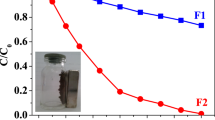
Abstract
Materials based on iron oxide were prepared via a closed system solution combustion synthesis (IOP), evaluating the effect of adding mango seed husk (T), peel (C), and a mixture of the portions (M) on the material properties obtained (IOT, IOC, and IOM, respectively). The structural (XRD, Mössbauer), morphological (SEM and TEM), optical (UV-VIS diffuse reflectance), textural (BET), and magnetic (VSM) properties of the materials obtained were evaluated. Different phase compositions (hematite, magnetite, and maghemite) were identified in the structure of the materials. Adding mango residue favored the formation of magnetite with superparamagnetic particles in the IOC sample. The solids were evaluated in the methylene blue photocatalysis at different concentrations, observing different effects on the discoloration rate. The IOP sample performed better at the lowest concentration (50 mg L−1) of the dye, with 98% discoloration after 90 min and a specific speed of 44.7 × 10−3 min−1, which can be explained by the heterojunction effect, making electronic recombination harder and increasing the quantum yield of the photocatalyst. At 70 mg L−1 concentration, the best catalyst was the IOM with 82% discoloration and a specific speed of 15.8 × 10−3 min−1, explained by the increase in the specific area due to the insertion of biomass, which improved adsorptive capacity, maintaining the observed heterojunction effects. Under sunlight, the best composite was IOC, with 90.5% discoloration and activity of 29.8 × 10−3 min−1, justified by combining favorable textural and optical properties.
Graphical Abstract










Similar content being viewed by others
Data Availability
I declare that all data generated in this study are available in the manuscript and supplementary material.
References
Al Hamedi, F.H., Kandhan, K., Liu, Y., Ren, M., Jaleel, A., Alyafei, M.A.M.: Waste water irrigation: A promising way for future sustainable agriculture and food security in the United Arab Emirates. Water 15, 2284 (2023). https://doi.org/10.3390/w15122284
Sousa J.C.G., Ribeiro A.R., Barbosa M.O., Pereira M.F.R., Silva A.M.T.: A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater. 15, 344:146–162 (2018). https://doi.org/10.1016/j.jhazmat.2017.09.058
Odling, G., Robertson, N.: Bridging the gap between laboratory and application in photocatalytic water purification. Catal. Sci. Technol. 9, 533–545 (2019). https://doi.org/10.1039/C8CY02438C
SinarMashuri, S.I., Ibrahim, M.L., Kasim, M.F., Mastuli, M.S., Rashid, U., Abdullah, A.H., Islam, A., AsikinMijan, N., Tan, Y.H., Mansir, N., MohdKaus, N.H., Yun Hin, T.-Y.: Photocatalysis for organic wastewater treatment: from the basis to current challenges for society. Catalysts 10, 1260 (2020). https://doi.org/10.3390/catal10111260
Hashimoto, K., Irie, H., Fujishima, A.: TiO 2 photocatalysis: a historical overview and future prospects. Jpn. J. Appl. Phys. 44, 8269–8285 (2005). https://doi.org/10.1143/JJAP.44.8269
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). https://doi.org/10.1038/238037a0
Rodríguez, E.M., Fernández, G., Álvarez, P.M., Hernández, R., Beltrán, F.J.: Photocatalytic degradation of organics in water in the presence of iron oxides: effects of pH and light source. Appl. Catal. B Environ. 102, 572–583 (2011). https://doi.org/10.1016/j.apcatb.2010.12.041
Khan, I., Saeed, K., Zekker, I., Zhang, B., Hendi, A.H., Ahmad, A., Ahmad, S., Zada, N., Ahmad, H., Shah, L.A., et al.: Review on methylene blue: its properties, uses, toxicity and photodegradation. Water 14, 242 (2022). https://doi.org/10.3390/w140202429
Ohtani, B., Amano, F., Malato, S., Guillard, C., Helali, S., Polo-López, M.I., Fernández-Ibáñez, P., Ohtani, B., Amano, F., Malato, S., Guillard, C.: Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 276, 31–40 (2014). https://doi.org/10.1016/j.jphotochem.2013.11.011
Giannakis, S., Liu, S., Carratalà, A., Rtimi, S., TalebiAmiri, M., Bensimon, M., Pulgarin, C.: Iron oxide-mediated semiconductor photocatalysis vs. heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. J. Hazard. Mater. 339, 223–231 (2017). https://doi.org/10.1016/j.jhazmat.2017.06.037
Ganguly, P., Byrne, C., Breen, A., Pillai, S.C.: Antimicrobial activity of photocatalysts: fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B Environ. 225, 51–75 (2018). https://doi.org/10.1016/j.apcatb.2017.11.018
Yang, L., Hakki, A., Wang, F., Macphee, D.E.: Photocatalyst efficiencies in concrete technology: the effect of photocatalyst placement. Appl. Catal. B Environ. 222, 200–208 (2018). https://doi.org/10.1016/j.apcatb.2017.10.013
Rocha, V.M.D.S., Pereira, M.D.G., Teles, L.R., Souza, M.O.D.G.: Effect of copper on the photocatalytic activity of semiconductor-based titanium dioxide (anatase) and hematite (α-Fe2O3). Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 185, 13–20 (2014). https://doi.org/10.1016/j.mseb.2014.02.004
Mohamed, H.H., Alomair, N.A., Akhtar, S., Youssef, T.E.: Eco-friendly synthesized α-Fe2O3/TiO2 heterojunction with enhanced visible light photocatalytic activity. J. Photochem. Photobiol. A Chem. 382, 1–12 (2019). https://doi.org/10.1016/j.jphotochem.2019.111951
Pang, Y.L., Lim, S., Ong, H.C., Chong, W.T.: Research progress on iron oxide-based magnetic materials: synthesis techniques and photocatalytic applications. Ceram. Int. 42, 9–34 (2016). https://doi.org/10.1016/j.ceramint.2015.08.144
Bora, L.V., Mewada, R.K.: Visible/solar light active photocatalysts for organic effluent treatment: fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 76, 1393–1421 (2017). https://doi.org/10.1016/j.rser.2017.01.130
Su, C.: Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: a review of recent literature. J. Hazard. Mater. 322, 48–84 (2017). https://doi.org/10.1016/j.jhazmat.2016.06.060
Alp, E., Aydogan, N.: A comparative study: synthesis of superparamagnetic iron oxide nanoparticles in air and N2 atmosphere. Colloids Surfaces A Physicochem. Eng. Asp. 510, 205–212 (2016). https://doi.org/10.1016/j.colsurfa.2016.06.033
Shamaila, S., Bano, T., Sajjad, A.K.L.: Efficient visible light magnetic modified iron oxide photocatalysts. Ceram. Int. 43, 14672–14677 (2017). https://doi.org/10.1016/j.ceramint.2017.07.193
da Silva, C.P., dos Santos, A.V., Oliveira, A.S., Souza, M.O.G.: Synthesis of composites and study of the thermal behavior of sugarcane bagasse/iron nitrate mixtures in different proportions. J. Therm. Anal. Calorim. 131, 611–620 (2017). https://doi.org/10.1007/s10973-017-6260-1
Sarkar, T., Tiwari, S., Rawat, K., Solanki, P.R., Bohidar, H.B.: Hydrophilic, fluorescent and superparamagnetic iron oxide-carbon composite nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 514, 218–225 (2017). https://doi.org/10.1016/j.colsurfa.2016.11.061
Oliveira, L.C.A., Rios, R.V.R.A., Fabris, J.D., Garg, V., Sapag, K., Lago, R.M.: Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon N. Y. 40, 2177–2183 (2002). https://doi.org/10.1016/S0008-6223(02)00076-3
Ramos, L.T. dos S., Santos, L.M., Machado, A.E.H., Souza, M.O.G.: Synthesis, characterization and photocatalytic activity of a composite based on TiO2 and active carbon prepared from mango pit. Sci. Plena. 17, 1–16 (2021). https://doi.org/10.14808/sci.plena.2021.077201
Arshad, A., Iqbal, J., Ahmad, I., Israr, M.: Graphene/Fe3O4 nanocomposite: interplay between photo-Fenton type reaction, and carbon purity for the removal of methyl orange. Ceram. Int. 44, 1 (2017). https://doi.org/10.1016/j.ceramint.2017.08.157
Souza, M.O.G., dos Santos, M.V.R., Castro, L.M.F., da Silva, C.P.: Production and in situ transformation of hematite into magnetite from the thermal decomposition of iron nitrate or goethite mixed with biomass. J. Therm. Anal. Calorim. 133, 1731–1739 (2019). https://doi.org/10.1007/s10973-019-08639-1
Souza, M.O.G., Rebouças, L.M., de Castro, L.M.F.: Characterization and thermal decomposition study of mango residue biomass (Mangifera indica L.). J. Therm. Anal. Calorim. 137, 1811–1816 (2019). https://doi.org/10.1007/s10973-019-08540-x
Pereira da Silva, C., Souza, M.O.G., Santos, W.N.L., Silva, L.O.B.: Optimization of the production parameters of composites from sugarcane bagasse and iron salts for use in dye adsorption. Sci. World J. 2019, 1–12 (2019). https://doi.org/10.1155/2019/8173429
Asuha, S., Zhao, S., Jin, X.H., Hai, M.M., Bao, H.P.: Effects of synthetic routes of Fe–urea complex on the synthesis of γ-Fe2O3 nanopowder. Appl. Surf. Sci. 255, 8897–8901 (2009). https://doi.org/10.1016/j.apsusc.2009.06.082
Monteiro, D.S., da Guarda Souza, M.O.: Thermal decomposition of precursors and iron oxide properties. J. Therm. Anal. Calorim. 123, 955–963 (2016). https://doi.org/10.1007/s10973-015-4840-5
Novitskaya, E., Kelly, J.P., Bhaduri, S., Graeve, O.A.: A review of solution combustion synthesis: an analysis of parameters controlling powder characteristics. Int. Mater. Rev. 66, 188–214 (2021). https://doi.org/10.1080/09506608.2020.1765603
Ianoş, R., Tăculescu, A., Păcurariu, C., Lazău, I.: Solution combustion synthesis and characterization of magnetite, Fe3O4. Nanopowders. J. Am. Ceram. Soc. 95, 2236–2240 (2012). https://doi.org/10.1111/j.1551-2916.2012.05159.x
Varma, A., Mukasyan, A.S., Rogachev, A.S., Manukyan, K.V.: Solution combustion synthesis of nanoscale materials. Chem. Rev. 116, 14493–14586 (2016). https://doi.org/10.1021/acs.chemrev.6b00279
Kopp Alves, A., Bergmann, C.P., Berutti, F.A.: Novel synthesis and characterization of nanostructured materials. Springer, Berlin (2013)
Jain, S.R., Adiga, K.C., PaiVerneker, V.R.: A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust. Flame 40, 71–79 (1981). https://doi.org/10.1016/0010-2180(81)90111-5
Huang, M., Qin, M., Cao, Z., Jia, B., Chen, P., Wu, H., Wang, X., Wan, Q., Qu, X.: Magnetic iron nanoparticles prepared by solution combustion synthesis and hydrogen reduction. Chem. Phys. Lett. 657, 33–38 (2016). https://doi.org/10.1016/j.cplett.2016.05.043
Castro, L.M.C., Souza, M.O.G.: Effects of the addition of mango residue on solution combustion synthesis of iron oxides. J. Therm. Anal. Calorim. 147(13), 7183–7191 (2022). https://doi.org/10.1007/s10973-021-11031-7
Lima, G.L., Oliveira, R.W.L., de Jesus Neto, R.M., Gomes, A.M. de S., Fiuza Junior, R.A., Andrade, H.M.C., Mascarenhas, A.J.S.: Single Step synthesis of magnetic materials derived from biomass residues. Waste Biomass Valor. 12, 1039–1050 (2021). https://doi.org/10.1007/s12649-020-01003-7
Ianoş, R., Păcurariu, C., Muntean, S.G., Muntean, E., Nistor, M.A., Nižňanský, D.: Combustion synthesis of iron oxide/carbon nanocomposites, efficient adsorbents for anionic and cationic dyes removal from wastewaters. J. Alloys Compd. 741, 1235–1246 (2018). https://doi.org/10.1016/j.jallcom.2018.01.240
Wu, W., Jiang, C.Z., Roy, V.A.L.L.: Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 8, 19421–19474 (2016). https://doi.org/10.1039/C6NR07542H
Sauthier, M.C. da S., da Silva, E.G.P., Santos, B.R. da S., Silva, E.F.R., Caldas, J. da C., Minho, L.A.C., dos Santos, A.M.P., dos Santos, W.N.L.: Screening of Mangifera indica L functional content using PCA and neural networks (ANN). Food Chem. 273, 115–123 (2019). https://doi.org/10.1016/j.foodchem.2018.01.129
Orozco, R.S., Hernández, P.B., Morales, G.R., Núñez, F.U., Villafuerte, J.O., Lugo, V.L., Ramírez, N.F., Díaz, C.E.B., Vázquez, P.C.: Characterization of lignocellulosic fruit waste as an alternative feedstock for bioethanol production. BioResources 9, 1873–1885 (2014). https://doi.org/10.15376/biores.9.2.1873-1885
Girod, M., Vogel, S., Szczerba, W., Thünemann, A.F.: How temperature determines formation of maghemite nanoparticles. J. Magn. Magn. Mater. 380, 163–167 (2015). https://doi.org/10.1016/j.jmmm.2014.09.057
Fathi, H., Masoudpanah, S.M.M., Alamolhoda, S., Parnianfar, H.: Effect of fuel type on the microstructure and magnetic properties of solution combusted Fe3O4 powders. Ceram. Int. 43, 7448–7453 (2017). https://doi.org/10.1016/j.ceramint.2017.03.017
Múzquiz-Ramos, E.M., Guerrero-Chávez, V., Macías-Martínez, B.I., López-Badillo, C.M., García-Cerda, L.A.: Synthesis and characterization of maghemite nanoparticles for hyperthermia applications. Ceram. Int. 41, 397–402 (2014). https://doi.org/10.1016/j.ceramint.2014.08.083
Babay, S., Mhiri, T., Toumi, M.: Synthesis, structural and spectroscopic characterizations of maghemite γ-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 1085, 286–293 (2015). https://doi.org/10.1016/j.molstruc.2014.12.067
Shokrollahi, H.: A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 426, 74–81 (2017). https://doi.org/10.1016/j.jmmm.2016.11.033
Darezereshki, E., Bakhtiari, F., Alizadeh, M., Behradvakylabad, A., Ranjbar, M.: Direct thermal decomposition synthesis and characterization of hematite (α-Fe2O3) nanoparticles. Mater. Sci. Semicond. Process. 15, 91–97 (2012). https://doi.org/10.1016/j.mssp.2011.09.009
Păcurariu, C., Paşka, O., Ianoş, R., Muntean, S.G.: Effective removal of methylene blue from aqueous solution using a new magnetic iron oxide nanosorbent prepared by combustion synthesis. Clean Technol. Environ. Policy 18, 705–715 (2016). https://doi.org/10.1007/s10098-015-1041-7
Elizalde-González, M.P., Hernández-Montoya, V.: Characterization of mango pit as raw material in the preparation of activated carbon for wastewater treatment. Biochem. Eng. J. 36, 230–238 (2007). https://doi.org/10.1016/j.bej.2007.02.025
Davies, G., McGregor, J.: Hydrothermal synthesis of biomass-derived magnetic carbon composites for adsorption and catalysis. ACS Ômega 6, 33000–33009 (2021). https://doi.org/10.1021/acsomega.1c05116
Silva, F.S., Nascimento, S.S., Santos, A.V., da Guarda Souza, M.O.: Study of the thermal decomposition of mixtures sugarcane bagasse/titanium dioxide. J. Therm. Anal. Calorim. 148, 37–47 (2023). https://doi.org/10.1007/s10973-022-11583-2
Kadirova, Z.C., Hojamberdiev, M., Katsumata, K.I., Isobe, T., Matsushita, N., Nakajima, A., Okada, K.: Fe2O3-loaded activated carbon fiber/polymer materials and their photocatalytic activity for methylene blue mineralization by combined heterogeneous-homogeneous photocatalytic processes. Appl. Surf. Sci. 402, 444–455 (2017). https://doi.org/10.1016/j.apsusc.2017.01.131
Mohamed, M.M., Bayoumy, W.A.A., Goher, M.E.E., Abdo, M.H.H., Mansour El-Ashkar, T.Y.Y.: Optimization of α-Fe2O3 @Fe3O4 incorporated N-TiO2 as super effective photocatalysts under visible light irradiation. Appl. Surf. Sci. 412, 668–682 (2017). https://doi.org/10.1016/j.apsusc.2017.03.200
Castro, J.D.S., das Virgens, C.F.: Thermal decomposition of Nephelium lappaceum L. peel. J. Therm. Anal. Calorim. 138, 3541–3549 (2019). https://doi.org/10.1007/s10973-019-08289-3
Carvalho, M.S.S., Virgens, C.F.F.: Effect of alkaline treatment on the fruit peel of Pachira aquatic Aubl.: physico-chemical evaluation and characterization. Microchem. J. 143, 410–415 (2018). https://doi.org/10.1016/j.microc.2018.08.021
Tyapkin, P.Y., Petrov, S.A., Chernyshev, A.P., Larichev, Y.V., Kirik, S.D., Gribov, P.A., Uvarov, N.F.: Properties of iron oxides inserted into SBA-15 mesoporous silica. Mater. Today Proc. 4, 11392–11395 (2017). https://doi.org/10.1016/j.matpr.2017.09.015
Hadadian, S., Masoudpanah, S.M., Alamolhoda, S.: Solution combustion synthesis of Fe3O4 powders using mixture of CTAB and citric acid fuels. J. Supercond. Nov. Magn. 32, 353–360 (2019). https://doi.org/10.1007/s10948-018-4685-9
Radpour, M., Alamolhoda, S., Masoudpanah, S.M.: Effects of pH value on the microstructure and magnetic properties of solution combusted Fe3O4 powders. Ceram. Int. 43, 13729–13734 (2017). https://doi.org/10.1016/j.ceramint.2017.07.085
Guardia, P., Riccardo Di Corato, R., Lartigue, L., Wilhelm, C., Espinosa, A., Garcia-Hernandez, M., Gazeau, F., Manna, L., Teresa Pellegrino, T.: Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. CS Nano 6(4), 3080–3091 (2012). https://doi.org/10.1021/nn2048137
Bhavani, P., Reddy, N.R., Reddy, I.V.S., Sakar, M.: Manipulation over phase transformation in iron oxide nanoparticles via calcination temperature and their effect on magnetic and dielectric properties. IEEE Trans. Magn. 53(9), 1–5 (2017). https://doi.org/10.1109/TMAG.2017.2715320
Marschall, R.: Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 24, 2421–2440 (2014). https://doi.org/10.1002/adfm.201303214
Wang, Y., Wang, Q., Zhan, X., Wang, F., Safdar, M., He, J.: Visible light driven type II heterostructures and their enhanced photocatalysis properties: a review. Nanoscale 5, 8326 (2013). https://doi.org/10.1039/c3nr01577g
Sun, B., Zhou, W., Li, H., Ren, L., Qiao, P., Xiao, F., Wang, L., Jiang, B., Fu, H.: Magnetic Fe2O3/mesoporous black TiO2hollow sphere heterojunctions with wide-spectrum response and magnetic separation. Appl. Catal. B Environ. 221, 235–242 (2018). https://doi.org/10.1016/j.apcatb.2017.09.023
Fu, H., Sun, S., Yang, X., Li, W., An, X., Zhang, H., Dong, Y., Jiang, X., Yu, A.: A facile coating method to construct uniform porous α-Fe2O3@TiO2 core-shell nanostructures with enhanced solar light photocatalytic activity. Powder Technol. 328, 389–396 (2018). https://doi.org/10.1016/j.powtec.2018.01.067
Hitam, C.N.C., Jalil, A.A.: A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manage. 258(110050), 1–19 (2020). https://doi.org/10.1016/j.jenvman.2019.110050
Colmenares, J.C., Luque, R.: Heterogeneous photocatalytic nanomaterials: prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 43, 765–778 (2014). https://doi.org/10.1039/C3CS60262A
Funding
The authors have not disclosed any funding. Coordination for higher Education Staff Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors would like to thank the Applied Chemistry Postgraduate Program (PGQA) of Universidade do Estado da Bahia (UNEB) for the granted infrastructure and the Higher Education Personnel Improvement Coordination (Capes) for their financial support.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The nanomaterials were based on iron oxide and carbonaceous material.
• The composites showed the typical magnetic behavior of non-stoichiometric magnetite.
• The microscopy results denote a modification of the morphology, maintaining the degree of crystallinity with the insertion of biomass residue.
• The photocatalytic activity was appreciable in all tested systems, emphasizing the system’s use of sunlight.
Statement of novelty
This study presents a new ecologically friendly preparation route employing combustion syntheses in a closed system of new composites based on iron oxide and carbonaceous material, using mango waste seed husk (T), peel (C), and mixture of the portions (M), with suitable properties for application in heterogeneous photocatalysis with UV and solar radiation, constituting an important contribution to the treatment of effluents contaminated with organic pollutants, especially textile dyes.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Castro, L.M.F., Huaman, N.R.C. & da Guarda Souza, M.O. Solution Combustion Synthesis of New Composites Based on Iron Oxide and Mango Waste for Heterogeneous Photocatalysis with UV and Solar Radiation. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02511-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02511-6