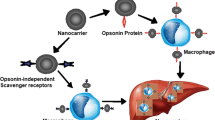
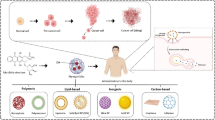
Abstract
This review provides a comprehensive examination of ibrutinib (Ibr), highlighting its wide-ranging utilization in the treatment of diverse malignancies and its potential as a viable target for solid tumor therapy on account of its ability to inhibit numerous kinases, such as Bruton’s tyrosine kinase (BTK), which is an essential component of the B-cell receptor signaling pathway and the microenvironment of cancer. The attention is directed toward the most recent developments in nanotechnology and its growing impact on the transformation of cancer therapeutics. The article examines the potential of nanotechnology in advancing approaches to enhance the efficacy of cancer treatments, overcome drug resistance, and enable the development of novel immunotherapies. An in-depth investigation into novel approaches, including nanoencapsulation and nanocarrier-mediated delivery, reveals their potential contributions to improving the therapeutic results of Ibr. The review is situated within a multidisciplinary framework. It highlights the potential uses of these emerging nanotechnological approaches to address obstacles, minimize adverse effects, and counteract potential resistance associated with Ibr treatment. This highlights nanotechnology’s profound potential in precision oncology, particularly in enhancing the therapeutic environment for hematologic cancers.






Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Sung, H., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A cancer Journal for Clinicians, 71(3), 209–249.
George, B., et al. (2020). Ibrutinib resistance mechanisms and treatment strategies for B-cell lymphomas. Cancers, 12(5), 1328.
Wen, T., et al. (2021). Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia, 35(2), 312–332.
Koehrer, S., & Burger, J. A. (2023). Chronic lymphocytic leukemia: Disease biology. Acta Haematologica.
Garg, N., et al. (2022). Bruton’s tyrosine kinase inhibitors: The next frontier of B-cell-targeted therapies for cancer, autoimmune disorders, and multiple sclerosis. Journal of Clinical Medicine, 11(20), 6139.
Mishra, A. K., et al. (2022). Macrophages as a potential immunotherapeutic target in solid cancers. Vaccines, 11(1), 55.
Szklener, K., et al. (2022). Ibrutinib in the treatment of solid tumors: Current state of knowledge and future directions. Cells, 11(8), 1338.
Zhang, S. Q., et al. (2015). Mechanisms of ibrutinib resistance in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. British Journal of Haematology, 170(4), 445–456.
Bojarczuk, K., et al. (2015). B-cell receptor signaling in the pathogenesis of lymphoid malignancies. Blood Cells Molecules and Diseases, 55(3), 255–265.
Khalaf, K., et al. (2021). Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Frontiers in Immunology, 12, 656364.
Ran, F., et al. (2022). Review of the development of BTK inhibitors in overcoming the clinical limitations of ibrutinib. European Journal of Medicinal Chemistry, 229, 114009.
Paydas, S. (2019). Management of adverse effects/toxicity of ibrutinib. Critical Reviews in Oncology/Hematology, 136, 56–63.
Mittal, D., Niveria, K., & Verma, A. K. (2022). Nanotechnology-based targeted delivery systems for protein kinase inhibitors in cancer therapy. Protein kinase inhibitors (pp. 779-Elsevier). 747.
Pourmadadi, M., et al. (2023). Novel epirubicin-loaded nanoformulations: Advancements in polymeric nanocarriers for efficient targeted cellular and subcellular anticancer drug delivery. Inorganic Chemistry Communications, 110999.
Fathi-Karkan, S., et al. (2023). Recent advancements in the targeted delivery of etoposide nanomedicine for cancer therapy: A comprehensive review. European Journal of Medicinal Chemistry, 115676.
Egbuna, C., Găman, M. A., & Jeevanandam, J. (2022). Applications of nanotechnology in drug discovery and delivery. Elsevier.
Smidova, V., et al. (2021). Nanomedicine of tyrosine kinase inhibitors. Theranostics, 11(4), 1546.
Li, J., et al. (2023). Development and application of nanomaterials, nanotechnology and nanomedicine for treating hematological malignancies. Journal of Hematology & Oncology, 16(1), 1–26.
Tshweu, L., et al. (2013). Nanoencapsulation of water-soluble drug, lamivudine, using a double emulsion spray-drying technique for improving HIV treatment. Journal of Nanoparticle Research, 15, 1–11.
Alimardani, V., et al. (2023). Nanotechnology-based cell-mediated delivery systems for cancer therapy and diagnosis. Drug Delivery and Translational Research, 13(1), 189–221.
Chehelgerdi, M., et al. (2023). Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Molecular Cancer, 22(1), 169.
Mehta, M., et al. (2023). Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Materials Au.
Blenke, E. O., et al. (2023). The storage and in-use stability of mRNA vaccines and therapeutics: Not a cold case. Journal of Pharmaceutical Sciences, 112(2), 386–403.
Sun, D., & Lu, Z. R. (2023). Structure and function of cationic and ionizable lipids for nucleic acid delivery. Pharmaceutical Research, 40(1), 27–46.
Zoulikha, M., & He, W. (2022). Targeted drug delivery for chronic lymphocytic leukemia. Pharmaceutical Research, 39(3), 441–461.
Jain, H., et al. (2022). Topical delivery of Bruton’s tyrosine kinase inhibitor and curcumin-loaded nanostructured lipid carrier gel: Repurposing strategy for the psoriasis management. Pharmaceutical Development and Technology, 27(9), 975–988.
Ashar, F., et al. (2022). Preparation and optimization of ibrutinib-loaded nanoliposomes using response surface methodology. Polymers, 14(18), 3886.
Ponnaganti, M., & Babu, A. K. (2021). Preparation, characterization and evaluation of chitosan nanobubbles for the targeted delivery of ibrutinib. Nveo-Natural Volatiles & Essential Oils Journal| Nveo, 5017–5037.
Qiu, Q., et al. (2018). Novel self-assembled ibrutinib-phospholipid complex for potently peroral delivery of poorly soluble drugs with pH-dependent solubility. An Official Journal of the American Association of Pharmaceutical Scientists, 19, 3571–3583.
Rangaraj, N., et al. (2020). QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: Evaluation using chylomicron flow blocking approach. Drug Delivery and Translational Research, 10, 1476–1494.
Vijetha, K. A., & Reddy, M. S. Design and optimization by response surface methodology and lymphatic uptake study of lipid-based drug delivery systems of ibrutinib.
Kumari, A., Yadav, S. K., & Yadav, S. C. (2010). Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces, 75(1), 1–18.
Song, P., et al. (2022). Preparation and evaluation of ibrutinib lipid-based formulations. Journal of Drug Delivery Science and Technology, 77, 103912.
Vilar, G., Tulla-Puche, J., & Albericio, F. (2012). Polymers and drug delivery systems. Current drug Delivery, 9(4), 367–394.
Khalid, M., & El-Sawy, H. S. (2017). Polymeric nanoparticles: Promising platform for drug delivery. International Journal of Pharmaceutics, 528(1–2), 675–691.
Raju, G. S. R., et al. (2022). Nanoparticles mediated tumor microenvironment modulation: Current advances and applications. Journal of Nanobiotechnology, 20(1), 274.
Tiwari, J. N., Tiwari, R. N., & Kim, K. S. (2012). Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Progress in Materials Science, 57(4), 724–803.
Fatima, F., & Anwer, M. K. (2023). Development and characterization of ibrutinib-loaded ethylcellulose-based nanosponges: Cytotoxicity assay against MCF-7 cell lines. Applied Sciences, 13(8), 4984.
Alshetaili, A. S., et al. (2019). Enhanced oral bioavailability of ibrutinib encapsulated poly (lactic-co-glycolic acid) nanoparticles: Pharmacokinetic evaluation in rats. Current Pharmaceutical Analysis, 15(6), 661–668.
Patel, M., et al. (2023). Core shell lipid-polymer hybrid nanoparticles for oral bioavailability enhancement of ibrutinib via lymphatic uptake. An Official Journal of the American Association of Pharmaceutical Scientists, 24(6), 142.
Murugesan, R., et al. (2021). Ibrutinib conjugated surface-functionalized multiwalled carbon nanotubes and its biopolymer composites for targeting prostate carcinoma. Journal of Materials Science, 56, 18684–18696.
Armutcu, C. (2022). The investigation of parameters affecting Ibrutinib release from chitosan/tripolyphosphate/carbon nanofiber composite microspheres. Turkish Journal of Chemistry, 46(5), 1632–1641.
Shakeel, F., Iqbal, M., & Ezzeldin, E. (2016). Bioavailability enhancement and pharmacokinetic profile of an anticancer drug ibrutinib by self-nanoemulsifying drug delivery system. Journal of Pharmacy and Pharmacology, 68(6), 772–780.
Zhao, L., et al. (2020). Chitosan/sulfobutylether-β-cyclodextrin nanoparticles for ibrutinib delivery: A potential nanoformulation of novel kinase inhibitor. Journal of Pharmaceutical Sciences, 109(2), 1136–1144.
Fuster, M. G., et al. (2023). Folic acid-modified ibrutinib-loaded silk fibroin nanoparticles for cancer cell therapy with over-expressed folate receptor. Pharmaceutics, 15(4), 1186.
Li, C., et al. (2021). Sialic acid conjugate-modified liposomal platform modulates immunosuppressive tumor microenvironment in multiple ways for improved immune checkpoint blockade therapy. Journal of Controlled Release, 337, 393–406.
Dhiman, S., Kaur, A., & Sharma, M. (2022). Fullerenes for anticancer drug targeting: Teaching an old dog a new trick. Mini Reviews in Medicinal Chemistry, 22(22), 2864–2880.
Saliev, T. (2019). The advances in biomedical applications of carbon nanotubes. C, 5(2), 29.
Singh, J., et al. (2023). Carbon nanostructures as therapeutic cargoes: Recent developments and challenges. C, 9(1), 3.
Jha, R., et al. (2020). Smart carbon nanotubes for drug delivery system: A comprehensive study. Journal of Drug Delivery Science and Technology, 58, 101811.
de Vries, R., et al. (2016). Stable isotope-labelled intravenous microdose for absolute bioavailability and effect of grapefruit juice on ibrutinib in healthy adults. British Journal of Clinical Pharmacology, 81(2), 235–245.
Lee, C. S., Rattu, M. A., & Kim, S. S. (2016). A review of a novel, Bruton’s tyrosine kinase inhibitor, ibrutinib. Journal of Oncology Pharmacy Practice : Official Publication of the International Society of Oncology Pharmacy Practitioners, 22(1), 92–104.
Zhang, Y., et al. (2020). Applications of hyperspectral imaging in the detection and diagnosis of solid tumors. Translational Cancer Research, 9(2), 1265.
Yadav, H. K. S., & Raizaday, A. (2016). Inorganic nanobiomaterials for medical imaging, in Nanobiomaterials in medical imaging (pp. 365–401). Elsevier.
Davodabadi, F., et al. (2023). Aptamer-functionalized quantum dots as theranostic nanotools against cancer and bacterial infections: A comprehensive overview of recent trends. Biotechnology Progress, 39(5), e3366.
Yaqoob, S. B., et al. (2020). Gold, silver, and palladium nanoparticles: A chemical tool for biomedical applications. Frontiers in Chemistry, 8, 376.
Mahmoodi Chalbatani, G. (2019). Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. International Journal of Nanomedicine, 3111–3128.
Dasari, G. K., Sunkara, S., & Gadupudi, P. C. R. (2020). One-step synthesis of magnetically recyclable palladium loaded magnesium ferrite nanoparticles: Application in synthesis of anticancer drug PCI-32765. Inorganic and Nano-Metal Chemistry, 50(9), 753–763.
Sánchez-Coronilla, A., et al. (2020). Theoretical study on the interactions between ibrutinib and gold nanoparticles for being used as drug delivery in the chronic lymphocytic leukemia. Journal of Molecular Liquids, 316, 113878.
Yang, Z., et al. (2015). Drug and gene co-delivery systems for cancer treatment. Biomaterials Science, 3(7), 1035–1049.
Indoria, S., Singh, V., & Hsieh, M. F. (2020). Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. International Journal of Pharmaceutics, 582, 119314.
Gupta, N., et al. (2019). Nanomaterials-based siRNA delivery: Routes of administration, hurdles and role of nanocarriers. Nanotechnology in modern animal biotechnology: Recent trends and future perspectives, 67–114.
Bor, G., Mat Azmi, I. D., & Yaghmur, A. (2019). Nanomedicines for cancer therapy: Current status, challenges and future prospects. Therapeutic Delivery, 10(2), 113–132.
Teo, P. Y., et al. (2016). Co-delivery of drugs and plasmid DNA for cancer therapy. Advanced drug Delivery Reviews, 98, 41–63.
Yang, Z., et al. (2023). Co-delivery of ibrutinib and hydroxychloroquine by albumin nanoparticles for enhanced chemotherapy of glioma. International Journal of Pharmaceutics, 630, 122436.
Kuo, H. P., et al. (2017). Combination of ibrutinib and ABT-199 in diffuse large B-cell lymphoma and follicular lymphoma. Molecular Cancer Therapeutics, 16(7), 1246–1256.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
R.B. and S.F-K. conceptualized and designed the study. S.R. and Z.M. contributed in the writing, review, and editing of the manuscript. A.R. and S.G. experimented and gathered data and prepared the text, figures, and table. The manuscript was reviewed and approved for submission by all authors.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behzadmehr, R., Fathi-karkan, S., Razzaq, S. et al. Unleashing the Potential of Ibrutinib-Loaded Nanoparticles for Cancer Treatment—A Comprehensive Review. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01445-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01445-6