS). However, in our study, the primary outcome focused on the first response to TACE. The univariate logistic regression analysis indicated that the number of tumors was a potential risk factor for nonresponse to TACE, but in the multivariate logistic regression analysis, the p value was not associated with the response to the first TACE. Kim et al. [9] found that multiple tumors were predictive factors for a failure to achieve a CR. The distinct point of our study compared to the previous study was that we combined CR and PR into one group, and we divided the multiple numbers of tumors into single and multiple tumors based on one lesion rather than three lesions. A similar result was reported in a study in which the tumor number was not a predictive factor for the first response to TACE [39]. Superselection of the tumor feeding artery was performed in every lesion, which contributed to the treatment response of every lesion being relatively independent. The predictive value of the number of tumors for the first response to TACE requires more research for confirmation. Hepatoportal arteriovenous fistula (HAVF) might redistribute the arterial flow into portal venous flow, which might weaken the first response and safety of TACE. In our study, HAVF was embolized before the iodized oil emulsion was injected to reduce the effect of TACE. However, the patients with HAVF tended to exhibit a poor response to TACE in our study, and another study also obtained a similar result [40]. Patients receiving TACE with an integrated tumor capsule had a better prognosis [41] and a machine-learning model to predict extrahepatic spread or vascular invasion also incorporated the tumor capsule [38]. The liver function of patients with HCC must be assessed to determine the treatment method. AST, ALB PT, and DBIL are serum biomarkers to reveal the liver status of patents with HCC. Therefore, we selected those indices for LASSO and logistic regression analyses. Finally, ALB and DBIL were selected for the construction of the TACF model. In our study, a low ALB level was associated with an unsatisfactory response to TACE, consistent with the results of previous studies [21, 42].
The assessment of liver function in patients with liver cancer often relies on the widely employed C–P class and ABIL score [28, 29]. The C–P score encompasses two subjective evaluation factors: hepatic encephalopathy and ascites severity. On the other hand, the calculation of the ABIL score criteria is relatively complex and requires the use of a calculator for its application. The AUROC values for both the C–P class and ABIL score were found to be below 0.6, with their calibration curves exhibiting inferior performance when compared to the TACF model. These outcomes imply that using liver function alone as a predictor for the effectiveness of initial TACE treatment may not yield satisfactory results. The BCLC staging system integrates liver function and tumor burden to categorize patients, displaying superior predictive efficacy for assessing the effectiveness of initial TACE treatment compared to relying solely on liver function scores, albeit lower than the TACF score. However, it is crucial to emphasize that the TACF score primarily focuses on patients undergoing their first TACE treatment, specifically evaluating outcomes following the initial TACE session. The evaluation of survival rates for patients receiving their first TACE treatment remains uncertain at present. Nevertheless, the TACF score demonstrates commendable discriminatory and calibration abilities in predicting the effectiveness of initial TACE treatment. In decision curve analysis, it exhibits higher net benefit values when contrasted with the C–P class, ABIL score, and BCLC staging system, implying that employing the TACF score as a guide for the initial treatment of liver cancer patients can yield greater clinical benefits. Significantly, in comparison to the Child–Pugh classification, ALBI score, and BCLC staging, the TACF staging system offers direct guidance for tailoring treatment strategies to potential TACE patients across various stages. This approach enables accurate treatment planning for individuals with advanced-stage liver cancer, utilizing widely accessible objective serum and imaging assessments.
Based on our calculations, for patients classified as intermediate risk after undergoing TACE treatment, we recommend considering adjunctive targeted therapy or immunotherapy. However, for high-risk patients, we advise against proceeding with TACE treatment and instead suggest focusing solely on systemic therapy. These recommendations aim to optimize the treatment approach based on the individual risk profile of each patient.
This study had several potential limitations. First, the TACF model was established based on a retrospective study. Second, although the patients were selected from two independent departments, the predictive model was developed and validated in one medical center, which might lead to the issue of the suitability of the TACF model for patients with intermediate-stage HCC before TACE who are treated at another medical center. Third, HBV infection is the main cause of HCC in China, but HCV infection and alcohol abuse are the main causes in other regions [24], which might result in different imaging characteristics of tumor. Thirdly, Despite the inclusion of variables like tumor size, HAVF, integrality of the tumor capsule, TBIL, and ALB in the TACF model, all of which are associated with the prognosis of liver cancer patients, this study did not consider patient survival as the primary outcome. As a result, the TACF model’s ability to forecast the survival duration of liver cancer patients has not been evaluated, underscoring the need for further investigation to assess its predictive efficacy in terms of patient survival. Fourthly, Due to the disparity in cost between drug-eluting beads TACE and conventional TACE, a significant proportion of patients at our medical center choose the more economical conventional TACE treatment. Therefore, the establishment of the TACF model is based on conventional TACE patients, and further research is needed to validate its applicability to drug-eluting beads TACE.
5 Conclusions
We derived and validated a simple-to-use scoring model (TACF score) incorporating objective imaging characteristics and liver function to predict the response to the first TACE among patients with intermediate-stage HCC. The TACF score significantly classify patients into three risk groups according to the first response to TACE. The risk predictive model had adequate discrimination, validation and clinical utility and could be used to screen the appropriate patients with intermediate-stage HCC before TACE based on the individual response risk.
Data availability
The original contributions presented in the study can be directed to the corresponding authors.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TACE:
-
Transarterial chemoembolization
- LASSO:
-
Least absolute shrinkage and selection operator
- TRIPOD:
-
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis
- BCLC:
-
Barcelona Clinic Liver Cancer
- ALBI:
-
Albumin–bilirubin
- C–P:
-
Child–Pugh
- Hb:
-
Hemoglobin
- RBC:
-
Red blood count
- WBC:
-
White blood cell count
- PLT:
-
Platelet
- ALB:
-
Albumin
- GLB:
-
Globulin
- TBIL:
-
Total bilirubin
- DBIL:
-
Direct bilirubin
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- K+ :
-
Potassium
- PT:
-
Prothrombin time
- INR:
-
International normalized ratio
- AFP:
-
α-fetoprotein
- HAVF:
-
Hepatic arteriovenous fistula
- PVTT:
-
Portal vein tumor thrombus
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- PD:
-
Progressive disease
- SD:
-
Stable disease
- CR:
-
Complete response
- PR:
-
Partial response
- DSA:
-
Digital subtraction angiography
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- OS:
-
Overall survival
- GVIF:
-
Generalized variance inflation factor
- ROC:
-
Receiver operating characteristic curve
- AIC:
-
Akaike information criterion
- AUROC:
-
Area under the receiver operating characteristic
- DCA:
-
Decision curve analysis
References
Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–53.
Venkatesh SK, Chandan V, Roberts LR. Liver masses: a clinical, radiologic, and pathologic perspective. Clin Gastroenterol Hepatol. 2014;12:1414–29.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–50.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
Maesaka K, Sakamori R, Yamada R, Tahata Y, Urabe A, Shigekawa M, Kodama T, Hikita H, Tatsumi T, Takehara T. Hypovascular hepatic nodules as a predictive factor for transcatheter arterial chemoembolization refractoriness in hepatocellular carcinoma. Hepatol Res. 2020;50:365–73.
Xue M, Wu Y, Fan W, Guo J, Wei J, Wang H, Tan J, Wang Y, Yao W, Zhao Y, Li J. Prognostic value of TP53 mutation for transcatheter arterial chemoembolization failure/refractoriness in HBV-related advanced hepatocellular carcinoma. Cancer Res Treat. 2020;52:925–37.
Xu LX, He MH, Dai ZH, Yu J, Wang JG, Li XC, Jiang BB, Ke ZF, Su TH, Peng ZW, Guo Y, Chen ZB, Chen SL, Peng S, Kuang M. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann Oncol. 2019;30:990–7.
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–16.
Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim DY, Ahn SH, Kim MD, Park SI, Won JY, Lee DY, Han KH. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304–10.
Hatta W, Tsuji Y, Yoshio T, Kakushima N, Hoteya S, Doyama H, Nagami Y, Hikichi T, Kobayashi M, Morita Y, Sumiyoshi T, Iguchi M, Tomida H, Inoue T, Koike T, Mikami T, Hasatani K, Nishikawa J, Matsumura T, Nebiki H, Nakamatsu D, Ohnita K, Suzuki H, Ueyama H, Hayashi Y, Sugimoto M, Yamaguchi S, Michida T, Yada T, Asahina Y, Narasaka T, Kuribasyashi S, Kiyotoki S, Mabe K, Nakamura T, Nakaya N, Fujishiro M, Masamune A. Prediction model of bleeding after endoscopic submucosal dissection for early gastric cancer: BEST-J score. Gut. 2021;70:476–84.
Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, Ma ZL, Liu ZY. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. 2016;34:2157–64.
Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, Mu W, Yin G, Li H, Zhao H, Li J, Zhang C, Zhu X, Wu J, Li J, Gong W, Li Z, Lin Z, Pan X, Shi H, Shao G, Liu J, Yang S, Zheng Y, Xu J, Song J, Wang W, Wang Z, Zhang Y, Ding R, Zhang H, Yu H, Zheng L, Gu W, You N, Wang G, Zhang S, Feng L, Liu L, Zhang P, Li X, Chen J, Xu T, Zhou W, Zeng H, Zhang Y, Huang W, Jiang W, Zhang W, Shao W, Li L, Niu J, Yuan J, Li X, Lv Y, Li K, Yin Z, Xia J, Fan D, Han G, China HCC-TACE Study Group. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70:893–903.
Kadalayil L, Benini R, Pallan L, O’Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565–70.
Kim BK, Shim JH, Kim SU, Park JY, Kim DY, Ahn SH, Kim KM, Lim YS, Han KH, Lee HC. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: development of a prediction model. Liver Int. 2016;36:92–9.
Liu D, Liu F, Xie X, Su L, Liu M, Xie X, Kuang M, Huang G, Wang Y, Zhou H, Wang K, Lin M, Tian J. Accurate prediction of responses to transarterial chemoembolization for patients with hepatocellular carcinoma by using artificial intelligence in contrast-enhanced ultrasound. Eur Radiol. 2020;30:2365–76.
Guo Z, Zhong N, Xu X, Zhang Y, Luo X, Zhu H, Zhang X, Wu D, Qiu Y, Tu F. Prediction of hepatocellular carcinoma response to transcatheter arterial chemoembolization: a real-world study based on non-contrast computed tomography radiomics and general image features. J Hepatocell Carcinoma. 2021;8:773–82.
Vosshenrich J, Zech CJ, Heye T, Boldanova T, Fucile G, Wieland S, Heim MH, Boll DT. Response prediction of hepatocellular carcinoma undergoing transcatheter arterial chemoembolization: unlocking the potential of CT texture analysis through nested decision tree models. Eur Radiol. 2021;31:4367–76.
Sun Y, Bai H, Xia W, Wang D, Zhou B, Zhao X, Yang G, Xu L, Zhang W, Liu P, Xu J, Meng S, Liu R, Gao X. Predicting the outcome of transcatheter arterial embolization therapy for unresectable hepatocellular carcinoma based on radiomics of preoperative multiparameter MRI. J Magn Reson Imaging. 2020;52:1083–90.
Kuang Y, Li R, Jia P, Ye W, Zhou R, Zhu R, Wang J, Lin S, Pang P, Ji W. MRI-based radiomics: nomograms predicting the short-term response after transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma patients with diameter less than 5 cm. Abdom Radiol. 2021;46:3772–89.
Zhang L, Jiang Y, Jin Z, Jiang W, Zhang B, Wang C, Wu L, Chen L, Chen Q, Liu S, You J, Mo X, Liu J, Xiong Z, Huang T, Yang L, Wan X, Wen G, Han XG, Fan W, Zhang S. Real-time automatic prediction of treatment response to transcatheter arterial chemoembolization in patients with hepatocellular carcinoma using deep learning based on digital subtraction angiography videos. Cancer Imaging. 2022;22:23.
Dong Z, Lin Y, Lin F, Luo X, Lin Z, Zhang Y, Li L, Li ZP, Feng ST, Cai H, Peng Z. Prediction of early treatment response to initial conventional transarterial chemoembolization therapy for hepatocellular carcinoma by machine-learning model based on computed tomography. J Hepatocell Carcinoma. 2021;8:1473–84.
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–31.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
N.H.C.o.t.P.s.R.o.C. Bureau of Medical Administration, [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:367–88.
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Inarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–8.
Shetty K, Rybicki L, Carey WD. The Child–Pugh classification as a prognostic indicator for survival in primary sclerosing cholangitis. Hepatology. 1997;25:1049–53.
Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–28.
Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72:558–69.
Sadatsafavi M, Adibi A, Puhan M, Gershon A, Aaron SD, Sin DD. Moving beyond AUC: decision curve analysis for quantifying net benefit of risk prediction models. Eur Respir J. 2021;58:2101186.
Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10.
Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P, Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43.
Chen SH, Wan QS, Zhou D, Wang T, Hu J, He YT, Yuan HL, Wang YQ, Zhang KH. A simple-to-use nomogram for predicting the survival of early hepatocellular carcinoma patients. Front Oncol. 2019;9:584.
Liu S, Liu KC, Lv WF, Lu D, Zhu XH, Jiang B, Tan YL, Wang GX. The efficacy and prognostic factors of the combination of TACE and apatinib for the treatment of BCLC stage C hepatocellular carcinoma. Front Med. 2021;8: 774345.
Niu XK, He XF. Development of a computed tomography-based radiomics nomogram for prediction of transarterial chemoembolization refractoriness in hepatocellular carcinoma. World J Gastroenterol. 2021;27:189–207.
Jin Z, Chen L, Zhong B, Zhou H, Zhu H, Zhou H, Song J, Guo J, Zhu X, Ji J, Ni C, Teng G. Machine-learning analysis of contrast-enhanced computed tomography radiomics predicts patients with hepatocellular carcinoma who are unsuitable for initial transarterial chemoembolization monotherapy: a multicenter study. Transl Oncol. 2021;14: 101034.
Peng J, Huang J, Huang G, Zhang J. Predicting the initial treatment response to transarterial chemoembolization in intermediate-stage hepatocellular carcinoma by the integration of radiomics and deep learning. Front Oncol. 2021;11: 730282.
Wu H, Zhao W, Zhang J, Han J, Liu S. Clinical characteristics of hepatic arterioportal shunts associated with hepatocellular carcinoma. BMC Gastroenterol. 2018;18:174.
Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ, Guo RP, Lin XJ, Lau WY. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol. 2015;63:122–30.
Chen M, Cao J, Hu J, Topatana W, Li S, Juengpanich S, Lin J, Tong C, Shen J, Zhang B, Wu J, Pocha C, Kudo M, Amedei A, Trevisani F, Sung PS, Zaydfudim VM, Kanda T, Cai X. Clinical-radiomic analysis for pretreatment prediction of objective response to first transarterial chemoembolization in hepatocellular carcinoma. Liver Cancer. 2021;10:38–51.
Acknowledgements
The datasets generated during and analyzed during study are available from the corresponding author on a reasonable request.
Funding
This work was supported by the Applied research incubation program of Jiangxi Province, China (20212BAG70026).
Author information
Authors and Affiliations
Contributions
J-WZ, S-HC, and R-GL designed the study. D-DN, X-YG, Q-HC and J-LH performed data collection. Q-TD, L-LB, G-HG and YC performed data collection of imaging characteristics and confirmation of result of TACE. S-HC wrote and critically read through the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by Ethics Committee of the First Affiliated Hospital of Nanchang University in accordance with the 1964 Declaration of Helsinki and its later amendments. The ethical approval number for this study is (2023) CDYFYYLK (02-040). The study is a retrospective observational study and has undergone ethical review and has been approved to be conducted in accordance with the approved protocol and under the condition of informed consent exemption. The ethics committee that granted the waiver of informed consent is the Nanchang University First Affiliated Hospital Medical Research Ethics Committee.
Approval of the research protocol
Not applicable.
Registry and the registration no. of the study/trial
Not applicable.
Animal studies
Not applicable.
Research involving recombinant DNA
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12672_2023_803_MOESM1_ESM.tif
Supplementary file 1—Supplemental Figure 1: Correlation analysis among variables in candidate models. ALB: albumin; DBIL: direct bilirubin; HAVF: hepatic arteriovenous fistula. (TIF 1689 KB)
12672_2023_803_MOESM2_ESM.tif
Supplementary file 2—Supplemental Figure 2: ROC curve analyses of candidate models and comparing the discrimination ability of BCLC staging, C–P class and ALBI grading in training group at different time periods. ROC: receiver operating characteristic curve; BCLC: Barcelona Clinic Liver Cancer; ALBI: albumin–bilirubin; C–P: Child–Pugh. (TIF 1409 KB)
12672_2023_803_MOESM3_ESM.tif
Supplementary file 3—Supplemental Figure 3: Calibration plot of candidate models, including TACF model (A), BCLC staging (B), C–P class (C), ALBI grading (D) in training group at different time periods. BCLC: Barcelona Clinic Liver Cancer; ALBI: albumin–bilirubin; C–P: Child–Pugh. (TIF 3731 KB)
12672_2023_803_MOESM4_ESM.tif
Supplementary file 4—Supplemental Figure 4: Information regarding missing values in overall (A), training (B) and validation group (C). TACE: transarterial chemoembolization; Hb: hemoglobin; RBC: red blood count; WBC: white blood cell count; PLT: platelet; ALB: albumin; GLB: globulin; TBIL: total bilirubin; DBIL: direct bilirubin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; K+: potassium; PT: prothrombin time; INR: international normalized ratio; AFP: a-fetoprotein; HAVF: hepatic arteriovenous fistula; PVTT: portal vein tumor thrombus; BCLC: Barcelona Clinic Liver Cancer; ALBI: albumin–bilirubin; C–P: Child–Pugh. (TIF 4630 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, JW., Nie, DD., Huang, JL. et al. Prediction model of no-response before the first transarterial chemoembolization for hepatocellular carcinoma: TACF score. Discov Onc 14, 184 (2023). https://doi.org/10.1007/s12672-023-00803-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00803-2