Abstract
The addition of metal oxide nanoparticles (NPs) to microalgae cultures have become of great interest since they can directly and/or indirectly interact with microalgae resulting in enhanced production of biomass and high-value added products. The physicochemical characteristics of the NPs, i.e., crystal phase, can differently influence microalgae metabolism, thus the synthesis of optimal NPs is mandatory to improve the value of microalgae biomass.This study aimed at assessing the effect of Fe2O3 NPs on Chlorella spp. cultures with and without carbonate supplementation to confirm their beneficial effect. First, Fe2O3 NPs were synthetized and two different annealing temperatures were implemented, 450 °C (Fe2O3-450) and 1000 °C (Fe2O3-1000), to obtain Fe2O3 NPs with different crystal phases. The NPs crystal phase was analyzed by X-ray diffraction and the presence of the γ- and α- phases on both NPs were confirmed. However, the Fe2O3-450 NPs presented a higher content of the γ- phase. Subsequently, the effect of the crystal phase of Fe2O3 NPs was assessed in Chlorella spp. cultures. The addition of Fe2O3-1000 NPs resulted in inhibition of Chlorella spp. On the other hand, the addition of Fe2O3-450 NPs recorded the carbohydrate enhancements > 91% in Chlorella spp. cultures. The indirect effect of the synthetized NPs was assessed in microalgae cultures without carbonate supplementation and the same tendency was observed. This study proves that Fe2O3-450 NPs directly and indirectly interact with microalgae, and can be used as a strategy to produce high-value biomass even in environments without carbonate supplementation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are considered as a promising source of biomass able to accumulate large amounts of high value-added products such as antioxidants, pigments, lipids and/or carbohydrates that can be transformed into biofuels such as biodiesel and bioethanol [1,2,3]. Microalgae production for energetic proposes has become a major study scenario and many efforts have been made to improve biomass productivity coupled to production of high-value products [4,5,6]. The most used methods to increase lipid content in microalgae culture is inducing a stress environment during microalgae growth, mostly by nutrient starvations, light stress, temperature stress and CO2 stress, however a stressful environment typically reduces growth rate [7]. Recently, the addition of nanoparticles (NPs) to microalgae cultures has demonstrated to improve biomass production in species such as Platymonas subcordiforus [8], Chlorella sp. [9], and Chlamydomonas reinhardtii [10, 11] which resulted in biomass enhancements when Co, Al2O3, CuO and Cr2O3 NPs were added, respectively.
The addition of metallic oxide NPs to microalgae cultures have gained popularity in the past years, and even if their use is a controversial topic or little is known of their mechanism of interaction with microalgae, it is believed that they can act as direct and indirect stimulants of microalgae cultures. For instance, they can directly stimulate the Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) enzyme; or can act as nutrient supplements, ROS (reactive oxygen species) generators, electron transports [12,13,14,15], which can result in an improved microalgae growth and production of high-value products. On the other hand, other authors have stated that NPs can indirectly improve the metabolism of microalgae by enhancing the CO2 transfer to the culture media since metallic oxides are known for reacting with CO2 and water to form carbonates [12, 16, 17]. For instance, Jeon et al. [18] demonstrated that the addition of SiO2 NPs to Chlorella vulgaris culture improved the mass transfer of CO2 to the cells, resulting in an improved kLa and lipid production. Moreover, the use of nanoporous materials, such as polymeric nanofibers, has been implemented to improve the contact time between microalgae and CO2, which can serve as a technique to improve the mass transfer of CO2 to the cells [12, 19]. Furthermore, it has been demonstrated that the addition of metal oxide NPs to polymeric nanofibers can create nanocomposites that increase the CO2 adsorption efficiencies [12, 19, 20], resulting in an improved biomass production. Thereby, the addition of NPs can be considered as a promising option to increase the concomitant production of microalgal biomass and high value-added metabolites by direct and indirect mechanisms of interaction.
Recently, Fe2O3 NPs have gained popularity since they are thermodynamically stable and are non-toxic for some species, moreover, it is believed that Fe ions are dispersed in the culture media, and can serve as nutrient supplements for microalgae [21]. Recently, Rana et al. [14] demonstrated the potential of Fe2O3 NPs on lipid production in Chlorella pyrenoidosa. However, it is known that the tolerance of NPs on microalgae is species specific [8, 14] and typically high NP concentration (> 50 mg L−1) inhibits microalgae growth [10, 22]. Interestingly, other characteristics of the NPs, such as size, crystal phase, oxidation stage, etc., influence differently microalgae growth and metabolite production. For instance, Vargas-Estrada et al. [23] recently reported that the physicochemical characteristics of carbon coated zero-valent iron NPs influenced differently the metabolism of Chlorella sorokiniana cultures. On the other hand, Lei et al. [24] studied the effect of particle size, crystal phase, oxidation state and environmental aging of different iron NPs and observed that the crystal phase of Fe2O3 NPs had a different effect on C. pyrenoidosa. According to these authors, the α- phase of Fe2O3 NPs presented a higher toxicity on C. pyrenoidosa than the γ- phase. Interestingly, Kayani et al. [25], synthetized Fe2O3 NPs and observed that the annealing temperature (400 and 1000 °C) influenced the crystal phase of the NPs. Indeed, even if the α- phase was present at both annealing temperatures, the γ- phase was present at higher concentrations when the annealing temperature was 400 °C. In this context, the proper Fe2O3 NPs synthesis conditions can lead to optimal NPs with the right physicochemical characteristics i.e., crystal phase, that can significantly enhance the value of microalgae biomass.
Thereby, the aim of this study was to synthesize the optimal Fe2O3 NPs to improve microalgae biomass production coupled to high-value products. Additionally, the indirect effect of the synthetized NPs was assessed in microalgae cultures without carbonate supplementation to confirm the beneficial effects of the synthetized Fe2O3 NPs.
Experimental Methods
Nanoparticles
Fe2O3 Nanoparticle Synthesis
Fe2O3 NPs were prepared by sol–gel method combined with hydrothermal treatment under controlled conditions. Ferric Nitrate (Fluka), ethylene glycol (sigma Aldrich) and nitric acid (Fermont) were used without any purification treatment. Fe2O3 colloidal solution was prepared by mixing ethylene glycol (50 mL) with Ferric Nitrate (3 g) in a flat bottom flask under constant agitation for 90 min. For hydrolysis, a solution of 0.1 M of citric acid in 50 mL of deionized water was mixed with 1 mL of nitric acid (used as a catalyst in the process of hydrolysis), which was added to the flask dropwise and left under constant stirring for 24 h at 50 °C. After the aging period of 24 h, the solution was placed in a glass beaker and kept in vigorous stirring for 5 h at 70 °C to make stable sol. The sol was then heated at 85 °C under maintained magnetic stirring until the water content was evaporated and a brown semi-solid gel was formed. This was followed by aging for 72 h for the condensation and polymerization reactions. The formed gel was then dried by putting it in an oven at 100 °C for 2 h. The dried gel was then annealed at temperatures of 450 and 1000 °C for 2 h to obtain the corresponding NPs, and will be referred as Fe2O3-450 and Fe2O3-1000, respectively.
Nanoparticle Characterization
To determine the structural features, surface images, elemental composition, phase formation and crystalline composition of Fe2O3 NPs dry powder samples were examined. TEM analyses were obtained using a JEOL JEM-1400 plus at 100 kV according to [26]. The surface images of the synthetized Fe2O3 NPs were obtained using scanning electron microscopy (SEM) (Hitachi FE-SEM S-5500) at different magnifications, and the elemental composition of the NPs was obtained with an energy-dispersive X-ray spectrometer Bruker Quantax 200 for EDS elemental analysis integrated to the FE-SEM S-5500 according to [27]. Finally, the phase formation and crystalline structure of the synthesized Fe2O3 NPs were determined by X-ray diffraction (XRD, Rigaku, DMAX 2200). The spectrum was obtained at the 2θ range of 10° to 70° with a goniometer scanning rate of 10° min−1 according to [27].
Nanoparticle Stock Solution
Fresh stock solutions with concentrations of 200 mg L−1 of each Fe2O3 NPs were prepared with sterilized microalgae culture media to facilitate their aggregation to microalgae cultures. To prevent changes in the physicochemical composition of the NPs by sterilizing conditions (121 °C, 15 min), the culture media, spatulas, volumetric flasks and the watch glasses were sterilized before preparing the stock solutions and weighting the NPs. Afterwards, the stock solutions were prepared under a sterile environment in a laminar flow hood. Before the solution was aggregated, the stock solutions were sonicated for 15 min to avoid NPs agglomeration.
Microalgae Strain and Culture
In this study, two different microalgae strains were used in order to elucidate the viability of the Fe2O3 NPs. The strain Chlorella vulgaris was selected for its robustness and was kindly donated by the UNICACH, Chiapas, Mexico, and a consortium of microalgae and nitrifying bacteria mainly dominated by Chlorella sp., which was donated by the Metropolitan Autonomous University (UAM), Mexico City, Mexico, which was isolated from the water channels of Xochimilco, Mexico. In this particular study, no change in the composition of the consortium was observed, and the dominant species was Chlorella sp. thus, it will be referred as Chlorella sp. Both C. vulgaris and Chlorella sp. were kept in an incubator (Thermo Scientific) at 19 °C with light: dark cycles of 15:9 h respectively in Erlenmeyer flasks covered with a cotton plug. C. vulgaris was cultivated in BG-11 and Chlorella sp. was cultivated in Bayfolan forte (Bayer CropScience) at a concentration of 1 mL L−1.
Experimental Set-up
Batch cultures were carried out in Erlenmeyer flasks of 500 mL. The working volume was kept constant (500 mL) by adding sterile deionized water before taking samples to cover losses from evaporation. Each Erlenmeyer flask was inoculated with microalgae culture at a 10% (v v−1) ratio.
Chlorella vulgaris was cultivated in BG-11 and Chlorella sp. was cultivated in Bayfolan forte (Bayer CropScience), at a concentration of 1 mL L−1, according to the following experimental design. First, to determine the optimal Fe2O3 NPs for Chlorella spp. cultures, the Fe2O3 NPs were added to reach a final concentration of 10 mg L−1 in the Erlenmeyer flasks. Three different conditions were assessed: (1) control (Chlorella spp. without NPs); (2) 10 mg L−1 Fe2O3-450; and (3) 10 mg L−1 Fe2O3-1000. After obtaining the optimal Fe2O3 NPs for microalgae cultivation, the selected Fe2O3 NPs were added at different concentrations (5 and 20 mg L−1) to determine the optimal Fe2O3 concentration for microalgae culture (Fig. 1). Each condition was run in duplicates. The flasks were incubated under cool-light with an intensity of 800 lm in light: dark cycles of 12:12 h respectively and air was pumped to avoid culture sedimentation.
Analytical Determinations
Biomass Concentration and Chlorophyll a
Microalgae biomass concentration was measured by cell density which was determined by optical microscopy with an improved Neubauer chamber. Dry weight was measured at the beginning and at the end of the exponential phase by filtering 10 mL of the sample through 47 mm glass fiber filters (Whatman. GE Healthcare Life Sciences) and drying them all night at 105 °C, according to standard methods [28]. Chlorophyll a content was determined by spectrophotometry, briefly 1 mL of sample was centrifuged at 10,000 rpm for 5 min, after that the supernatant was discarded and 1 mL of a solution of methanol: water 90:10 (v v−1) was added. Then, the sample was heated in a water bath at 75 °C for 20 min, and then centrifuged at 10,000 rpm for 5 min, the supernatant was carefully removed and the optical density was measured at a wavelength of 663 nm and 645 nm. The chlorophyll a content was calculated according to Eq. (1):
Growth Parameters and CO2 Bio-fixation Rate
The biomass productivity (Px, mg L−1 d−1) was calculated according to equation Eq. (2):
where Xt is the biomass concentration (mg L−1) at time t (d) and X0 is the concentration of biomass at time t0 (d).
The specific growth rate ( \(\mu\), d−1) was calculated according to Eq. (3):
The CO2 bio-fixation rate (R, mg L−1 d−1) was calculated according to Eq. (4):
where MCO2 is the molar mass of CO2 (g mol−1) and MC the molar mass of carbon (g mol−1), and Xcbm is the carbon fraction in the microalgal biomass (50%, w w−1) [19].
Alkalinity and Dissolved Inorganic Carbon (DIC)
Alkalinity was determined by acid titration at the beginning and at the end of the experiment. pH was measured using a digital pH meter (HANNA instruments) and the DIC concentration was calculated according to [29].
Biomass Composition
To determine the carbohydrate and lipid content in the microalgae biomass at the end of the experiment the microalgae biomass was centrifuged at 3500 rpm for 15 min, frozen at − 30 °C (Equitec) and finally freeze-dried (− 110 °C, 0.049 hPa) (Labconco, USA). The carbohydrate content was determined by the phenol-sulfuric acid method [30] using d-glucose as the standard and lipids were determined according to [31].
Microalgae Sample Preparation for TEM Analysis
Microalgae were collected in Eppendorf tubes and fixed for 24 h at 40 °C with 3% glutaraldehyde in 0.1 M cacodylate buffer, washed overnight in 0.2 M cacodylate buffer and postfixed with 0.1 M OsO4 in 0.1 M cacodylate buffer for 1 h at 40 °C. Several washes equal to the previous ones were carried out. Pellets were obtained and transferred to smaller tubes to dehydrate in increasing concentrations of ethanol (30%, 50%, 70%), making 2 changes of 30 min at each concentration, until reaching 96% and absolute ethanol. In the second change of absolute ethanol, the pellets were removed from the tubes and transferred to vials. This was followed by a change of solvent with propylene oxide (2 times for 30 min). For infiltration, the pellets were left in a 1:1 mixture of epoxy resin: propylene oxide overnight at room temperature. The propylene oxide was evaporated to concentrate the resin. Several changes of resin were made under agitation until the complete extraction of the oxide. The pellets were oriented in molds with complete resin and polymerized in an oven at 60 °C for 36 h. The blocks obtained were cut in an ultramicrotome and sections with a thickness of 60 to 90 nm were obtained and collected on 300 mesh copper grids. The grids with the sections were counterstained with 2% uranyl acetate for 20 min and with 2% lead citrate for 15 min. Finally, the samples were observed in the JEOL Model JEM-1010 transmission electron microscope operated at 80Kv. The images were obtained with an ORIUS model GATAN camera that is coupled to the microscope and with the Digital Micrograph program.
Statistical Analysis
To determine the effect of the NPs on microalgae growth and biomass composition, the results were analyzed by ANOVA followed by Tuckey’s test considering α = 0.05.
Results and Discussion
Characterization of the Fe2O3 Nanoparticles
The structural features of Fe2O3 NPs have been examined by SEM analyses and TEM analyses. Both results obtained by TEM and SEM show the good aggregation of spherical/rhombic structures between 30 and 50 nm diameter. Polycrystalline nature of nanoparticles with hexagonal plates and spherical shaped particles are visualized in Fig. 2. The peaks in EDX spectrum confirm the presence of Iron and Oxygen, their atomic percentages are shown in Table 1.
The phase formation and crystalline structure of synthesized Fe2O3 NPs was determined by X-ray diffraction recorded by scanning 2θ in the range of 20–70°, with grazing incidence angle of 0.5° recorded at room temperature. Figure 3 shows the powder X-ray diffraction pattern of α-Fe2O3 nanoparticles.
All diffraction peaks are in agreement with Joint Committee on Powder Diffraction Standards (JCPDS) X-ray data file. At 450 °C the major XRD peak is observed at 2θ = 33.08° while the second major peak is recorded at 2θ = 35.54°. Other peaks are found at 2θ = 53.97° 2θ = 57.55° and 62.42°. The planes for corresponding angles are (104), (311), (116), (110) and (214), respectively. When annealing temperature was raised to 1000 °C the XRD peaks were registered at 2θ = 33.13°, 35.60°, 49.42°, 54.04°, 62.39°, and 63.95. At this temperature the corresponding planes are (104), (311), (024), (116), (214), and (300) [32]. The spectra of nanoparticles at both annealing temperatures agree with the standard card corresponding to α phase of iron oxide (hematite). The peak at 35.54° refers to the presence of γ-Fe2O3 (maghemite) phase, while the other peaks refer to the α-Fe2O3 phase. Hence, hematite structure is achieved at annealing temperature of 450 °C, but main peaks related to this specific crystal phase are best defined when the annealing temperature is increased to 1000 °C. These results are in agreement with [25, 33], who observed similar peaks at similar angles. According to [25] when Fe2O3 nanoparticles are annealed at 400 °C the α- and γ- phase are present. On the other hand, when Fe2O3 are annealed at 1000 °C a phase change from γ- to α- takes place, and hematite is the dominant phase, which is in agreement with our results obtained by X-ray diffraction.
Effect of Fe2O3 Nanoparticles on Chlorella vulgaris
Biomass Growth and Chlorophyll Content
The crystal phase of the Fe2O3 NPs plays an important role on microalgae growth, as stated by Lei et al. [24], the α- phase presents higher toxicity than the γ- phase in Chlorella pyrenoidosa. In this way, the influence of Fe2O3 NPs herein annealed at different temperatures was assessed in C. vulgaris to find the optimum Fe2O3 NPs for microalgae growth and macromolecule accumulation, the results are shown in Fig. 4.
After 9 days of cultivation, the addition of 10 mg L−1 of Fe2O3-450 to C. vulgaris cultures did not influence microalgae growth compared to the control, while the addition of 10 mg L−1 of Fe2O3-1000 showed an inhibitory effect at day 9 (Fig. 4a). Indeed, the crystal composition of the Fe2O3-1000 NPs significantly influenced C. vulgaris growth, which is in agreement with the results previously reported by Lei et al. [24]. It is important to highlight that since a mixture of both crystal phases (α- and γ-) were present in the Fe2O3-450 NPs no significant difference in C. vulgaris growth, compared to the control, was observed. Similar findings have been also observed by Bibi et al. [22], who reported a toxic effect by the addition of α-Fe2O3 NPs to C. vulgaris cultures. TEM micrographs confirmed the toxicity of the Fe2O3 NPs to C. vulgaris cultures (Fig. 5). For one hand, the addition of Fe2O3-450 show NPs agglomeration and a few deformed cells (Fig. 5b), but no significant changes compared to the control (Fig. 5a). On the other hand, the addition of Fe2O3-1000 shows deformed cells, agglomeration of NPs and the adhesion of NPs to the algal cells (Fig. 5c), which is in agreement with the observations of Bibi et al. [22], who also reported deformed cells by the addition of α-Fe2O3 NPs to microalgae cultures. The deformation of microalgae cells and cell lysis significantly affected microalgae growth in the present study. It is important to highlight that the deformation and lysis of microalgae cells has been previously attributed to the formation of ROS [21], in this way, this confirms the fact that the α- phase of the Fe2O3 NPs present significant toxicity on microalgae cultures. Additionally, the concomitant NPs agglomeration and NP adhesion to algal cells has been considered as toxic effects since inhibition for lack of light availability or nutrient limitation is carried out [21, 34, 35]. In this context, the inhibition herein observed in C. vulgaris culture by the addition of α-Fe2O3 NPs was attributed to the formation of ROS and lack of availability due to the formation of NPs agglomerates and the adhesion of NPs to C. vulgaris cells. Interestingly, C. vulgaris cultivated with Fe2O3-1000 presented a higher biomass productivity (Px), higher biofixation rate (R), but no significant difference was observed between the assays for the specific growth rate (µ) (Table 2). Even if the Px and R values observed herein obtained are lower than the values reported by Vaz et al. [20], which are 115 mg L−1 and 216 mg L−1 d−1 respectively, it is important to addressed that those authors added nanofibers which are widely used to improve CO2 adsorption. On the other hand, Bibi et al. [22] did not observe significant changes in microalgae growth when α-Fe2O3 NPs were added at low concentrations (< 50 mg L−1), which suggests that other NPs physicochemical characteristics significantly influence microalgae growth, and this is in agreement with the previously reported by Vargas-Estrada et al. [36].
On the other hand, the DICfinal of the assays containing Fe2O3-1000 NPs was higher than the DICfinal of the control. As it has been stated elsewhere [16], the presence of metallic oxides enables the CO2 removal as they react with CO2 and water to form carbonates, thus the obtained results suggest that the NPs could have induced the dissolution of CO2 into the culture broth in the form of carbonates, resulting in enhanced kinetic parameters. Interestingly, the R values recorded herein, more specifically by the addition of 10 mg L−1 of Fe2O3-1000 NPs, confirm the fact that this phenomenon occurred. In this way, Fe2O3-1000 NPs, significantly enhanced C. vulgaris growth, which was mediated by a higher CO2 uptake however, the long exposure of C. vulgaris to these particular NPs (dominating by the α- phase) resulted in toxic effect leading to a cell density loss of up to 57% on day 9. Vargas-Estrada et al. [23] reported similar findings using Fe2O3 NPs and observed that the presence of Fe2O3 NPs enhanced the CO2 concentration in closed systems of Chlorella sorokiniana cultures.
Moreover, chlorophyll content is used to indicate the photosynthesis rate and/or the cell division of microalgae [37] and in this particular study, the chlorophyll content in C. vulgaris decreased when Fe2O3-1000 were added while the chlorophyll content in C. vulgaris with Fe2O3-450 did not present a significant change (Fig. 4c). It has been previously stated that a loss in chlorophyll a content may be due to inhibition in the electron transport chain specially in the donor center, whereas an increase of chlorophyll a content should be due to the inhibition in the acceptor side [34]. Thus, the reduction of chlorophyll herein indicates that C. vulgaris quantum yield of PSII was interfered by the presence of Fe2O3-1000, confirming the toxic effect of Fe2O3-1000 to C. vulgaris cultures, mainly attributed to the dominating α- phase.
Time course of the cell density (a) and (b); and chlorophyll content (c) and (d) of Chlorella vulgaris cultivated in BG-11 medium under different conditions assessed: control (green circles), 10 mg L−1 Fe2O3-450 (blue triangles), 10 mg L−1 Fe2O3-100 (yellow cruces); 5 mg L−1 Fe2O3-450 (red squares); 20 mg L−1 Fe2O3-450 (navy blue diamonds)
Indeed Fe2O3-450 NPs were selected as the optimal NPs for C. vulgaris growth and subsequently different concentrations (5 and 20 mg L−1) were assessed. After 9 days of cultivation, the addition of 5 mg L−1 Fe2O3-450 did not show a significant difference in cell density, however the exponential phase was prolonged (Fig. 4b). Moreover, the chlorophyll content was significantly lower by the addition of the NPs, suggesting that this concentration influences the pigment synthesis (Fig. 4d). On the other hand, the addition of 20 mg L−1 resulted in lower cell density and chlorophyll content, suggesting that this particular dose can inhibit C. vulgaris growth, which is in accordance with the previously reported by [22]. Furthermore, there was no statistical difference in the Px (Table 3) between the assays, however assays containing 20 mg L−1 of Fe2O3-450 recorded the lowest Px values. Additionally, the µ and the R values decreased as the NPs concentration increased, suggesting that the concentration of NPs interfered in the growth of C. vulgaris, contrary results have been previously observed by Rana et al. [14], who observed that 20 mg L−1 of Fe2O3 NPs enhanced the biomass productivity of C. pyrenoidosa, confirming that NPs tolerance is species specific.
Biochemical Composition
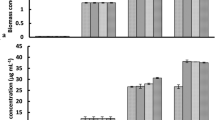
The addition of Fe2O3 NPs stimulated carbohydrate content of C. vulgaris according to the type of NPs added (Fig. 6). The carbohydrate content of C. vulgaris increased from 18.67 to 41.27% and 44.83% (% dw) when 10 mg L−1 of Fe2O3-450 and Fe2O3-1000 respectively were added. Interestingly, the carbohydrate content did not present a statistical difference compared to the control (18.67% dw) when 5 mg L−1 were added, whereas the carbohydrate content significantly increased up to 53.09% (% dw) when 20 mg L−1 of Fe2O3-450 were added. Thus, the carbohydrate content in C. vulgaris increased with the increasing concentrations of Fe2O3-450. Indeed, results suggest that concentrations ≥ 10 mg L−1 stimulate carbohydrate accumulation in C. vulgaris. Similar findings have been recently reported by Vargas-Estrada et al. [23], who reported increasing carbohydrate content in C. sorokiniana with increasing concentrations of Fe2O3 NPs, additionally, the authors reported a maximum enhancement up to 47% (compared to the control) when 70 mg L−1 of Fe2O3 NPs were added.
On the other hand, the lipid content of C. vulgaris increased from 11.25% (control) to 14.8% and 21.5% when 10 mg L−1 of Fe2O3-450 and Fe2O3-1000 respectively were added. The higher lipid content in C. vulgaris induced by the addition of Fe2O3-1000 NPs confirms the fact that these particular NPs led to the formation of ROS, and C. vulgaris accumulated higher quantity of lipids as a protection response [23]. Moreover, the addition of 5 mg L−1 and 20 mg L−1 of Fe2O3-450 also entailed a statistically higher lipid content than the control, indeed the lipid content increased from 11.25% (control) to 16.9% and 17.6% for 5 mg L−1 and 20 mg L−1 respectively. Even if the lipid content in C. vulgaris did not present a systematic trend with increasing concentrations, the higher lipid content was recorded when 20 mg L−1 of Fe2O3-450 NPs were added. In this way, it can be said that the higher concentration of NPs stimulated the lipid content, likely due to the formation of ROS [14], since the interaction of light and oxygen in presence of moisture produces oxidizing radicals (OH−) and ROS, and higher concentrations of NPs led to higher concentration of generated ROS.
Indeed, even if the addition of Fe2O3-450 NPs resulted in a carbohydrate and lipid enhancement, the carbohydrate enhancement was significantly higher, suggesting that the NPs influenced C. vulgaris metabolism. Carbohydrate’s and lipid’s main component is carbon, hence providing a source of this element can increase their production [38]. Thus, high CO2 concentration improves the carboxylase activity of RuBisCO enzyme, which is known as the key enzyme for CO2 fixation, resulting in an improved photosynthetic activity [39]. Additionally, the RuBisCO enzyme can transform the CO2 into precursors of carbohydrates, which are accumulated as a reserve of energy for cell growth [12]. Thereby, from the results obtained herein, the addition of NPs improved C. vulgaris metabolism likely by the formation of carbonated species. However, at this point it is important to mention that the DICfinal did not present a significant change between the assays but the significantly higher carbohydrate content in C. vulgaris when 10 and 20 mg L−1 of Fe2O3-450 NPs were added, suggesting that these NPs could have enhanced the CO2 source to C. vulgaris. Vaz et al. [20] reported similar findings when nanofibers containing Fe2O3 NPs were added to Chlorella fusca cultures. Thus, the use of Fe2O3 NPs played a crucial role in C. vulgaris metabolism.
a Carbohydrate (black) and lipid (light grey) content of Chlorella vulgaris cultivated with 10 mg L−1 of the corresponding Fe2O3 NPs; b C. vulgaris cultivated with 5 and 20 mg L−1 of Fe2O3-450; c Chlorella sp. cultivated with 10 mg L−1 of the corresponding Fe2O3 NPs; d Chlorella sp. cultivated with 5 mg L−1 and 20 mg L−1 of Fe2O3-450
Effect of Fe2O3 Nanoparticles on Chlorella Sp.
Biomass Growth and Chlorophyll Content
After 9 days of cultivation, Chlorella sp. cultivated in Bayfolan Forte presented an increment in cell density from 1.46 E + 07 cells mL−1 to 2.27 E + 07 cells mL−1 when 10 mg L−1 of Fe2O3-450 were added. On the other hand, the addition of 10 mg L−1 of Fe2O3-1000 did not show a significant difference in cell density (1.61 E + 07 cell mL−1). Even if the cell density of Chlorella sp. cultivated with Fe2O3-1000 was lower compared to the Fe2O3-450, the growth was not statistically different compared to the control (Fig. 7a), suggesting that the addition of Fe2O3-1000 NPs did not negatively affect the cell density of Chlorella sp.
Time course of the cell density (a) and (b) and chlorophyll content of Chlorella vulgaris; (c) and (d) of Chlorella sp. cultivated in Bayfolan forte medium under different conditions assessed: control (green circles), 10 mg L−1 Fe2O3-450 (light blue triangles), 10 mg L−1 Fe2O3-100 (yellow cruces); 5 mg L−1 Fe2O3-450 (red squares); 20 mg L−1 Fe2O3-450 (navy blue diamonds)
Furthermore, the Px of Chlorella sp. significantly decreased by 65% when 10 mg L−1 of Fe2O3-1000 NPs was added to the culture, as well as the µ and the R values (Table 2). These results are in agreement with the previously observed ones with C. vulgaris culture, in this way, it can be said that Fe2O3-1000 NPs resulted in inhibition of both microalgae species. TEM micrographs show the interactions between Chlorella sp. and the two types of Fe2O3 NPs (Fig. 5). The addition of 10 mg L−1 of Fe2O3-450 show the agglomeration of NPs (Fig. 5e) but no significant changes in the morphology of the cells were observed compared to the control (Fig. 5d). On the other hand, the TEM micrographs confirmed cell deformation and cell lysis on Chlorella sp. exposed to Fe2O3-1000 NPs (Fig. 5f). As discussed above, the toxicity of Fe2O3-1000 NPs is attributed to the dominating α- phase, which lead to the formation of ROS and is in agreement with [24]. Similarly, Rana et al. [14] reported toxic effects of α- Fe2O3 NPs on Chlorella sorokiniana even at low doses (2 mg L−1), on the other hand, Bibi et al. [22] observed a toxic effect of α- Fe2O3 on C. vulgaris at doses > 50 mg L−1. In this way, even if the resistance to α- Fe2O3 exposure is species specific, it is important to highlight that the crystal phase of the NPs plays an important role and the α- phase has demonstrated a toxic effect on microalgae.
On the other hand, the addition of 10 mg L−1 of Fe2O3-450 NPs did not have a significant difference compared to the control. Moreover, the DICfinal did not present a significant difference between the assays, but the R value recorded when 10 mg L−1 of Fe2O3-450 NPs were added was higher, suggesting that the addition of the Fe2O3-450 NPs could have entailed the carbonate formation and uptake by Chlorella sp. Additionally, the assays containing 10 mg L−1 of Fe2O3-450 NPs increased their cell density, confirming the fact that the Fe2O3-450 NPs stimulated Chlorella sp. growth. At this point it is important to highlight that Chlorella sp. cultures were not supplemented with carbonate, thereby, the higher growth recorded by the addition of Fe2O3-450 NPs suggests that these NPs influenced the formation of carbonates that were rapidly consumed by Chlorella sp. [20, 40]. Therefore, Fe2O3-450 NPs were selected as the optimal NPs for Chlorella sp. culture, and different concentrations (5 and 20 mg L−1) were elucidated to find the optimum concentration to enhance Chlorella sp. growth. The addition of 5 mg L−1 Fe2O3-450 did not enhance biomass growth and the addition of 20 mg L−1 resulted in biomass loss on day 7 (Fig. 7b). Even if the addition of 20 mg L−1 Fe2O3-450 resulted in a cell density loss, the addition of Fe2O3-450 entailed higher R values regardless of the concentration added (Table 3). In this way, the addition of Fe2O3-450 NPs entailed the carbonate formation. Which is in agreement with the results obtained with C. vulgaris and with those reported by [12, 17] that NPs can improve the mass transfer of CO2 to the liquid phase. It is important to highlight that even if in the present study no significant amount of CO2 was sparged to the microalgae cultures, the CO2 contained in air can support a low microalgae growth. It is well known that CO2 dissolves in water according to Eq. (5) [29].
Indeed, CO2 and HCO3− are the preferred carbon sources of microalgae, and depending on the pH of the culture media, the concentration of dissolved CO2 can increase or decrease. When the HCO3− concentration increases, the carboxysome accumulation increases as well, resulting in an activated microalgae photosynthetic activity [21]. In this context, it can be said that, since the microalgae cell density was enhanced by the addition of Fe2O3-450 NPs as well as the R values in both microalgae species, the NPs influenced the CO2 transfer to microalgae. Nonetheless, it is important to highlight that to the best of the authors´ knowledge, this specific mechanism has not been well described, and further research is needed to explain this mechanism.
The chlorophyll content of Chlorella sp. suffered a decay from 4.28 mg L−1 to almost negligible (0.05 mg L−1) when 10 mg L−1 Fe2O3-1000 NPs were added, however, when Fe2O3-450 were added, Chlorella sp. presented a higher chlorophyll content (14.68 mg L−1) compared to the control (4.28 mg L−1) (Fig. 7c). Thus, both Fe2O3 NPs had an impact on Chlorella sp., but Fe2O3-1000 NPs confirmed their toxicity since they led to a chlorophyll loss in Chlorella sp. cultures. Indeed, these results confirmed the toxicity of the crystal phase of Fe2O3-1000 NPs which is in agreement with [24], likely due to the high oxidation activity of the NPs, which led to the formation of ROS that resulted in deformed microalgal cells and cell lysis.
On the other hand, the chlorophyll content in the control and the assays containing 20 mg L−1 of Fe2O3-450 NPs behaved similarly and suffered a decay on day 7 (Fig. 7d), this particular behavior could be explained by the fact that: (1) cell density loss as a result of the toxic effect caused by the addition of 20 mg L−1 Fe2O3-450 NPs; (2) in the control conditions microalgae reduced its light needs, which has been previously observed by Marchello et al. [41], and is in accordance with the observed herein, since microalgae cell density did not decay. On the other hand, the addition of 5 mg L−1 of Fe2O3-450 NPs resulted in a chlorophyll enhancement, suggesting that low doses of Fe2O3-450 NPs stimulated pigment synthesis in Chlorella sp. This behavior is in accordance with the results reported by Marchello et al. [41], where the addition of low concentrations of TiO2 NPs (7.9 × 10−4–7.9 × 10−2 mg L−1) resulted in increased chlorophyll a content in C. sorokiniana cultures.
Biochemical Composition
The addition of 10 mg L−1 of Fe2O3-1000 did not present a significant difference in the carbohydrate content of Chlorella sp. On the other hand, the addition of 10 mg L−1 of Fe2O3-450 NPs to Chlorella sp. culture resulted in a significant carbohydrate enhancement of 91%, increasing from 16.98 to 32.50% (% dw) (Fig. 6c). It has been previously stated that microalgae possess a carbon concentrating mechanism that prepares the cells to gather and accumulate inorganic carbon from surroundings and the concentration of CO2 in the culture broth affects the starch content [2, 39]. Hence, since the culture media was not carbonate supplemented and the only carbon source was provided by the sparged air, the results suggest that the addition of Fe2O3-450 NPs influenced the CO2 dissolution in the culture media, which significantly enhanced the carbohydrate metabolism of Chlorella sp. resulting in a high carbohydrate accumulation. At this point, it is important to highlight that the culture media of Chlorella sp. did not have a carbonate supplementation, indeed both cell density and carbohydrate accumulation were enhanced by the addition of 10 mg L−1 Fe2O3-450 NPs. This is in accordance with similar assays with C. vulgaris, where the carbohydrate content was increased by 121%. Interestingly, the carbonate supplementation influenced the carbohydrate content and higher contents were recorded with carbonate than without supplementation (41.27 and 32.65% dw respectively). This fact confirms that the higher carbonate availability for C. vulgaris cultures resulted in higher carbohydrate content, notwithstanding, the addition of 10 mg L−1 Fe2O3 NPs significantly stimulated carbohydrate content in Chlorella sp. likely mediated by a carbonate formation.
Moreover, the lipid content of Chlorella sp. did not present a significant change when 10 mg L−1 of Fe2O3-450 NPs were added to the culture. Nevertheless, the addition of 10 mg L−1 of Fe2O3-1000 resulted in a lipid decrement from 11.25 to 3.25% (dw), which is contrary to the results reported by Bibi et al. [22], who recorded lipid enhancements by 39.7 and 25.5 when 50 and 100 mg L−1 of α-Fe2O3 NPs were added to C. vulgaris cultures. This confirms the fact that NPs tolerance is species specific and some microalgae strains are more resistant than others. For instance, Rana et al. [14] reported that C. pyrenoidosa was more resistant to α-Fe2O3 NPs than C. sorokiniana. Moreover, the production of ROS has been stated as one toxicity pathway that causes cellular damages such as loss of membrane fluidity and the oxidation of unsaturated lipids [21, 42]. Indeed, lipid peroxidation has been identified when the ROS concentration increases. For instance, Li et al. [43] observed that cobalt ions (Co2+) induced lipid peroxidation in Pavlova viridis as a result of an increased ROS production. Moreover, it has been stated that Fe2O3 NPs led to the formation of ROS by Fenton reactions that cause damage to microalgae cells [13]. Therefore, the reduction in the lipid content recorded in Chlorella sp. cultures can be mainly attributed to cell-self-protection from ROS generated by the Fe2O3-1000 NPs. This fact confirms that the toxicity of Fe2O3-1000 NPs on microalgae is likely due to the formation of ROS, which significantly damage microalgae cells. This particular toxicity of the Fe2O3-1000 NPs is mainly attributed to their crystal phase, which presented a dominating α-phase compared to the Fe2O3-450 NPs.
Interestingly, the addition of 5 mg L−1 Fe2O3-450 resulted in a lipid enhancement from 18% (control) to 26.75% (% dw) whereas the addition of 20 mg L−1 Fe2O3-450 NPs resulted in a lipid decrement (15.7% dw) (Fig. 6d), which can be related to lipid oxidation resulting from an oxidative stress created by the formation of ROS mediated by the higher Fe2O3-450 NPs concentration. Indeed, from these results it can be said that Fe2O3-450 NPs created a stressful environment for Chlorella sp., however low doses (5 mg L−1) of Fe2O3-450 NPs were tolerable for Chlorella sp. and stimulated lipid production, while the addition of higher doses ( ≥ 10 mg L−1) did not have a significant change compared to the control. Even if the results recorded herein are contrary to the results reported by Bibi et al. [22], where lipid contents up to 39.7% were recorded, it is important to highlight that those high lipid contents were recorded after 30 days of culture time, whilst the time of cultivation herein was 9 days. Indeed, at short cultivation time it is possible to use Fe2O3 NPs as a technique to increase microalgae value, especially when carbonate is not supplemented. For instance, the addition of 20 mg L−1 of Fe2O3-450 NPs increased the carbohydrate content from 9.92 to 19.75% (% dw) while no significant enhancement was observed when 5 mg L−1 of Fe2O3-450 NPs were added. Nonetheless, 10 mg L−1 of Fe2O3-450 NPs was the optimal dose for carbohydrate production. Indeed, Fe2O3-450 NPs influenced Chlorella sp. metabolism, resulting in high carbohydrate content, which was mediated by a higher R value. Thereby, the recorded results suggest that Fe2O3-450 NPs induced CO2 transfer to culture media by the formation of carbonates that were taken by microalgae. However, more research is needed to confirm this theory. Nonetheless, it is important to highlight that Fe2O3-450 NPs at low doses can be used as a technique to support the production of high-value biomass in environments without carbonate supplementation.
Conclusions
The annealing temperature of the Fe2O3 nanoparticles influenced the crystal phase formation of the nanoparticles. Fe2O3-450 nanoparticles presented a higher concentration of the γ-phase than the Fe2O3-1000 nanoparticles. The crystal phase influenced differently Chlorella spp. growth, and the Fe2O3-1000 nanoparticles presented a higher toxicity in microalgae cultures. On the other hand, Fe2O3-450 nanoparticles influenced the growth and carbohydrate accumulation of Chlorella spp. at concentrations ≥ 10 mg L−1. The indirect beneficial effects of the synthetized nanoparticles were confirmed in microalgae cultures without carbonate supplementation. Indeed, Fe2O3-450 nanoparticles can be used as a strategy to produce high-value biomass in environments without carbonate supplementation.
Data Availability
On request.
Change history
01 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12649-024-02454-y
References
Sakthi Vignesh, N., Vimali, E., Sangeetha, R., Arumugam, M., Ashokkumar, B., Ganeshmoorthy, I., Varalakshmi, P.: Sustainable biofuel from microalgae: application of lignocellulosic wastes and bio-iron nanoparticle for biodiesel production. Fuel 278, 118326 (2020). https://doi.org/10.1016/j.fuel.2020.118326
Chandra, N., Shukla, P., Mallick, N.: Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatal. Agric. Biotechnol. 26, 101632 (2020). https://doi.org/10.1016/j.bcab.2020.101632
Yap, J.K., Sankaran, R., Chew, K.W., Munawaroh, H., Ho, H.S., Banu, S.H.R., Show, J.: Advancement of green technologies: a comprehensive review on the potential application of microalgae biomass. Chemosphere 281, 130886 (2021). https://doi.org/10.1016/j.chemosphere.2021.130886
Tan, X.B., Zhao, X.C., Yang, L., Bin: Strategies for enhanced biomass and lipid production by Chlorella pyrenoidosa culture in starch processing wastewater. J. Clean Prod. 236, 117671 (2019). https://doi.org/10.1016/j.jclepro.2019.117671
Rashid, N., Ryu, A.J., Jeong, K.J., Lee, B., Chang, Y.K.: Co-cultivation of two freshwater microalgae species to improve biomass productivity and biodiesel production. Energy Convers. Manag. 196, 640–648 (2019). https://doi.org/10.1016/j.enconman.2019.05.106
Magalhães, I.B., Ferreira, J., de Siqueira Castro, J., de Assis, L.R., Calijuri, M.L.: Technologies for improving microalgae biomass production coupled to effluent treatment: a life cycle approach. Algal. Res. (2021). https://doi.org/10.1016/j.algal.2021.102346
Mulgund, A.: Increasing lipid accumulation in microalgae through environmental manipulation, metabolic and genetic engineering: a review in the energy NEXUS framework. Energy Nexus 5, 100054 (2022). https://doi.org/10.1016/j.nexus.2022.100054
Chen, X., Zhang, C., Tan, L., Wang, J.: Toxicity of co nanoparticles on three species of marine microalgae. Environ. Pollut. 236, 454–461 (2018). https://doi.org/10.1016/J.ENVPOL.2018.01.081
Ji, J., Long, Z., Lin, D.: Toxicity of oxide nanoparticles to the green algae Chlorella Sp.. Chem. Eng. J. 170, 525–530 (2011). https://doi.org/10.1016/j.cej.2010.11.026
Pedroso, S., Helena, R., Costa, R., Popovic, R., Gerson, W.: Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquatic Toxicol. 143, 431–440 (2013). https://doi.org/10.1016/j.aquatox.2013.09.015
Henning, C., Perreault, F., Oukarroum, A., Pedroso, S., Popovic, R., Gerson, W.: Effect of chromium oxide (III) nanoparticles on the production of reactive oxygen species and photosystem II activity in the green alga Chlamydomonas reinhardtii. Sci. Total Environ. 565, 951–960 (2016). https://doi.org/10.1016/j.scitotenv.2016.01.028
Vaz, B.S., Vieira Costa, A., Greque de Morais, J.: Physical and biological fixation of CO2 with polymeric nanofibers in outdoor cultivations of Chlorella Fusca LEB 111. Int. J. Biol. Macromol. 151, 1332–1339 (2020). https://doi.org/10.1016/j.ijbiomac.2019.10.179
Kadar, E., Rooks, P., Lakey, C., White, D.A.: The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci. Total Environ. 439, 8–17 (2012). https://doi.org/10.1016/j.scitotenv.2012.09.010
Rana, M.S., Bhushan, S., Sudhakar, D.R., Prajapati, S.K.: Effect of iron oxide nanoparticles on growth and biofuel potential of Chlorella spp.. Algal. Res. 49, 101942 (2020). https://doi.org/10.1016/j.algal.2020.101942
Gunawan, C., Sirimanoonphan, A., Yang, W., Marquis, C.P., Amal, R.: Submicron and nano formulations of titanium dioxide and zinc oxide stimulate unique cellular toxicological responses in the green microalga Chlamydomonas reinhardtii. J. Haz. Mater. 260, 984–992 (2013). https://doi.org/10.1016/j.jhazmat.2013.06.067
Mulu, E., Arimi, M.M.M., Ramkat, R.C.: A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sustain. Energy Rev. 145, 111081 (2021). https://doi.org/10.1016/j.rser.2021.111081
Xu, P., Li, J., Qian, J., Wang, B., Liu, J., Xu, R., Chen, P., Zhou, W.: Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere 319, 137987 (2023). https://doi.org/10.1016/j.chemosphere.2023.137987
Jeon, H.S., Park, S.E., Ahn, B., Kim, Y.K.: Enhancement of biodiesel production in Chlorella vulgaris cultivation using silica nanoparticles. Biotechnol. Bioprocess. Eng. 22, 136–141 (2017). https://doi.org/10.1007/s12257-016-0657-8
da Silva Vaz, B., da Mastrantonio, S., Costa, D.J., de Morais, M.G.: Green alga cultivation with nanofibers as physical adsorbents of carbon dioxide: evaluation of gas biofixation and macromolecule production. Bioresour. Technol. (2019). https://doi.org/10.1016/j.biortech.2019.121406
Vaz, B.S., Costa, J.A.V., de Morais, M.G.: Innovative nanofiber technology to improve carbon dioxide biofixation in microalgae cultivation. Bioresour. Technol. 273, 592–598 (2019). https://doi.org/10.1016/j.biortech.2018.11.054
Vargas-Estrada, L., Longoria, A., Arias, D.M., Okoye, P.U., Sebastian, P.J.: Role of nanoparticles on microalgal cultivation : a review. Fuel 280, 118598 (2020). https://doi.org/10.1016/j.fuel.2020.118598
Bibi, M., Zhu, X., Munir, M., Angelidaki, I.: Bioavailability and effect of α-Fe2O3 nanoparticles on growth, fatty acid composition and morphological indices of Chlorella vulgaris. Chemosphere 282, 131044 (2021). https://doi.org/10.1016/j.chemosphere.2021.131044
Vargas-Estrada, L., Hoyos, E.G., Sebastian, P., Munoz, R.: Influence of mesoporous iron based nanoparticles on Chlorella sorokiniana metabolism during photosynthetic biogas upgrading. Fuel (2023). https://doi.org/10.2139/ssrn.4187585
Lei, C., Zhang, L., Yang, K., Zhu, L., Lin, D.: Toxicity of iron-based nanoparticles to green algae : effects of particle size, crystal phase, oxidation state and environmental aging *. Environ. Pollut. 218, 505–512 (2016). https://doi.org/10.1016/j.envpol.2016.07.030
Kayani, Z.N., Arshad, S., Riaz, S., Naseem, S.: Synthesis of Iron oxide nanoparticles by sol-gel technique and their characterization. IEEE Trans. Magn. 50, 1 (2014). https://doi.org/10.1109/TMAG.2014.2313763
Ayala-Fonseca, L.A., Amieva, E.J.-C., Rodriguez-Gonzalez, C., Angeles-Chavez, C., De la Rosa, E., Castaño, V.M., Salas, P.: Enhanced Raman effect of solvothermal synthesized reduced graphene oxide/titanium dioxide nanocomposites. ChemistrySelect 5, 3789–3797 (2020). https://doi.org/10.1002/slct.202000335
Lobato-Peralta, D.R., Duque-Brito, E., Orugba, H.O., Arias, D.M., Cuentas-Gallegos, A.K., Okolie, J.A., Okoye, P.U.: Sponge-like nanoporous activated carbon from corn husk as a sustainable and highly stable supercapacitor electrode for energy storage. Diam. Relat. Mater. 138, 110176 (2023). https://doi.org/10.1016/j.diamond.2023.110176
APHA-AWWA-WPCF: Standard methods for the examination of water and wastewater, Washington: (1999)
Rosa, G.M., da, Moraes, L., Cardias, B.B., de Souza, M.R.A.Z., Costa, J.A.V.: Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 192, 321–327 (2015). https://doi.org/10.1016/j.biortech.2015.05.020
Dubois, M., Smith, F., Rebers, P.A., Gilles, K.A., Hamilton, J.K.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956). https://doi.org/10.1021/ac60111a017
Li, G., Zhang, J., Li, H., Hu, R., Yao, X., Liu, Y., Zhou, Y., Lyu, T.: Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere (2020). https://doi.org/10.1016/j.chemosphere.2020.128578
Kuai, J., Zhang, H.: Research on generation and polishing mechanisms of nano grain a-Fe2O3 in precision electrolytic in process dressing (ELID) grinding. Procedia Manuf. 37, 425–430 (2019). https://doi.org/10.1016/j.promfg.2019.12.069
Raja, K., Mary Jaculine, M., Jose, M., Verma, S., Prince, A.A.M., Ilangovan, K., Sethusankar, K., Das, J.: Sol–gel synthesis and characterization of α-Fe2O3 nanoparticles. Superlattices Microstruct. 86, 306–312 (2015). https://doi.org/10.1016/j.spmi.2015.07.044
Sendra, M., Yeste, M.P., Gatica, J.M., Moreno-Garrido, I., Blasco, J.: Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere 179, 279–289 (2017). https://doi.org/10.1016/J.CHEMOSPHERE.2017.03.123
Fathi, P., Sadeghi, G., Hosseini, M.-J., Farahmandkia, Z., Mehrasbi, M.R.: Effects of copper oxide nanoparticles on the Chlorella algae in the presence of humic acid. SN Appl. Sci. 2, 140 (2020). https://doi.org/10.1007/s42452-019-1812-6
Vargas-Estrada, L., Hoyos, E.G., Sebastian, P.J., Mu, R.: Elucidating the role of nanoparticles on photosynthetic biogas upgrading : influence of biogas type, nanoparticle concentration and light source. Algal. Res. (2022). https://doi.org/10.1016/j.algal.2022.102899
Liang, S.X.T., Wong, L.S., Dhanapal, A.C.T.A., Djearamane, S.: Toxicity of metals and metallic nanoparticles on nutritional properties of microalgae. Water Air Soil Pollut. 231, 52 (2020). https://doi.org/10.1007/s11270-020-4413-5
Varaprasad, D., Raghavendra, P., Sudha, N.R., Sarma, L.S., Parveen, S.N., Chandana, P.S., Chandra, M.S., Chandrasekhar, T.: Bioethanol production from green alga Chlorococcum minutum through reduced graphene oxide-supported platinum-ruthenium (Pt-Ru/RGO) nanoparticles. Bioenergy Res. (2021). https://doi.org/10.1007/s12155-021-10282-4
González-Fernández, C., Ballesteros, M.: Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol. Adv. 30, 1655–1661 (2012). https://doi.org/10.1016/j.biotechadv.2012.07.003
Cheng, J., Zhu, Y., Li, K., Lu, H., Shi, Z.: Calcinated MIL-100(fe) as a CO2 adsorbent to promote biomass productivity of Arthrospira platensis cells. Sci. Total Environ. 699, 134375 (2020). https://doi.org/10.1016/j.scitotenv.2019.134375
Marchello, A.E., Barreto, D.M., Lombardi, A.T.: Effects of titanium dioxide nanoparticles in different metabolic pathways in the freshwater microalga Chlorella sorokiniana (Trebouxiophyceae). Water Air Soil Pollut. 229, 48 (2018). https://doi.org/10.1007/s11270-018-3705-5
Déniel, M., Errien, N., Daniel, P., Caruso, A., Lagarde, F.: Current methods to monitor microalgae-nanoparticle interaction and associated effects. Aquat. Toxicol. 217, 105311 (2019). https://doi.org/10.1016/J.AQUATOX.2019.105311
Mei, L.I., Qin, Z., Hu, C., Li, C., Liu, Z., Kong, Z.: Cobalt and manganese stress in the microalga Pavlova viridis (Prymnesiophyceae): effects on lipid peroxidation and antioxidant enzymes. J. Environ. Sci. 19, 1330–1335 (2007). https://doi.org/10.1016/S1001-0742(07)60217-4
Acknowledgements
The funding for this work was given through the project IN108922. Laura Vargas-Estrada would like to acknowledge the National Council of Science and Technology (CONACYT), Mexico, for her PhD scholarship (No. 736224). The authors acknowledge the help received from Dr. Claramaría Rodríguez-González (CIDETEQ) and Dr. L. Palma-Tirado (Instituto de Neurobiología-UNAM) for the SEM/TEM analysis and Ms. Maria Luisa Ramon and Mr. Rogelio Moran for XRD/SEM analysis.
Funding
The funding for this work was given through the project IN108922. Laura Vargas-Estrada would like to acknowledge the National Council of Science and Technology (CONACYT), Mexico, for her PhD scholarship (Grant No. 736224).
Author information
Authors and Affiliations
Contributions
LV: Design and experiments, writing; RD: Design, writing; PJS: Supervision, editing.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest or competing interests for this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original publication of the article, the graphical abstract has been processed incorrectly. The corrected graphical abstract is provided below.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vargas-Estrada, L., Domínguez-Espíndola, R.B. & Sebastian, P.J. The Influence of Fe2O3 Nanoparticles on Chlorella spp. Growth and Biochemicals Accumulation. Waste Biomass Valor 15, 3281–3295 (2024). https://doi.org/10.1007/s12649-023-02378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02378-z