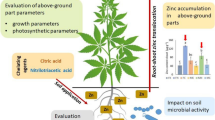
Abstract
Purpose
The potential of industrial hemp (Cannabis sativa L.) for phytoremediation of copper-contaminated Mediterranean soils was investigated. The accumulation of copper in hemp’s parts and the effect of contaminated soil on the production of cannabidiol (CBD) were, also, of primary concern.
Methods
Two soil types, a Clay Loam & a Sandy Clay Loam, were exposed to two levels of Cu contamination, with low & high Cu concentrations. The soils’ total and available Cu content, along with Cu in plant tissues, were determined. Height, fresh aboveground biomass and leaf chlorophyll content were further evaluated. Furthermore, the amount of cannabidiol (CBD) in the hemp flowers was determined.
Results
Hemp appeared to be highly resistant, as can successfully grow in both soil types having low or high Cu levels. The maximum Cu content was detected in the highest part of the plant roots, with a progressive decline towards the upper parts of the plant (CuRoots > CuShoots > CuLeaf), in both soil types. It has been observed that more than 50% of the plant’s copper concentration is accumulated in the roots. Furthermore, the hemp plants cultivated in high Cu-contaminated soils produced greater amounts of cannabidiol (CBD).
Conclusion
Ηemp proved to be a promising plant for phytostabilization in Cu-contaminated soils, as its above-ground biomass is almost free of metals and can be used further for fiber production. The presence of Cu in soils did not appear to disrupt the production of the important secondary metabolite CBD, but rather increased following increasing soil Cu content.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
In recent years, the need for remediation and valorization of heavy metal-contaminated soils is imperative. In this direction, eco-friendly and cost-effective methods are proposed, with phytoremediation at the top of the list of suggested approaches. Industrial hemp (Cannabis sativa L.) seems to be tolerant to heavy metal polluted soils, accumulating them primarily in its roots and contributing to the phytostabilization mechanism. As a result, its above-ground part, which is primarily used for rope production, is metal-free. Furthermore, the production of high-value cannabidiol (CBD) appears to increase as the concentration of metals in cannabis-growing soil increases. Therefore, hemp cultivation is promising for Mediterranean soils, as it produces high amounts of CBD in response to metal pollution stress and remediates polluted soils by accumulating the pollutants in the roots, leaving the stems clean for further utilization in the rope industry.
Introduction
Heavy metals cover a group of metals and metalloids having an atomic density greater than 5 g cm−3 that can be dangerous in both plants and animals [1]. Twenty of the aforementioned metals behave as nutrients when present in small amounts (trace elements), but when their concentrations are above a certain threshold, they become exceedingly toxic to organisms [2, 3]. Therefore, the use of the term Potential Toxic Elements (PTE) has been considered imperative in recent [4, 5]. Due to their failure to decompose and tendency for remaining in their metallic or complex forms for a long period of time, PTEs are among the most hazardous contaminants in the environment [6, 7]. Although their origin in the environment is attributed to both natural and anthropogenic sources, rapid industrial development, deregulated urbanization, and intensive use or waste of fertilizers appear to have played the most critical role in the spread of the pollution problem worldwide [5, 8, 9].
Soil is a direct or indirect recipient of resistant pollutants, like metals, resulting in negative consequences both on soil health and the living organisms in its environment [10, 11]. However, the overall amount of a metal deposited in soil is not equal to the amount of bioavailable metals, i.e., metals that can be directly taken by plants [2, 12]. As a result, a distinction is drawn between ‘total’ and ‘available’ to plants concentrations. Conventional approaches for the remediation of heavy metal-contaminated soils include the addition of suitable media, high surface area solids, and plant residues, which enhance the organic matter and fertility of the soils [13]. Metal uptake by plants is the basis of an ever-growing strategy for restoring, remediating or improving contaminated soils. PTEs uptake in plants occurs mostly through the roots and leaf surface [2, 4]. The remediation of contaminated soils is a complex process that aims either to completely eliminate metals or to reduce their concentration to the acceptable limits indicated by legislation [4, 12]. Several existing soil remediation techniques, despite their effectiveness, are considered restrictive due to their high operating costs [14]. Moreover, the control of their processes is extremely demanding and often exposes the environment to further risk, while at the same time causing visual disturbance due to the aesthetic alteration of the landscape [13]. For all the aforementioned reasons, in the last decades, phytoremediation has emerged as a promising method [4].
Phytoremediation is defined as the process in which plants are used to relieve, stabilize, transport, or degrade pollutants from soil, sediments, surface water, and groundwater [15]. The main mechanisms of phytoremediation include the accumulation (phytoextraction, rooting), degradation (rooting, phytodegradation), immobilization (hydraulic control and phytostabilization), and diffusion (phytoprotection) of contaminants [16]. These mechanisms are used either individually or in combination by plants, depending on the pollutant type [17]. Phytoremediation is commonly applied using hyperaccumulating plants, i.e., plants that accumulate high amounts of metals in their aboveground biomass [18, 19]. In general, however, in order for a plant to be considered suitable for phytoremediation, it is essential that it fulfill certain characteristics, such as rapid growth, high biomass, an extensive root system, resistance to high metal concentrations, a high translocation factor within the plant tissue, adaptability to certain environments, and easy agricultural management [4, 20]. Nevertheless, combining all of the above characteristics in a single plant is often impossible, so two main strategies are usually followed: either the use of supersaturation or the use of high biomass plants in combination with metal solubilizing agents [18]. Cannabis sativa L. displays some of the above-mentioned features and has received a lot of interest in recent years for phytoremediation purposes [4, 12, 21].
Copper (Cu) is a metal widely used in industry but also an essential micronutrient for the growth of both plants and animals [1]. This metal contributes significantly to the physiological function of plants, particularly in seed production, water regulation, and disease resistance [22]. Nonetheless, at high concentrations, it provokes toxicity in plants, animals, and humans, with consequent severe environmental effects. Within the plant tissues, it accumulates mainly in the roots, with a slight upward trend towards the shoots. Rehman et al. concluded that this is probably related to the effort of plants to prevent the movement of Cu to the photosynthetic tissues [23].
Hemp (Cannabis Sativa L.) is a multifunctional plant with a wide range of industrial and pharmaceutical uses [4, 12]. Its applications can be found in four main sectors: industry (use and manufacture of new materials), agri-food, medical/pharmaceutical, and recreation [21]. The long history of cannabis in the medical and pharmaceutical sectors can be attributed to the valuable cannabinoids it provides. Among these, cannabidiol and tetrahydrocannabidiol are considered to be the most significant [24]. In particular, canabidiol is known for its anticancer, anti-inflammatory, anticonvulsant, antiepileptic, analgesic, anxiolytic, and neuroprotective properties [24]. The cultivation of cannabis, both industrial and mainly pharmaceutical, has for many years been the subject of debate and faced legal challenges due to the production of the psychotropic substance tetrahydrocannabidiol (THC). The evolving legal status of medicinal cannabis for pharmaceutical purposes is important as it affects both clinical and patient access to cannabis-based treatments [4, 12]. Furthermore, after completing phytoremediation, industrial hemp may be regarded as a “resource” for future exploitation because the buildup of heavy metals in the plant tissue has no impact on the plant’s development or the amount of useful components it produces [25].
This study aimed to evaluate the potential of hemp plant for remediation purposes in Cu-loaded soils with different particle size compositions. Copper accumulation within the plant tissue, in the plant roots along with in its aboveground biomass, is of particular concern. Furthermore, the effect of soil Cu levels on the amount of the produced cannabidiol (CBD) was investigated.
Materials and Methods
Experimental Design and Cu-soil Spiking
The experiment was carried out at the University of Thessaly farm in the region of Velestino (near Volos), in central Greece. For this purpose, thirty 20-liter pots were used. In the 15 pots, soil directly collected from the university farm was filled up to a final volume of 10 L, while in the remaining 15 plant containers, a mixture consisted of sand and farm soil was placed at a ratio of 1:10. The experimental design included 2 levels of copper, 100 ppm and 200 ppm, with 5 replicates each, along with a control sample for each soil type, with 5 replicates. The coding of the treatments was formulated as follows: CL = soil collected from the university farm; SCL = the 1:10 mixture; A = the Low copper concentration (100 ppm Cu) treatments; B = High copper concentration (200 ppm Cu) treatments; and C = the control treatments. In this way, the following coding were obtained: CL-A, CL-B, CL-C, SCL-A, SCL-B, and SCL-C.
For the contamination of the soils, solutions of copper nitrate Cu(NO3)2 ware used. The proper mass of the high purity salt has been added to 1 L volumetric flask and was fulfilled with water in order to achieve the proper Cu++ solutions. In each pot, a black plastic bag was used and a volume of 200 mL of the above prepared solutions were added. The bags were tightly closed, and the soil was stirred manually. This was followed by incubation with the bags sealed for 2 weeks, to prevent evaporation, having the agitation repeated every 2–3 days. After this period of time, the soil was placed back into the plant containers for sowing. The chosen variety was Felina 32, as the plant is usually one-meter high, an ideal high for a pot experiment. The growth period of the plant lasted 18 weeks. During this period the plant was completely developed and subsequently grubbed up in order to carry out soil and chemical analyses of the plant tissues.
Hemp Seeding, Plant Thinnings and Growth Parameters Measurements
Ten hemp seeds were put in each pot. The seeds were kept in the refrigerator and before use they were suspected to wash for ten minutes with NaClO, in order to have no microbiological impacts [4].
The chlorophyll levels were measured using the portable chlorophyll meter SPAD-502, Minolta Co., Ltd. In this instrument, the resulting values are considered to be proportional to the concentration of chlorophyll in the leaves. The procedure was carried out in the following way: three measurements were recorded in the upper part of each plant, and the average of these was calculated. Respectively, three measurements were recorded in the lower part of each plant, and, similarly, the average of these was calculated. The chlorophyll measurement was carried out four times during the experiment, at 20-day intervals.
Soil Chemical Analyses
The soil samples were analyzed before and after hemp cultivation. They were transported to the soil laboratory, air-dried and prepared for soil analyses [5, 12, 26]. They were sieved through a < 2 mm pore sieve and subjected to chemical analyses, as previously described [4, 13, 26]. Soil pH along with Electrical Conductivity (EC) values were evaluated in a soil:water (1:1) mixture, organic matter content was measured using the Walkley Black method, using K2CrO4 as an oxidizing factor. The CaCO3 content was measured using a proper calcimeter, while the Bouyoucos method was used in order to determine the granulimetric soil composition.
Soil Cu available and total concentrations were evaluated using the DTPA and Aqua Regia extraction methods respectively. The extraction of available Cu contents was performed by DTPA solution [7.4 g CaCl2·2H2O, 9.835 g DTPA (diethylo-triaminopenta-acetic acid), and 74.5 g triethanolamine in 5 L of deionized water] in a 1:2 soil to solution ratio (20 g of soil and 40 mL of the extracting solution). The samples were shaken for 2 h, then filtered and diluted 1:10 volume. Total Cu contents were extracted by Aqua Regia mixture using a close system with plastic container. The procedure was carried out using 1 g (± 0.1) of dry soil blended with 9 mL of concentrated HCl, 3 mL of concentrated HNO3, and 4 mL of diluted HNO3 at 5%. The digestion was performed at 140 °C for 4.5 h, and after filtration, the samples were diluted in ratio 1:100. The available & total Cu concentrations were determined by an atomic absorption spectrophotometer with a flame or a graphite furnace equipment. For the evaluation of the analytical method a Certified Reference Material (CRM) (No 141R, calcareous loam soil) from BCR (Community Bureau of Reference) was analyzed along with the soil samples. The recovery ratio ranged from 91.7 to 101.6%.
Plant Analyses
After harvesting, each plant was placed in a 60 °C incinerator for three days to ensure moisture removal from its tissues [4, 12, 27]. Afterwards, every dry plant was dissected into 3 points: upper, middle, and lower (bottom), and then, carefully divided into the following subsections: upper leaves (60–90 cm), middle leaves (30–60 cm), upper shoot (60–90 cm), middle shoot (30–60 cm), lower shoot (0–30 cm), and upper root (0–5 cm), lower root (5–10 cm). The individual sections were pulverized separately, first with an electric grinder and then with a porcelain mortar, and afterwards they were placed in a numbered plastic bag. The total Cu concentrations of the individual plant parts were quantified by the Aqua Regia extraction method [12]. For the evaluation of the analytical methods a NIST SRM 1573a—(Tomato Leaves) certified material was analyzed along with the plant tissues. The recovery ratio ranged from 88.8 to 104.1%.
Extraction and Quantification of Cannabidiol (CBD)
Cannabidiol (CBD) were determined by high-performance liquid chromatography after plant tissues extraction with acetonitrile.
A 0.25 g mount of powdered plant tissues (flowers) was extracted with 20 mL of acetonitrile in a 50 mL glass vial with teflonated caps by vortexing for 1 min and thereafter sonicated in an ultrasonic bath for 20 min. Then it was allowed to settle at room temperature for 10 min and the supernatant extract was filtered through a 0.45 μm Titan 2 HPLC Nylon membrane filter, and placed into 2 mL amber vials in order to be analyzed.
Chromatographic analyses were performed by an HP 1100 liquid chromatograph (Hewlett Packard GmbH, Waldbronn, Germany), equipped with a variable wavelength UV detector and the HP ChemStation LC 3D chromatography manager data acquisition and processing system with the possibility of obtaining UV spectra at selected retention times of chromatograms. The analytical column was a Reprosil Gold 100 (Dr Maisch GmbH) C18 column (250 × 4.6 mm I.D, 5.0 μm particle size). The mobile phase was 60% methanol and 40% water solution containing 0.1% formic acid. The flow rate was 1.0 mL/min, the chromatographic injection volume was 20 µL, the optimum detection was obtained at 228 nm and the column temperature was maintained at 40 °C.
Quantification was performed using the external standard method, measuring the peak areas of the cannabidiol. Calibration solutions was prepared from a cannabidiol standard solution of 1.0 mg/mL in methanol (Sigma, Darmstadt, Germany) after appropriate dilutions.
Indices of Copper Accumulation and Transport in Soil to Plant System
The ability of the hemp to accumulate Cu, along with Cu potential transport from the roots to the aboveground parts, was calculated based on the three following indices [4, 28]:
Bioaccumulation Factor (BAF), which estimates the transfer of the metal from the soil to the roots: BAF = root Cu concentration (mg kg−1)/available soil Cu concentration (mg kg−1)). Plants are considered to be hyperaccumulators when BAF > 1, while they are considered to be blockers when BAF < 1.
Translocation Factor (TF), which is used to estimate the transport of the metal from the roots to the shoots: TF = Cu concentration in shoots (mg kg−1)/Cu concentration in the roots (mg kg−1). Metal hyperaccumulators exhibit TF > 1.
Transfer Coefficient (TC), which is used to evaluate the transfer of metals from the soil to the aboveground biomass: TC = Cu concentration in the aboveground biomass (mg kg−1)/total Cu concentration in the soil (mg kg−1) determined by the Aqua Regia method.
Statistical Analysis
The impact of the two factors (soil type and varying levels of contamination) on the growth characteristics of hemp was investigated via 2-way ANOVA statistical analysis with SPSS. The Microsoft Office Excel package (v.11, 2022) was used to study and evaluate statistically the results. The mean, maximum, and minimum values, along with the standard deviation among treatments, were obtained for each data set. Tukey t-test was used to find the statistically significant difference, between treatments, both at 95 & 99% levels of significance.
Results and Discussion
Soil Properties
Both soil samples are slightly alkaline, with low electrical conductivity values, as shown in Table 1. The CL soil is an alkaline clay loam soil with 32% clay, 28% sand, and 40% silt, while SCL soil is an alkaline sandy clay loam soil with 28.2% clay, 50.5% sand, and 21.3% silt. The first soil sample has almost 49% higher organic matter content than the second one, indicating a healthier soil [13].
Soil and Plant Cu Content
In the following Τable 2, the soil Cu availability, expressed as a percentage of available to total Cu concentration, is presented.
The ratio of the available to the total Cu concentration, ranges between 8.5 and 9.9%. Copper availability seems to higher in the SCL (mixture of farmland soil and sand) than in the farmland soil (CL). Furthermore, Cu availability ratio increased as the percentage of added Cu(NO3)2 increased.
Both available and total Cu concentrations in soil samples before and after hemp cultivation in each treatment, are presenting in the following Fig. 1.
The varying levels of contamination were accurately reflected in the treatments’ total and available Cu, in the expected order: B > A > C, indicating that the high contaminated soil had higher total and available concentrations both in CL & SCL soils. The control soil was not entirely devoid of Cu, possibly due to the long-term use of agricultural fertilizers in the farm land obtained. In both soil types, there was a decline seen in both total and available Cu concentrations after the cultivation of hemp, which was naturally expected, as Cu accumulated in parts of the plant moving from the soil.
The copper concentration in the hemp sections, both in roots and overgrown parts, grown in CL soil are presented in the following Fig. 2.
As expected in treatment B where the highest copper nitrate was added, the amount of copper taken up by the plant was higher.
According to the data presented in Figs. 2 and 3 and a progressive decrease in Cu concentration is observed towards the upper parts of the cannabis plant, with the highest concentration being detected in the upper roots. The lower (bottom) roots, consisting mainly of delicate and thin rhizomes that could not be fully preserved during the destructive measurements, led to an underestimation of Cu concentration. The lower (bottom) and middle shoots have the second- highest amounts, whereas the leaves have the lowest concentrations. The hemp flowers have almost no detectable Cu concentrations.
The following Table 3 displays the percentages of copper concentrations distributed throughout the plant.
Indices of Copper Accumulation and Transport Within the Plant Tissue
Bioaccumulation Factor (BAF)
Given the above- mention data from Table 4, it becomes obvious that the bioaccumulation factor (BAF) of all treatments in both soil types is > 1. Thus, under the conditions of this experiment, hemp behaves as a Cu supersaturant, retaining sufficient copper in its roots for gradual soil remediation over time [28].
Translocation Factor (TF)
As far as Translocation Factor concern, the treatments of both soil types present values < 1, indicated the weak tendency of Cu to move into the upper biomass (Table 5). Cu seems to be stabilized in the roots rather than in the stems, or leaves, leaving the above grown hemp part almost unaffected by copper contamination.
Transfer Coefficient (TC)
In accordance to the Translocation Factor the values of the Transfer Coefficient Index indicate only a slight migration of copper to the upper biomass (Table 6).
Cannabis sativa (L.) Growth Parameters
The following Fig. 4 illustrates the results of Cu soil addition on the hemp growth parameters.
Cu appears to have a beneficial effect on plant growth. Plants of the highest contamination level (B) show the highest values, followed by plants of contamination level A, while control plants (C) show the lowest growth. The impact of the two parameters (soil type and contamination level) on plant growth can be better understood using a statistical two-way analysis of variance (2-way ANOVA). Thus, the soil type had a statistically significant effect on plant height, aboveground biomass, and SPAD index values. This information, when combined with the above data, leads to the conclusion that plants in CL soil developed more vigorously than plants in SCL soil.
Statistical analysis, also demonstrated that the level of contamination had a substantial effect on all developmental features. The effect was positive, as shown by the graphs, since the values were in the following order: B > A > M. Again, greater Cu concentrations in comparison to the Control treatment, contributed to an increase in plant biomass, height, and chlorophyll.
Further post-hoc analysis (LSD test, P = 0.05) was carried out to determine which Cu treatments were statistically significantly different, concerning growth trait measurements. For each growth measurement, the treatments that presented differences were the following: height = A–C, B–C; aboveground biomass = A–B, B–C; SPAD = B–C.
Levels of Cannabidiol (CBD) in Hemp Tissues
Chromatographic analysis of CBD in plant tissues provided satisfactory chromatograms of extracts, without interferences at the CBD elution time (Fig. 5). Quantitative data were obtained through the calibration curve by injecting CBD standard solutions. Calibration curve was confirmed every two weeks and both detector’s response (RSD < 5%) and retention time remained stable throughout the experimental duration.
The CBD concentrations determined in the hemps flowers are presented in the following Table 7, along with the following Fig. 6.
Table 7; Fig. 6 present the results from the analysis of the quantification of cannabidiol using the dry leaf biomass. When soil Cu increases, an increase in cannabidiol levels is observed. CBDs concentration follows the order of Cu soil treatments, B (high) > A (low) > C, in both soil types studied. It appears, therefore, that high Cu concentrations in the soil and thus in plant tissues trigger the production of higher levels of cannabidiol.
Discussion
Soil Properties
Soil samples varied in terms of sand content, as the second sample was obtained by mixing the original sample with sand at a ratio of 1:10. The study of Table 1 reveals that the soil samples do not differ significantly among them with regard to their pH value. Alloway [1], as well as numerous researchers, have observed and demonstrated in their experiments that soil reaction is a determinant factor in the physicochemical behavior of the soil environment. The first sample has a higher pH value, higher clay content, and higher organic matter content. Therefore, the sample having a particle size composition such that it is classified as ClayLoam soil possesses all the prerequisites to be more chemically active than the second soil sample, which is classified as SandyClayLoam soil [1, 2]. Napoletano et al. [3] and Papadimou et al. [5] consolidated their observations and results of their experiments on soils that had higher values of electrical conductivity, mainly due to anthropogenic interference. In the present study, the soil samples had low electrical conductivity values, i.e. low presence of salts in the soil solution [1, 2]. The values of organic matter percentage indicate that the soil samples are typical representative agricultural soils from the area of Thessaly. Golia & Diakoloukas [9], studied a great number of soils derived from agricultural areas, belonging to four soil orders and observed that organic matter values are usually lower than 3% which was also noticed in the present study.
Soil and Plant Copper Content
According to Table 2 that the availability of copper depends on the particle size distribution of the soil samples. It can be observed that in the first soil, which shows a higher clay content, Cu seems to have a lower percentage of availability compared to the second soil mixture. The metal cations tend to bind to the clay fragments, resulting in a lower fraction of available-total metal concentration in clay soils compared to sandy soils [1, 2, 11]. The researchers Vega et al. [29], studied the relationships between heavy metals content and soil properties and concluded that the availability fraction, i.e. available to total concentration, usually occupies percentages around 10%. In the present study, the percentages range from 8.5 to 9.9%, indicating that in both soil samples, the availability of copper to plants is governed by the physicochemical parameters of the soils. The 1st soil sample has a higher soil pH value, so lower availability of copper is expected. Also, the fact of higher organic matter value in the 1st soil sample also contributes to the binding of copper by organic matter groups, forming chelate complexes and diminishing its availability [2, 12, 13].
Furthermore, the additional copper nitrate provided during fertilization or our pollution experiment, might lead to a considerable leaching [30], particularly of the nitrate anions. An increase in copper uptake is observed from the study of Figs. 2 and 3 as the amount of copper added to the soil increases. Table 3 depicts the percentages of Cu concentration as distributed in the different parts of the plant. It is obvious that by far the highest Cu concentration is found in the root. In fact, the sum of the percentages in both parts of the root exceeds 50% in both soil types, at both contamination levels, and in the control samples. In addition, it is evident that the percentage of copper decreases as it progresses towards the top of the plant. The hemp leaves used to extract CBD appear to contain no more than 2.6% of the total amount of metal taken up by hemp. Therefore, in soils containing up to 200 ppm copper, in a period of less than five months, it is possible to achieve soil phytoremediation using hemp. It is possible to stabilize in the root of the cultivated hemp plant more than 50% of the copper concentration collected by the plant, while the remaining parts of the plant (shoots and leaves) have much lower copper concentrations.
The gradual decrease in Cu concentration towards the upper parts of the hemp plant, in combination with the increased concentration of the roots, are justified by the generally immobile nature of copper within the plant tissue [31]. The observation that copper is present mainly in the roots of the cannabis plant is consistent with similar experiments [12, 14, 32,33,34], as well as the fact that the bottom and middle shoots have the second-highest Cu concentration [35]. Metal mobility is worth studying, as it is governed by the physico-chemical parameters of the soil and varies depending on whether the soil is agricultural or urban [36, 40]. Similar findings were observed for several other plants, such as the common thistle, where a higher metal concentration was observed in the root and significantly less or undetectable in the plant’s flower when grown in Cd-contaminated soils [41].
Angelova and her colleagues [32] found during their study a significant heavy metals accumulation and distribution potential fibre crops (flax, cotton and hemp). Golia et al. 2021 & 2023 [4, 12] observed that there are metals that move more vigorously and faster in the underground tissues than in the above-ground tissues of plants.
Indices of Copper Accumulation and Transport Within the Plant Tissue
The indices used in this study, as well as in similar investigations, constitute a means of indirectly and directly evaluating a plant’s ability to transfer one or more metals from the soil to the plant and to allocate them within the plant mass. The absolute value of the indices, however, is only a first approximation, as the study of the concentration of the metal in all parts of the plant is a crucial issue. The bioaccumulation factor (BAF) values demonstrated the potential of hemp to act as a metal accumulator. Hemp appears to survive satisfactorily under conditions of high metal concentration [4, 12, 33] and to be able to carry out its physiological functions satisfactorily [34]. Moreover, hemp, like other oil-bearing plants, possesses a large phytomass, fulfilling an additional requirement of plants suitable for phytoremediation, i.e. that they can accumulate metals in their tissues.
The translocation factor (TF) values revealed the weak tendency of copper to be transported to the aboveground parts of the plant. The researchers Petrova et al. in a controlled conditions experiment with laboratory contaminated solution [39], as well as Ćaćić et al. in a greenhouse experiment [33] conducted in alkaline and acidic soils, found that Cannabis Sativa (L.) strongly accumulates metals in her roots, rather than in the shoots, or leaves. So, in light of its high biomass output and its confirmed ability to absorb copper in its roots, this plant represents a prime candidate for phytoremediation.
Cannabis sativa (L.) Growth Parameters
Figure 4 depicts the variation of growth parameters of industrial hemp plants. The changes in the green weight of the aboveground part of the plant, height, and photosynthetic capacity are plotted for the two soil samples and the two treatments of copper levels in the soils.
High copper concentrations were found to appear beneficial effects on the growth of hemp plants, according to the measurements of the growth parameters. Since copper is a trace element for plants, the higher dose in this case enhanced their growth. Due to its interference with many cellular processes (such as photosynthesis, electron transport, etc.), Cu serves as a micronutrient in multiple biological processes and is a crucial component in many metalloproteins [31]. In other words, the high copper levels in neither of the two soil samples seemed to have a negative effect on either the green weight, height, or photosynthetic capacity. These observations are particularly important as the plant, regardless of whether it is grown in soil with different clay or organic matter content, i.e. regardless of whether the soil is fertile or not, can withstand high levels of copper and seems to use it to its advantage. Wu et al. [25] and Golia et al. [4] showed that plant functions are not inhibited by the presence of metals. On the contrary, the plant increases its weight and height and improves its photosynthetic capacity as copper levels increase.
Levels of Cannabidiol (CBD) in Hemp Tissues
The composition of cannabidiol appears to be proportional to the amount of copper in the soils. Adding more copper to both soil samples seems to increase the production of the secondary metabolite, the valuable cannabidiol. In the soil having CL particle size distribution, the amount of cannabidiol produced increased by 38.9 and 50.7% when smaller and larger amounts of copper were added to the soil, respectively. When the highest amount of copper was added (level B), the SCL soil’s ability to produce cannabidiol increased. Actually, when Cu was added to levels A and B, respectively, increases of 50.2 & 61.3% were observed. The effect of soil physicochemical properties plays a crucial role in the accumulation of metals in plant parts [21, 29]. However, they also seem to determine the production of secondary metabolites. Also, the values of produced cannabidiol seem to be related to the levels of copper in the soil more than to the levels of copper in the plant parts.
Lastly, the fact that the higher soil metal concentration led to the production of a higher amount of cannabidiol is in agreement with a previous study [34] were was observed that higher CBD content has been synthesized in the flowers of cannabis grown in contaminated soil rather than in uncontaminated soil. All plants have defense mechanisms, such as the production of secondary metabolites, in response to excess metals, especially heavy metals, in their tissues. Berni et al. [41] concluded that this mechanism could explain the observed increase of secondary metabolite production in plants treated with high levels of copper during their cultivation. Furthermore, Anjitha and his partners found that exposure of plants to heavy metals promote the biosynthesis of pharmaceutically important compounds [42]. On the other hand, in the same research has been concluded that plants can mitigate heavy metal toxicity by modulating their secondary metabolism.
As a result, investigating the impact of soil heavy metal pollution levels on secondary metabolite yield by plants grown in them is important. Additional experiments with simultaneous metals are required in the future, in order to achieve the highest possible production of cannabidiol contributing to soil system remediation. However, scientists should consider the long-term effects of this copper accumulation pattern on soil health and also take this into account for cultivating hemp. The accumulation of copper in the soil over time and the potential effects of the accumulation on subsequent crops and soil ecosystems should be addressed [13]. Τhe sustainable use of medicinal plants for continuous phytoremediation is a long-standing question. Ιnvestigating the potential impact of accumulated metals on the quality, yield and safety of the CBD produced could provide valuable information for both environmental and pharmaceutical purposes.
Furthermore, enhanced CBD production in response to increasing soil copper levels is a particularly interesting outcome. Future studies could explore the hypothesis that CBD production may represent a genetic response to stress induced by its cultivation in toxic environments. This potentially could be accomplished by investigating biochemical indices in both medicinal and energy or industrial plants.
Conclusions
Hemp was revealed to be a tolerant plant, growing successfully in soils with varied particle size distributions supplemented with two levels of Cu concentrations. Cu proved to have a favorable effect on the plants at both contamination levels, as evidenced by the plants’ growth characteristics, aboveground weight, height, and photosynthetic capability.
In CL (clay loam) soil copper availability was lower, compare to the SCL (sandy clay loam) soil, probably due to higher clay and organic matter content and alkaline soil reaction. The maximum Cu content was observed to accumulate in the upper part of the roots, and gradually decreased to the higher parts of the plant (CuRoot > CuShoot > CuLeaf). The trace element accumulation and transport indices also supported the tendency of copper to concentrate in the roots with a moderate increase towards the lower part of the shoot. This would make hemp an ideal candidate for phytoremediation, by means of phytostabilization, in Cu-contaminated soils, allowing further utilization of its uncontaminated aboveground biomass.
Finally, higher Cu treatments showed higher CBD concentrations, potentially as a defense mechanism against the increased metal concentration. As a result, in Cu-polluted soils, industrial hemp is a suitable plant for cultivation since it is resistant, has a short life cycle, and provides an enormous biomass. It may be seen of as a Cu-accumulator plant, capable for remediation purposes, generating metal-free stems appropriate for fiber industrial use, and producing increased CBD quantities, even if cultivated in heavily Cu-contaminated soils.
Data Availability
Data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alloway, B.J.: Sources of heavy metals and metalloids in Soils. In: Alloway, B. (ed.) Heavy Metals in Soils, Environmental Pollution, vol. 22, pp. 11–50. Springer, Dordrecht (2013). https://doi.org/10.1007/978-94-007-4470-7_2
Kabata-Pendias, Α: Trace elements. In: Soils and Plants, 4th edn. CRC Press, Boca Raton (2010)
Napoletano, P., Guezgouz, N., Di Iorio, E., Colombo, C., Guerriero, G., De Marco, A.: Anthropic impact on soil heavy metal contamination in riparian ecosystems of Northern Algeria. Chemosphere (2023). https://doi.org/10.1016/j.chemosphere.2022.137522
Golia, E.E., Bethanis, J., Ntinopoulos, N., Kaffe, G.G., Komnou, A.A., Vasilou, C.: Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Pharm. (2023). https://doi.org/10.1016/j.scp.2022.100961
Papadimou, S.G., Kantzou, O.D., Chartodiplomenou, M.A., Golia, E.E.: Urban soil pollution by heavy metals: effect of the lockdown during the period of COVID-19 on pollutant levels over a five-year study. Soil. Syst. (2023). https://doi.org/10.3390/soilsystems7010028
Shifaw, E.: Review of heavy metals pollution in China in agricultural and urban soils. J. Health Pollut. (2018). https://doi.org/10.5696/2156-9614-8.18.180607
Mao, X., Sun, J., Shaghaleh, H., Jiang, X., Yu, H., Zhai, S., Hamoud, Y.A.: Environmental assessment of soils and crops based on heavy metal risk analysis in Southeastern China. Agronomy (2023). https://doi.org/10.3390/agronomy13041107
Zwolak, A., Sarzyńska, M., Szpyrka, E., Stawarczyk, K.: Sources of soil pollution by heavy metals and their accumulation in vegetables: a review. Water Air Soil Pollut. (2019). https://doi.org/10.1007/s11270-019-4221-y
Golia, E.E., Diakoloukas, V.: Soil parameters affecting the levels of potentially harmful metals in Thessaly area, Greece: a robust quadratic regression approach of soil pollution prediction. Environ. Sci. Pollut. Res. (2022). https://doi.org/10.1007/s11356-021-14673-0
Kormoker, T., Kabir, M., Khan, R., Islam, S., Shammi, R.S., Abdullah, A.M., Proshad, R., Tamim, U., Sarker, E., Taj, T.I., Akter, A., Idris, A.M.: Road dust–driven elemental distribution in megacity Dhaka, Bangladesh: environmental, ecological, and human health risks assessment. Environ. Sci. Pollut. Res. (2022). https://doi.org/10.1007/s11356-021-17369-7
Soleimani, H., Mansouri, B., Kiani, A., Omer, A.K., Tazik, M., Ebrahimzadeh, G., Sharafi, K.: Ecological risk assessment and heavy metals accumulation in agriculture soils irrigated with treated wastewater effluent, river water, and well water combined with chemical fertilizers. Heliyon (2023). https://doi.org/10.1016/j.heliyon.2023.e14580
Golia, E.E., Angelaki, A., Giannoulis, K.D., Skoufogianni, E., Bartzialis, D., Cavalaris, C., Vleioras, S.: Evaluation of soil properties, irrigation and solid waste application levels on Cu and Zn uptake by industrial hemp. Agron. Res. (2021). https://doi.org/10.15159/AR.21.016
Golia, E.E.: The impact of heavy metal contamination on soil quality and plant nutrition. Sustainable management of moderate contaminated agricultural and urban soils, using low cost materials and promoting circular economy. Sustain. Chem. Pharm. (2023). https://doi.org/10.1016/j.scp.2023.101046
Guidi Nissim, W., Palm, E., Mancuso, S., Azzarello, E.: Trace element phytoextraction from contaminated soil: a case study under mediterranean climate. Environ. Sci. Pollut. Res. (2018). https://doi.org/10.1007/s11356-018-1197-x
Laghlimi, M., Baghdad, B., Hadi, H., Bouabdli, A.: Phytoremediation mechanisms of heavy metal contaminated soils: a review. Open. J. Ecol. (2015). https://doi.org/10.4236/oje.2015.58031
Sharma, J.K., Kumar, N., Singh, N.P., Santal, A.R.: Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: an approach for a sustainable environment. Front. Plant. Sci. (2023). https://doi.org/10.3389/fpls.2023.1076876
Kafle, A., Timilsina, A., Gautam, A., Adhikari, K., Bhattarai, A., Aryal, N.: Phytoremediation: mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. (2022). https://doi.org/10.1016/j.envadv.2022.100203
Sheoran, V., Sheoran, A.S., Poonia, P.: Factors affecting phytoextraction: a review. Pedosphere (2016). https://doi.org/10.1016/S1002-0160(15)60032-7
Rehman, Z., Junaid, M.F., Ijaz, N., Khalid, U., Ijaz, Z.: Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci. Total Environ. (2023). https://doi.org/10.1016/j.scitotenv.2023.161468
Tonelli, F.C.P., Tonelli, F.M.P., Lemos, M.S., de Nunes, M.: Mechanisms of phytoremediation. In: Bhat, R.A., Tonelli, F.M.P., Dar, G.H., Hakeem, K. (eds.) Mechanisms of phytoremediation, pp. 37–64. Academic Press, NY (2022)
Angelini, L.G., Tavarini, S., Cestone, B., Beni, C.: Variation in mineral composition in three different plant organs of five fibre hemp (Cannabis sativa L.) cultivars. Agrochimica 58(1), 1–18 (2014)
Wuana, R.A., Okieimen, F.E.: Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. (2011). https://doi.org/10.5402/2011/402647
Rehman, M., Liu, L., Wang, Q., Saleem, M.H., Bashir, S., Ullah, S., Peng, D.: Copper environmental toxicology, recent advances, and future outlook: a review. Environ. Sci. Pollut. Res. (2019). https://doi.org/10.1007/s11356-019-05073-6
Morales, P., Reggio, P.H.: CBD: a new hope? ACS Med. Chem. Lett. (2019). https://doi.org/10.1021/acsmedchemlett.9b00127
Wu, Y., Trejo, H.X., Chen, G., Li, S.: Phytoremediation of contaminants of emerging concern from soil with industrial hemp (Cannabis sativa L.): a review. Environ. Dev. Sustain. (2021). https://doi.org/10.1007/s10668-021-01289-0
Dimirkou, A., Ioannou, Z., Golia, E.E., Danalatos, N., Mitsios, I.K.: Sorption of cadmium and arsenic by goethite and clinoptilolite. Commun. Soil Sci. Plant Anal. (2009). https://doi.org/10.1080/00103620802623539
Habibollahi, M.H., Sharafi, K., Omer, A.K.: Analysis of minerals and toxic elements in commonly consumed herbal medicinedin Zahedan, Iran, and associated human health risk assessment. J. Food Prot. (2022). https://doi.org/10.4315/JFP-22-173
Islam, M.D., Hasan, M.M., Rahaman, A., Haque, P., Islam, M.S., Rahman, M.M.: Translocation and bioaccumulation of trace metals from industrial effluent to locally grown vegetables and assessment of human health risk in Bangladesh. SN Appl. Sci. (2020). https://doi.org/10.1007/s42452-020-3123-3
Vega, F.A., Covelo, E.F., Andrade, M.L., Marcet, P.: Relationships between heavy metals content and soil properties in minesoils. Anal. Chim. Acta (2004). https://doi.org/10.1016/j.aca.2004.06.073
Osman, K.T.: Management of Soil Problems. Springer, Chittagong (2018)
Koukoulakis, P., Kalavrouziotis, I., Kokkinos, P.: The Geochemical Behavior of Heavy Metals in the Soil. Tziola Publications, Thessaloniki (2018)
Angelova, V., Ivanova, R., Delibaltova, V., Ivanov, K.: Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind. Crops Prod. (2004). https://doi.org/10.1016/j.indcrop.2003.10.001
Ćaćić, M., Perčin, A., Zgorelec, Ž, Kisić, I.: Evaluation of heavy metals accumulation potential of hemp (Cannabis sativa L). J. Cent. Eur. Agric. (2019). https://doi.org/10.5513/JCEA01/20.2.2201
Quagliata, G., Celletti, S., Coppa, E., Mimmo, T., Cesco, S., Astolfi, S.: Potential use of copper-contaminated soils for hemp (Cannabis sativa L.) cultivation. Environments (2021). https://doi.org/10.3390/environments8110111
Ahmad, R., Tehsin, Z., Malik, S.T., Asad, S.A., Shahzad, M., Bilal, M., Shah, M.M., Khan, S.A.: Phytoremediation potential of hemp (Cannabis sativa L.): Identification and characterization of heavy metals responsive genes. Clean (Weinh) (2016). https://doi.org/10.1002/clen.201500117
Alexakis, D.E., Bathrellos, G.D., Skilodimou, H.D., Gamvroula, D.E.: Land suitability mapping using geochemical and spatial analysis methods. Appl. Sci. (2021). https://doi.org/10.3390/app11125404
Skilodimou, H.D., Bathrellos, G.D.: Natural and technological hazards in urban areas: assessment, planning and solutions. Sustainability (2021). https://doi.org/10.3390/su13158301
Papadimou, S.G., Barbayiannis, Ν, Golia, E.E.: Preliminary investigation of the use of Silybum marianum (L.) Gaertn. As a cd accumulator in contaminated mediterranean soils: the relationships among cadmium (cd) soil fractions and plant cd content. Euro-Mediterranean J. Environ. Integr. (2023). https://doi.org/10.1007/s41207-023-00430-x
Petrova, S., Benešová, D., Soudek, P., Vanĕk, T.: Enhancement of metal(loid)s phytoextraction by Cannabis sativa L. J. Food Agric. Environ. 10, 631–641 (2012)
Husain, R., Weeden, H., Bogush, D., Deguchi, M., Soliman, M., Potlakayala, S., Katam, R., Goldman, S., Rudrabhatla, S.: Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS ONE (2019). https://doi.org/10.1371/journal.pone.0221570
Berni, R., Luyckx, M., Xu, X., Legay, S., Sergeant, K., Hausman, J.F., Lutts, S., Cai, G., Guerriero, G.: Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. (2019). https://doi.org/10.1016/j.envexpbot.2018.10.017
Anjitha, K.S., Sameena, P.P., Puthur, J.T.: Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress (2021). https://doi.org/10.1016/j.stress.2021.100038
Acknowledgements
The authors would like to express their gratitude to Assistant Professor K. Giannoulis, Dr. D. Bartzialis, and Dr. A. Angelaki for their contribution to the experiment at the University of Thessaly Farm.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Conceptualization, EEG and NGT; methodology, EEG and NGT; software, CV and EEG; validation, EEG, CV and NGT formal analysis, EEG, CV; investigation, EEG, CV resources, EEG and NGT; data curation, EEG and NGT; writing-original draft preparation, EEG, CV and NGT; writing—review and editing, EEG, CV and NGT; visualization, EEG, CV and NGT; supervision, EEG and NGT.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Consent for Publication
All the authors approved the final manuscript and agreed to its submission to the Journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasilou, C., Tsiropoulos, N.G. & Golia, E.E. Phytoremediation & Valorization of Cu-contaminated Soils Through Cannabis sativa (L.) Cultivation: A Smart Way to Produce Cannabidiol (CBD) in Mediterranean Soils. Waste Biomass Valor 15, 1711–1724 (2024). https://doi.org/10.1007/s12649-023-02388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02388-x