Abstract
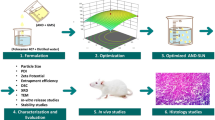
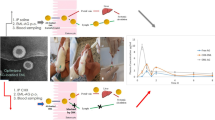
Ibrutinib (IBR) is the choice of drug for the treatment of chronic lymphocytic leukaemia (CLL) and mantle cell lymphoma (MCL). IBR has low oral bioavailability of 2.9% owing to its high first pass metabolism. Present study was aimed to develop the nanostructured lipid carriers (NLC) using glyceryl monostearate (GMS) as solid lipid and Capryol™ PGMC as liquid lipid. Plackett-Burman design (PBD) was applied to screen the significant factors; furthermore, these significant factors were subjected to optimisation using Central Composite design (CCD). The size, poly dispersity index (PDI) and entrapment efficiency (E.E.) of the developed NLC were 106.4 ± 8.66 nm, 0.272 ± 0.005 and 70.54 ± 5.52% respectively. Morphological evaluation using transmission electron microscope (TEM) and field emission scanning electron microscope (FESEM) revealed spherical particles. Furthermore, differential scanning calorimetry (DSC) indicates the formation of molecular dispersion of drug in the melted lipid matrix while Powder X-Ray Diffraction (PXRD) studies reveal the absence of crystalline drug peaks in the formulation diffractogram. In-vivo pharmacokinetics of NLC displayed an increase in Cmax (2.89-fold), AUC0-t (5.32-fold) and mean residence time (MRT) (1.82-fold) compared with free drug. Furthermore, lymphatic uptake was evaluated by chylomicron flow blocking approach using cycloheximide (CXI). The pharmacokinetic parameters Cmax, AUC0-t and MRT of NLC without CXI were 2.75, 3.57 and 1.30 folds higher compared with NLC with CXI. The difference in PK parameters without CXI indicates significant lymphatic uptake of the formulation. Hence, NLC can be a promising approach to enhance the oral bioavailability of drugs with high first-pass metabolism.

Graphical abstract














Similar content being viewed by others
References
Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma. 2013;54(11):2385–91.
Kokhaei P, Jadidi-Niaragh F, Sotoodeh Jahromi A, Osterborg A, Mellstedt H, Hojjat-Farsangi M. Ibrutinib-a double-edge sword in cancer and autoimmune disorders. J Drug Target. 2016;24(5):373–85.
Smith MR. Ibrutinib in B lymphoid malignancies. Expert Opin Pharmacother. 2015;16(12):1879–87.
Qiu Q, Lu M, Li C, Luo X, Liu X, Hu L, et al. Novel self-assembled ibrutinib-phospholipid complex for potently peroral delivery of poorly soluble drugs with pH-dependent solubility. AAPS PharmSciTech. 2018;19(8):3571–83.
Massó-Vallés D, Jauset T, Soucek L. Ibrutinib repurposing: from B-cell malignancies to solid tumors. Oncoscience. 2016;3(5–6):147.
Haura EB, Rix U. Deploying ibrutinib to lung cancer: another step in the quest towards drug repurposing. J Natl Cancer Inst. 2014;106(9):dju250.
Shakeel F, Iqbal M, Ezzeldin E. Bioavailability enhancement and pharmacokinetic profile of an anticancer drug ibrutinib by self-nanoemulsifying drug delivery system. J Pharm Pharmacol. 2016;68(6):772–80.
Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495(1):439–46.
Garg A, Bhalala K, Tomar DS. In-situ single pass intestinal permeability and pharmacokinetic study of developed Lumefantrine loaded solid lipid nanoparticles. Int J Pharm. 2017;516(1–2):120–30.
Xie S, Zhu L, Dong Z, Wang Y, Wang X, Zhou W. Preparation and evaluation of ofloxacin-loaded palmitic acid solid lipid nanoparticles. Int J Nanomedicine. 2011;6:547.
Gonçalves L, Maestrelli F, Mannelli LDC, Ghelardini C, Almeida A, Mura P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur J Pharm Biopharm. 2016;102:41–50.
Mishra DK, Dhote V, Bhatnagar P, Mishra PK. Engineering solid lipid nanoparticles for improved drug delivery: promises and challenges of translational research. Drug Deliv Transl Res. 2012;2(4):238–53.
Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine. 2013;8:2733.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems–an overview. Acta Pharm Sin B. 2013;3(6):361–72.
Nooli M, Chella N, Kulhari H, Shastri NR, Sistla R. Solid lipid nanoparticles as vesicles for oral delivery of olmesartan medoxomil: formulation, optimization and in vivo evaluation. Drug Dev Ind Pharm. 2017;43(4):611–7.
Shi X, Song S, Ding Z, Fan B, Huang W, Xu T. Improving the solubility, dissolution, and bioavailability of Ibrutinib by preparing it in a Coamorphous state with saccharin. J Pharm Sci. 2019;108:3020–8.
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–13.
Beloqui A, del Pozo-Rodríguez A, Isla A, Rodríguez-Gascón A, Solinís MÁ. Nanostructured lipid carriers as oral delivery systems for poorly soluble drugs. J Drug Deliv Sci Technol. 2017;42:144–54.
Sahu AK, Kumar T, Jain V. Formulation optimization of erythromycin solid lipid nanocarrier using response surface methodology. Biomed Res Int. 2014;2014:1–8.
Lin C-H, Chen C-H, Lin Z-C, Fang J-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25(2):219–34.
Radtke M, Müller RH. Nanostructured lipid drug carriers. New Drugs. 2001;2:48–52.
Müller RH, Alexiev U, Sinambela P, Keck CM. Nanostructured lipid carriers (NLC): the second generation of solid lipid nanoparticles. Percutaneous Penetration Enhancers Chem Methods Penetration Enhancement. Springer. 2016:161–85.
Nagaich U, Gulati N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: design and in vivo characterization. Drug Deliv Transl Res. 2016;6(3):289–98.
Rangaraj N, Pailla SR, Chowta P, Sampathi S. Fabrication of ibrutinib nanosuspension by quality by design approach: intended for enhanced oral bioavailability and diminished fast fed variability. AAPS PharmSciTech. 2019;20(8):326.
Kaithwas V, Dora CP, Kushwah V, Jain S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf B: Biointerfaces. 2017;154:10–20.
Shah NV, Seth AK, Balaraman R, Aundhia CJ, Maheshwari RA, Parmar GR. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: design and in vivo study. J Adv Res. 2016;7(3):423–34.
Negi LM, Jaggi M, Talegaonkar S. Development of protocol for screening the formulation components and the assessment of common quality problems of nano-structured lipid carriers. Int J Pharm. 2014;461(1–2):403–10.
Patwekar SL, Pedewad SR, Gattani S. Development and evaluation of nanostructured lipid carriers-based gel of isotretinoin. Part Sci Technol. 2018;36(7):832–43.
Natarajan J, Baskaran M, Humtsoe LC, Vadivelan R, Justin A. Enhanced brain targeting efficacy of olanzapine through solid lipid nanoparticles. Artif Cells Nanomed Biotechnol. 2017;45(2):364–71.
Wu P-S, Lin C-H, Kuo Y-C, Lin C-C. Formulation and characterization of hydroquinone nanostructured lipid carriers by homogenization emulsification method. J Nanomater. 2017;2017:1–7.
Singh B, Kapil R, Nandi M, Ahuja N. Developing oral drug delivery systems using formulation by design: vital precepts, retrospect and prospects. Expert Opin Drug Deliv. 2011;8(10):1341–60.
Kan S, Lu J, Liu J, Wang J, Zhao Y. A quality by design (QbD) case study on enteric-coated pellets: screening of critical variables and establishment of design space at laboratory scale. Asian J Pharm Sci. 2014;9(5):268–78.
Politis SN, Colombo P, Colombo G, Rekkas DM. Design of experiments (DoE) in pharmaceutical development. Drug Dev Ind Pharm. 2017;43(6):889–901.
Yerlikaya F, Ozgen A, Vural I, Guven O, Karaagaoglu E, Khan MA, et al. Development and evaluation of paclitaxel nanoparticles using a quality-by-design approach. J Pharm Sci. 2013;102(10):3748–61.
Kovács A, Berkó S, Csányi E, Csóka I. Development of nanostructured lipid carriers containing salicyclic acid for dermal use based on the quality by design method. Eur J Pharm Sci. 2017;99:246–57.
Beg S, Saini S, Bandopadhyay S, Katare O, Singh B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of olmesartan medoxomil employing multivariate statistical techniques. Drug Dev Ind Pharm. 2018;44(3):407–20.
Mishra V, Kesharwani P, Amin MC, Iyer A, editors. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. Academic Press; 2017.
Xia D, Shrestha N, van de Streek J, Mu H, Yang M. Spray drying of fenofibrate loaded nanostructured lipid carriers. Asian J Pharm Sci. 2016;11(4):507–15.
Gupta B, Poudel BK, Tran TH, Pradhan R, Cho H-J, Jeong J-H, et al. Modulation of pharmacokinetic and cytotoxicity profile of imatinib base by employing optimized nanostructured lipid carriers. Pharm Res. 2015;32(9):2912–27.
Martins S, Tho I, Souto E, Ferreira D, Brandl M. Multivariate design for the evaluation of lipid and surfactant composition effect for optimisation of lipid nanoparticles. Eur J Pharm Sci. 2012;45(5):613–23.
Esbensen KH, Guyot D, Westad F, Houmoller LP. Multivariate data analysis: in practice: an introduction to multivariate data analysis and experimental design. Multivariate Data Analysis; 2002.
Baumgartner R, Teubl BJ, Tetyczka C, Roblegg E. Rational design and characterization of a nanosuspension for intraoral administration considering physiological conditions. J Pharm Sci. 2016;105(1):257–67.
Elmowafy M, Ibrahim HM, Ahmed MA, Shalaby K, Salama A, Hefesha H. Atorvastatin-loaded nanostructured lipid carriers (NLCs): strategy to overcome oral delivery drawbacks. Drug Deliv. 2017;24(1):932–41.
Sharma A, Jaiswal S, Shukla M, Sharma M, Chauhan PMS, Rangaraj N, et al. HPLC–MS-MS method development and validation of antileishmanial agent, S010-0269, in hamster serum. J Chromatogr Sci. 2015;53(9):1542–8.
Bhalekar MR, Upadhaya PG, Madgulkar AR, Kshirsagar SJ, Dube A, Bartakke US. In-vivo bioavailability and lymphatic uptake evaluation of lipid nanoparticulates of darunavir. Drug Deliv. 2016;23(7):2581–6.
Dahan A, Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24(4):381–8.
Zhang C, Peng F, Liu W, Wan J, Wan C, Xu H, et al. Nanostructured lipid carriers as a novel oral delivery system for triptolide: induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int J Nanomedicine. 2014;9:1049.
Patel M, Sawant K. A quality by design concept on lipid based nanoformulation containing antipsychotic drug: screening design and optimization using response surface methodology. J Nanomed Nanotechnol. 2017;8(3):1–11.
Chouhan P, Saini T. D-optimal design and development of microemulsion based transungual drug delivery formulation of ciclopirox olamine for treatment of onychomycosis. Indian J Pharm Sci. 2016;78(4):498–511.
Patil-Gadhe A, Pokharkar V. Pulmonary targeting potential of rosuvastatin loaded nanostructured lipid carrier: optimization by factorial design. Int J Pharm. 2016;501(1–2):199–210.
Instruments M. Zetasizer nano series user manual. MAN0317. 2004;1:2004.
Singh A, Neupane YR, Panda BP, Kohli K. Lipid based nanoformulation of lycopene improves oral delivery: formulation optimization, ex vivo assessment and its efficacy against breast cancer. J Microencapsul. 2017;34(4):416–29.
Mandpe L, Pokharkar V. Quality by design approach to understand the process of optimization of iloperidone nanostructured lipid carriers for oral bioavailability enhancement. Pharm Dev Technol. 2015;20(3):320–9.
Su Y-L, Liu H-Z, Guo C, Wang J. Association behavior of PEO–PPO–PEO block copolymers in water or organic solvent observed by FTIR spectroscopy. Mol Simul. 2003;29(12):803–8.
Han L, Wang T. Preparation of glycerol monostearate from glycerol carbonate and stearic acid. RSC Adv. 2016;6(41):34137–45.
Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415(1–2):232–43.
Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47(1):139–51.
Liao H, Gao Y, Lian C, Zhang Y, Wang B, Yang Y, et al. Oral absorption and lymphatic transport of baicalein following drug–phospholipid complex incorporation in self-microemulsifying drug delivery systems. Int J Nanomedicine. 2019;14:7291–306.
Mishra A, Vuddanda PR, Singh S. Intestinal lymphatic delivery of praziquantel by solid lipid nanoparticles: formulation design, in vitro and in vivo studies. J Nanotechnol. 2014;2014:1–12.
Baheti A, Srivastava S, Sahoo D, Lowalekar R, Prasad Panda B, Kumar Padhi B, et al. Development and pharmacokinetic evaluation of industrially viable self-microemulsifying drug delivery systems (SMEDDS) for terbinafine. Curr Drug Deliv. 2016;13(1):65–75.
Trevaskis NL, Charman WN, Porter CJ. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60(6):702–16.
Acknowledgements
The authors are thankful to the NIPER-HYD and Department of Pharmaceuticals (DoP) for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All Institutional and National guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rangaraj, N., Pailla, S.R., Shah, S. et al. QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach. Drug Deliv. and Transl. Res. 10, 1476–1494 (2020). https://doi.org/10.1007/s13346-020-00803-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00803-7