Abstract
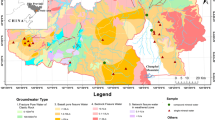
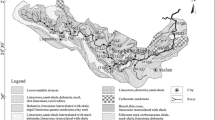
The Qingjiang River is the second largest tributary of the Yangtze River in Hubei Province and is also a typical karst catchment. Eighty-two important groundwater samples were collected during the high and low water periods of 2019. The results show the following. (1) The major hydrochemical types are Ca + Mg-HCO3 and Ca-HCO3, indicating that carbonate weathering is the main factor controlling the groundwater chemistry. (2) The results of inverse hydrochemical modeling reveal two kinds of groundwater–carbonate rock interactions. One is the codissolution of calcite and dolomite, and the other is dedolomitization, which is widespread in the dolomite aquifers. Furthermore, gypsum has a tendency to dissolve in each aquifer, and the common ion effect of Ca2+ caused by gypsum dissolution promotes dedolomitization. The modeling results suggest that major elements can be effectively used to trace the material source of the groundwater. (3) The chemical weathering of carbonate rock is mainly affected by carbonic acid, sulfuric acid, and nitric acid. After accounting for the impacts of evaporites and atmospheric input, the calculations show that the contribution of carbonic acid involved in carbonate weathering is 70.9% in the high water period and 70.0% in the low water period. Statistical analysis of karst spring discharge and the contributions of the acids involved in carbonate weathering reveals that the two are positively related. This result reflects the behaviors of sulfuric acid and nitric acid under the hydrodynamic conditions in different seasons. Therefore, carbonate weathering should be carefully evaluated in karst areas with abundant groundwater, and the role of groundwater in carbonate weathering is worthy of further study.









Similar content being viewed by others
References
Agrawal GD, Lunkad SK, Malkhed T (1999) Diffuse agricultural nitrate pollution of groundwaters in India. Water Sci Technol 39:67–75. https://doi.org/10.2166/wst.1999.0138
Ayora C, Cendon DI, Taberner C, Pueyo JJ (2001) Brine-mineral reactions in evaporite basins: Implications for the composition of ancient oceans. Geology 29:251–254. https://doi.org/10.1130/0091-7613(2001)029%3c0251:BMRIEB%3e2.0.CO;2
Baumann GO, Vital M, Melisa G, Sebastián G, Héctor M, Daniel EM (2019) Hydrogeochemical modeling and dedolomitization processes in the Patagonian Boulders and Patagonia Formation in the eastern Patagonia, Argentina. Environ Earth Sci. https://doi.org/10.1007/s12665-019-8583-7
Bullen TD, Kendall C (1998) Isotope tracers in catchment hydrology. In: Kendall C, McDonnell JJ (eds) Tracing of weathering reactions and water flowpaths: a multi-isotope approach. Elsevier Science, pp 611–646. https://doi.org/10.1016/B978-0-444-81546-0.50025-2
Chen H, Xiang TT, Zhou X, Xu CY (2012) Impacts of climate change on the Qingjiang watershed’s runoff change trend in China. Stochastic Environ Res Risk Assess 26:847–858. https://doi.org/10.1007/s00477-011-0524-2
Deng M, Tang MS (1993) Mechanism of dedolomitization and expansion of dolomitic rocks. Cem Concr Res 23(6):1397–1408
Du ZQ, Linghu B, Ling F, Li WB, Tian WD, Wang HL, Gui YM, Sun BY, Zhang XM (2012) Estimating surface water area changes using time-series Landsat data in the Qingjiang River basin, China. J Appl Remote Sens. https://doi.org/10.1117/1.jrs.6.063609
Evamy BD (1967) Dedolomitization and the development of rhombohedral pores in limestones. J Sediment Res 37:1204–1215
Frank S, Goeppert N, Ohmer M, Goldscheider N (2019) Sulfate variations as a natural tracer for conduit-matrix interaction in a complex karst aquifer. Hydrol Process. https://doi.org/10.1002/hyp.13400
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30. https://doi.org/10.1016/S0009-2541(99)00031-5
Garcia-Rios M, Cama J, Luquot L, Soler JM (2014) Interaction between CO2-rich sulfate solutions and carbonate reservoir rocks from atmospheric to supercritical CO2 conditions: experiments and modeling. Chem Geol 383:107–122. https://doi.org/10.1016/j.chemgeo.2014.06.004
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Hamdi R, Kusaka H, Doan Q, Cai P, He H, Luo G, Kuang W, Caluwaerts S, Duchêne F, Van Schaeybroek B, Termonia P (2004) The state-of-the-art of urban climate change modeling and observations. Earth Syst Environ 4:631–646. https://doi.org/10.1007/s41748-020-00193-3
Han GL, Liu CQ (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou province, China. Chem Geol 204:1–21. https://doi.org/10.1016/j.chemgeo.2003.09.009
Han GL, Liu CQ (2006) Strontium isotope and major ion chemistry of the rainwaters from Guiyang, Guizhou Province, China. Sci Total Environ 364:165–174. https://doi.org/10.1016/j.scitotenv.2005.06.025
Hartmann J, Jansen N, Dürr HH, Kempe S, Köhler P (2009) Global CO2 consumption by chemical weathering: What is the contribution of highly active weathering regions? Global Planet Change 69:185–194. https://doi.org/10.1016/j.gloplacha.2009.07.007
Huang QB, Qin XQ, Cheng RR, Li TF, Wu HY, Liao HW (2021) Investigation of the hydrogeochemical processes and regional evolution of karst groundwater in Liulin Spring catchment, northern China. Environ Earth Sci. https://doi.org/10.1007/s12665-020-09280-0
Jeong CH (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210. https://doi.org/10.1016/s0022-1694%2801%2900481-4
Jiang ZQ, Qin H, Ji CM, Feng ZK, Zhou JZ (2017) Two dimension reduction methods for multi-dimensional dynamic programming and its application in cascade reservoirs operation optimization. Water 9:634. https://doi.org/10.3390/w9090634
Kačaroğlu F (1999) Review of groundwater pollution and protection in karst areas. Water Air Soil Pollut 113:337–356. https://doi.org/10.1023/A:1005014532330
Karim A, Veizer J (2000) Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem Geol 170:153–177. https://doi.org/10.1016/S0009-2541(99)00246-6
Kump LR, Brantley SL, Arthur MA (2000) Chemical weathering, atmospheric CO2, and climate. Annu Rev Earth Planet Sci 28:611–667. https://doi.org/10.1146/annurev.earth.28.1.611
Li SR (1988) The tectonic factors conducive to the formations of evaporites during the indosinian in Hubei province and its adjacent areas are discussed. Acta Geol Sin 2:30–37 (in Chinese)
Li SL, Gaillardet J, Han GL, Calmels D, Liu C (2006) Sulfuric acid as a weathering agent of carbonate weathering constrained by δ13C: examples from Southwest China. Chin J Geochem 25:270–271. https://doi.org/10.1007/BF02840269
Li SL, Calmels D, Han GL, Gaillardet J, Liu C (2008) Sulfuric acid as an agent of carbonate weathering constrained by delta δ13CDIC: examples from Southwest China. Earth Planet Sci Lett 270:189–199. https://doi.org/10.1016/j.epsl.2008.02.039
Li XD, Liu CQ, Liu XL, Bao LR (2011) Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate weathering using dual-isotopic data from the Jialing River, Southwest China. J Asian Earth Sci 42:370–380. https://doi.org/10.1016/j.jseaes.2011.06.002
Li YQ, Li SJ, He DF, Gao J, Wang YC, Huang HY, Zhang JT, Zhang Y (2020) Middle Triassic tectono-sedimentary development of Sichuan Basin: insights into the cratonic differentiation. Geol J 56:1858–1878. https://doi.org/10.1002/gj.4033
Liu ZH, Li Q, Sun HL, Wang JL (2007) Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: soil CO2 and dilution effects. J Hydrol 337:207–223. https://doi.org/10.1016/j.jhydrol.2007.01.034
Liu ZH, Dreybrodt W, Liu H (2011) Atmospheric CO2 sink: silicate weathering or carbonate weathering? Appl Geochem 26:292–294. https://doi.org/10.1016/j.apgeochem.2011.03.085
Liu J, Wang H, Jin DW, Xu F, Zhao CH (2020) Hydrochemical characteristics and evolution processes of karst groundwater in Carboniferous Taiyuan formation in the Pingdingshan coalfield. Environ Earth Sci. https://doi.org/10.1007/s12665-020-8898-4
Ma HY (2016) Major ion chemistry of groundwater in the Sangong River Watershed Northwestern China. Environ Earth Sci. https://doi.org/10.1007/s12665-016-5321-2
Maher K, Chamberlain CP (2014) Hydrologic regulation of chemical weathering and the geologic carbon cycle. Science 343:1502–1504
Marco T, Barbara P, Marco P, Michele S (2013) Long-term spatio-temporal hydrochemical and 222Rn tracing to investigate groundwater flow and water–rock interaction in the Gran Sasso (central Italy) carbonate aquifer. Hydrogeol J 21:1447–1467. https://doi.org/10.1007/s10040-013-1023-y
Martin JB (2017) Carbonate minerals in the global carbon cycle. Chem Geol 449:58–72. https://doi.org/10.1016/j.chemgeo.2016.11.029
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 278:401–428. https://doi.org/10.1016/0198-0254(87)95902-4
Mohamed EA, Fathy A, Karim M, Talal A (2019) Hydrochemical equilibrium and statistical approaches as effective tools for identifying groundwater evolution and pollution sources in arid areas. Geosci J 23:299–314. https://doi.org/10.1007/s12303-018-0039-7
Moon S, Huh Y, Qin J, Pho NV (2007) Chemical weathering in the Hong (Red) River basin: rates of silicate weathering and their controlling factors. Geochim Cosmochim Acta 71:1411–1430. https://doi.org/10.1016/j.gca.2006.12.004
Moon S, Huh Y, Zaitsev A (2009) Hydrochemistry of the Amur River: weathering in a northern temperate basin. Aquat Geochem 15:497. https://doi.org/10.1007/s10498-009-9063-6
Nader FH, Swennen R, Keppens E (2008) Calcitization/dedolomitization of Jurassic dolostones (Lebanon): results from petrographic and sequential geochemical analyses. Sedimentology 55:1467–1485. https://doi.org/10.1111/j.1365-3091.2008.00953.x
Pawellek F, Frauenstein F, Veizer J (2002) (2002) Hydrochemistry and isotope geochemistry of the upper Danube River. Geochim Cosmochim Acta 66:3839–3853. https://doi.org/10.1016/S0016-7037(01)00880-8
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–923. https://doi.org/10.1029/TR025i006p00914
Pu JB, Cao M, Zhang YZ, Yuan DX, Zhao HP (2014) Hydrochemical indications of human impact on karst groundwater in a subtropical karst area, Chongqing, China. Environ Earth Sci 72:1683–1695. https://doi.org/10.1007/s12665-014-3073-4
Romanov D, Gabrovsek F, Dreybrodt W (2003) The impact of hydrochemical boundary conditions on the evolution of limestone karst aquifers. J Hydrol 276:240–253. https://doi.org/10.1016/S0022-1694(03)00058-1
Santhanam H, Karthikeyan ARM (2021) Saturation indices of aqueous mineral phases as proxies of seasonal dynamics of a transitional water ecosystem using a geochemical modeling approach. Model Earth Syst Environ 7:1813–1829. https://doi.org/10.1007/s40808-020-00910-x
Stradioto MR, Teramoto EH, Chang HK (2020) Rock-solute reaction mass balance of water flowing within an aquifer system with geochemical stratification. Appl Geochem 123:104784. https://doi.org/10.1016/j.apgeochem.2020.104784
Sun Y, Wan JW, Yang SY, Xue XH, Huang K (2016) Influences of water conservancy and hydropower projects on runoff in Qingjiang River upstream basin. J Earth Sci 27:110–116. https://doi.org/10.1007/s12583-016-0640-5
Sun PA, He SY, Yu S, Pu JB, Yuan YQ, Zhang C (2021) Dynamics in riverine inorganic and organic carbon based on carbonate weathering coupled with aquatic photosynthesis in a karst catchment, Southwest China. Water Res 189:116658. https://doi.org/10.1016/j.watres.2020.116658
Torres M, West AJ, Li GJ (2014) Sulphide oxidation and carbonate dissolution as a source of CO2 over geological timescales. Nature 507:346–349. https://www.nature.com/articles/nature13030
Vasić L, Živojinovic DŽ, Rajaković-Ognjanović V, Huang F, Cao JH (2021) The subthermal potential of karstic groundwater of Kučaj-Beljanica region in Serbia estimated by the multivariate analysis. Environ Earth Sci. https://doi.org/10.1007/s12665-021-09392-1
Wang ZY, Shen JF, Xu RC, Shi BX (1995) Karst landscapes and their evolution in reaches of the Qingjiang River watershed. Earth Sci 4:439–444 (in Chinese)
Wang Q, Yu S, Jiang PP, Sun PA (2021) Water chemical characteristics and influence of exogenous acids in the Yangtze River basin. Environ Sci. https://doi.org/10.1322/j.hjkx.202012040 (in Chinese)
Wen XH, Diao M, Wang D, Gao M (2012) Hydrochemical characteristics and salinization processes of groundwater in the shallow aquifer of Eastern Laizhou Bay, China. Hydrol Process 26:2322–2332. https://doi.org/10.1002/hyp.8362
Wu XC, Li CS, Sun B, Geng FQ, Gao S, Lv MH, Ma XY, Li H, Xing LT (2020) Groundwater hydrogeochemical formation and evolution in a karst aquifer system affected by anthropogenic impacts. Environ Geochem Health 42:2609–2626. https://doi.org/10.1007/s10653-019-00450-z
Xie YC, Huang F, Yang H, Yu S (2021) Role of anthropogenic sulfuric and nitric acids in carbonate weathering and associated carbon sink budget in a karst catchment (Guohua), southwestern China. J Hydrol 599:126287. https://doi.org/10.1016/j.jhydrol.2021.126287
Yu S, Du WY, Sun PA, He SY, Kuo YM, Yuan YQ, Huang J (2015) Study on the hydrochemistry character and carbon sink in the middle and upper reaches of the Xijiang River Basin, China. Environ Earth Sci 74:997–1005. https://doi.org/10.1007/s12665-014-3771-y
Yu L, Daniels LM, Mulders JJPA, Saldi GD, Harrison AL, Liu L, Oelkers EH (2019a) An experimental study of gypsum dissolution coupled to CaCO3 precipitation and its application to carbon storage. Chem Geol 525:447–461. https://doi.org/10.1016/j.chemgeo.2019.08.005
Yu ZL, Wu GJ, Laura K, Li F, Yan N, Qu DM, Liu XM (2019b) Seasonal variation of chemical weathering and its controlling factors in two alpine catchments, Nam Co basin, central Tibetan Plateau. J Hydrol 576:381–395. https://doi.org/10.1016/j.jhydrol.2019.06.042
Zhang LL, Zhao ZQ, Zhang W, Tao ZH, Huang L, Yang JX, Wu QX, Liu CQ (2016) Characteristics of water chemistry and its indication of chemical weathering in Jinshajiang, Lancangjiang and Nujiang drainage basins. Environ Earth Sci 75:506. https://doi.org/10.1007/s12665-015-5115-y
Zhang X, Xu ZF, Liu WJ, Moon S, Zhao T, Zhou XD, Zhang JY, Wu Y, Jiang H, Zhou L (2019) Hydro-geochemical and Sr isotope characteristics of the Yalong River Basin, Eastern Tibetan Plateau: implications for chemical weathering and controlling factors. Geochem Geophys Geosyst 20:1221–1239. https://doi.org/10.1029/2018GC007769
Zhong YS, Wang LC, Xu Y, Zhang YM, Liu CL (2020) Microfacies and multi-isotope records of Anisian sequences from the Upper Yangtze Block: possible responses to tectonics and climate-driven relative sea-level change. Int J Earth Sci 109:489–509. https://doi.org/10.1007/s00531-020-01817-9
Acknowledgements
The authors would like to express special thanks to the editors and the anonymous reviewers for offering valuable suggestions, which were helpful for improving the quality of the manuscript. Our special thanks are given to Yinong Peng, Suqing Deng, and Wei Sun for their help with field and laboratory work.
Funding
This work was supported by a project of the China Geological Survey (DD20190824) and the National Key Research and Developmental Program of China (2016YFC0502302).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, X., Zhou, H., Wan, J. et al. A method for quantifying the role of carbonic acid, sulfuric acid, and nitric acid in carbonate weathering after accounting for the effects of evaporites in the Qingjiang karst catchment. Environ Earth Sci 81, 473 (2022). https://doi.org/10.1007/s12665-022-10605-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10605-4