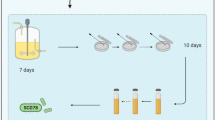
Abstract
Purpose
Glucose is one of the most important carbon and energy source for heterotrophic growth in all living organisms. However, glucose has been reported as a poor substrate to support the growth of hyperthermophilic archaea belonging to the order Thermococcales. To enhance glucose-assisted growth of Thermococcus onnurineus NA1, adaptive evolution process was applied. In an effort for industrial applications, glucose-adapted cells were further tested for H2 producing potential using food processing waste as a promising zero-value substrate containing polysaccharides composed of glucose.
Methods
Adaptive evolution of T. onnurineus NA1 was performed by transferring cells to fresh medium containing glucose until cell growth increased. Genome sequencing was conducted to identify genetic changes in adapted cells. H2 production in the parent strain and glucose-adapted cells was analyzed using either glucose or potato peel waste as substrate.
Results
The glucose-adapted cells, WG-100T, had 10.8-fold and 14.7-fold increases in cell density and glucose consumption, respectively, compared to the parent strain. Genome sequencing of WG-100T revealed a total of 17 genomic changes in genes, including those encoding transcription factors and several proteins involved in various transport systems. WG-100T produced H2 using potato peel waste through simultaneous saccharification and fermentation.
Conclusion
This study showed that the performance of the Thermococcales strain was improved by adaptive evolution, resulting in faster use of glucose. In addition, it was shown that the use of a hyperthermophile made it possible to produce biohydrogen without pretreatment of food processing waste for saccharification.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
A hyperthermophilic archaeon, engineered through adaptive evolution to improve glucose utilization, demonstrated the potential for H2 production from potato peel waste without saccharification pretreatment. This strategy is considered to be useful for expanding the scope of sugar-based cell factory that use a variety of biomass waste to produce value-added products.
Introduction
Hydrogen gas (H2) is attracting attention due to climate change and energy crisis. Currently, 96% of H2 is produced from fossil fuels, and thus technology to produce H2 using renewable resources is required. Biohydrogen production by dark fermentation using agricultural product residues or wastes derived from food processing is being considered as an alternative [1, 2]. Potato peel waste generates 70–140 thousand tons per year from industrial processes and contains a lot of carbohydrates such as starch and non-starch polysaccharides and other components such as protein and lignin [3, 4]. Therefore it has been considered as a promising zero-value substrate for the production of various value-added products such as biofuels, antioxidants and biosorbent [5,6,7,8,9]. However, in order for most microbes to use potato peels, a pretreatment process such as hydrolysis by acid or enzymes, pyrolysis or hydrothermal treatment is required for saccharification. An alternative may be the use of thermophiles or hyperthermophiles with thermophilic enzymes to degrade polysaccharides at high temperatures where saccharification and fermentation can occur simultaneously through substrate liquefaction.
Glucose, a key component of starch, is one of the important carbon and energy sources that support the heterotrophic growth of all living organisms [10]. Studies on growth of hyperthermophiles using glucose as a substrate have been mainly reported in Thermotoga and Sulfolobus species [11,12,13,14]. However, Thermotoga maritima has been reported to have a lower growth rate in the presence of glucose [15] than other sugars such as maltose and starch [16]. Studies on glucose-assisted growth of other hyperthermophiles are very limited, and slow growth of Pyrococcus furiosus and Aeropyrum pernix on glucose has been reported [17, 18]. Thermococcus onnurineus NA1 belonging to the order Thermococcales is known to produce biohydrogen using substrates such as starch, formate and carbon monoxide (CO) [19,20,21]. Many studies have been attempted to improve the performance of the species, and growth and H2 productivity have been significantly increased through adaptive laboratory evolution and genetic engineering [22,23,24,25]. Despite its versatile appetites, however, growth on glucose has never been determined.
In this study, we investigated the potential of T. onnurineus NA1 for growth on glucose and exploited an adaptive evolution process to enhance glucose-assisted growth. We further tested H2 production in glucose-adapted cells using potato peel waste.
Materials and Methods
Strain, Media, Potato Peel Waste and Culture Conditions
Thermococcus onnurineus NA1 isolated from a deep-sea hydrothermal vent area [26] was grown in a modified medium 1 (MM1) as a basal medium [20] and routinely cultured at 80 °C, pH 6.5. For cultivation in serum vials, MM1 + glucose medium was prepared by adding glucose stock solution to a final concentration of 2, 5, 10, 15 or 20 g/L of glucose in an anaerobic chamber (Coy Laboratory Products, Grass Lake, USA) containing an anoxic gas mixture (N2:H2 = 95:5). The glucose stock solution was prepared by dissolving 200 g/L of glucose in water and autoclaving. MM1 + carbohydrate media were prepared by adding 5 g/L of maltose, starch or maltodextrin. Na2S∙9H2O was added to a final concentration of 0.005% to remove dissolved oxygen from the medium.
Potato peel waste generated prior to cooking was supplied by a local restaurant. Dried potato peels (DPP) were prepared by drying potato peels at 60 °C for 24 h and grinding in the laboratory and were stored at −20 °C before use. For batch culture in serum vials, MM1 + DPP medium was prepared by adding DPP to a final concentration of 3.3, 5.0 or 6.6 g/L in serum vials and the pH was adjusted to 6.5 with 2 N HCl.
Batch culture in bioreactors was performed as previously described [25]. MM1 + glucose and MM1 + DPP media were prepared by adding 20 g/L of glucose or 3.3 g/L of DPP, respectively, and purging with pure argon gas (99.999%) at 80 °C for 60 min without autoclaving. The pH was controlled at 6.1–6.2 with 5 N NaOH.
Adaptive Laboratory Evolution
Adaptive evolution was performed in MM1 + glucose medium containing 20 g/L of glucose. After the parent strain was cultured in serum vials at 80 °C for 24 h, 2% (v/v) culture broth was transferred to fresh medium using a sterile syringe. This transfer step was repeated 100 times.
Bioinformatic Analysis and Genomic Analysis
The homology search of the amino acid sequence was performed using the Basic Local Alignment Search Tool (BLAST) against a nonredundant protein database from the National Center for Biotechnology Information (NCBI, Bethesda, USA). The multiple sequence alignment was performed using the T-Coffee program provided by the EMBL-EBI (https://www.ebi.ac.uk). Extraction and purification of genomic DNA was performed using phenol/chloroform/isoamyl alcohol (25:24:1, v/v). Genome sequencing was performed using Pacific Biosciences (Menlo Park, USA) single-molecule real-time (SMRT) sequencing technology with the 10-kb insert library at approximately 100x coverage [27]. Assembly and consensus polishing were performed as previously reported [28]. All mutations were verified by PCR and Sanger sequencing using the primers listed in the supplementary material Table S1.
Analytical Methods
Cell growth was monitored by measuring the optical density at 600 nm (OD600) using a UV-Visible spectrophotometer (BioPhotometer plus; Eppendorf, Hamburg, Germany). The concentrations of glucose and acetate were measured using an HPLC-refractive index detector (RID) system (YL 9100; YL Instrument Co., Anyang, Republic of Korea) equipped with a Rezex ROA column (300 × 7.8 mm; Phenomenex, Torrance, USA). The mobile phase in HPLC was 2.5 mM sulfuric acid at a flow rate of 0.6 mL/min. H2 concentration was measured using a YL6100 gas chromatograph (YL Instrument Co., Anyang, Republic of Korea) equipped with Molsieve 5 A and Porapak N columns (Supelco, Bellefonte, USA) and thermal conductivity and flame ionization detectors. Argon was used as a carrier gas at a flow rate of 30 mL/min. The total volume of outlet gas from the bioreactor was measured using a wet gas meter (Shinagawa, Tokyo, Japan).
Results and Discussion
Growth of T. onnurineus NA1 on Glucose
The hyperthermophilic archaeon T. onnurineus NA1 was tested whether it can grow using glucose as a substrate. Cell density gradually increased as the glucose concentration increased from 2 to 20 g/L (Fig. 1a). Glucose consumption was actually detected proportional to cell growth in the time-course profile (Fig. 1b). However, the cell densities were low and only small amounts of glucose were consumed as reported in P. furiosus and A. pernix [17, 18].
Cell growth of T. onnurineus NA1 on glucose. a Effect of glucose concentration on cell growth for the parent strain (black bars) and glucose-adapted WG-100T cells (gray bars). b Time-course profiles of cell growth for the parent strain in the presence (open circles) or absence of 20 g/L of glucose (closed circles) and remaining glucose (closed squares). Error bars indicate standard deviation of two (a) or three (b) biological replicates. OD600, optical density at 600 nm
Adaptive Evolution of T. onnurineus NA1 to Glucose
It has been reported that growth rate and glucose utilization in T. maritima were improved through adaptive evolution [29]. Therefore, the approach was applied to T. onnurineus NA1. Adaptation to glucose was performed by transferring the cells to a fresh medium containing 20 g/L of glucose after 24 h cultivation. A gradual increase in cell density and glucose consumption was observed as the serial transfer proceeded (data not shown). The 100th transferred cells, designated WG-100T, exhibited 2.9-5.7 times higher cell densities at all tested glucose concentrations compared to the corresponding values of the parent strain (Fig. 1a).
Further characterization of the phenotypic properties of the evolved cells was performed in batch culture using bioreactors where the pH was maintained at 6.1–6.2. The adapted cells displayed markedly improved growth with 10.8-fold increase in cell density (Fig. 2a) through a 14.9-fold increase in glucose consumption (Fig. 2b). Metabolite production of acetate (Fig. 2c) and H2 (Fig. 2d) increased 15.7- and 14.2-fold, respectively, in the adapted cell culture compared to the parent strain. The molar ratios of acetate and H2 produced per glucose consumed were 1.5 and 1.8 for the parent strain and 1.6 and 1.7 for the adapted cells. WG-100T showed 2.2-, 8.5-, 15.2- and 15.6-fold better performance at specific growth rate, maximum glucose consumption rate, maximum acetate production rate and maximum H2 production rate, respectively, compared to the parent strain (Table 1). In addition, in terms of biomass, acetate and H2, the productivity of WG-100T was 22.3-, 3.3- and 5.8-fold higher than that of the parent strain. The biomass productivity of 0.16 g/L/h achieved by WG-100T in simple batch culture is comparable to the high cell density achieved through fed-batch culture of Saccharolobus solfataricus P2 or Saccharolobus solfataricus Gθ [30, 31].
Batch culture profile of glucose-adapted WG-100T cells in a bioreactor. Cell density (a), residual glucose concentration (b), acetate concentration (c) and H2 concentration (d) were measured for the parent strain (open circles) and WG-100T (closed circles). Error bars indicate standard deviation of three independent cultivations. OD600, optical density at 600 nm
Genomic Analysis of the Adapted Cells
To understand how the adapted cells acquired the enhanced growth phenotype in the presence of glucose, the genetic variation in the genome was analyzed by genome sequencing. A total of 17 alterations were detected in the genome of the WG-100T (Table 2). Although most of the mutations were distinct from those identified in cells that evolved either under formate or CO conditions [22, 23], point mutations in the TON_0820 gene, encoding aromatic amino acid permease, were detected in all three conditions. Interestingly, the mutation locations were all different [22, 23]: the gene carried a frame-shift mutation during adaptive evolution under glucose condition. As the mutation has been confirmed to stimulate growth under CO condition, it may have a positive or negative effect on cell growth under glucose condition. It was found that several mutated genes are related to the transport systems: ABC-type transporter permease (TON_0597), proton/glutamate symporter (TON_0901), ABC-type maltodextrin transport system (TON_1795) and xanthine/uracil permease (TON_1957). TON_1795 and TON_1957 had frame-shift mutations, presumably resulting in non-functional gene products. TON_0597 gene encoding permease belongs to a gene cluster including genes encoding sugar-binding protein (TON_0594), ATPase (TON_0595), permease (TON_0596), which are components of the ATP-binding cassette (ABC)-type transporter system. The substrate specificity of TON_0594 is unclear, but it seems to be related with sugar. Mutations in the TON_0597 gene may increase the uptake of a substrate required for growth, leading to improved growth of WG-100T. TON_0901 shares 78.3% and 70.6% amino acid sequence identity with the sodium-coupled aspartate transporters of Thermococcus kodakarensis and Pyrococcus horikoshii, respectively, which have been functionally and structurally identified to specifically transport aspartate [32,33,34]. All residues that interact with the substrate aspartate are strictly conserved (Fig. S1), and thus mutation in TON_0901 may enhance cell growth by increasing aspartate uptake. It would be helpful to construct mutants with each mutation to determine the effect of the alterations at TON_0597 or TON_0901 on the observed phenotype of the adapted cells.
Furthermore multi-omics analyses are needed to pinpoint mechanisms for explaining the phenotype of evolved T. onnurineus NA1 cells. Changes in the expression levels of various genes, including those encoding transporters, may play a beneficial role. This may enable to identify glucose transport genes in T. onnurineus NA1. The putative glucose ABC transport gene cluster of Sulfolobus acidocaldarius has been predicted by gene expression analysis as it showed upregulation during growth in the presence of glucose [35]. The clear elucidation of mechanism behind the phenotypic change in the WG-100T awaits further study.
Utilization of Saccharides of the Adapted Cells
Based on the fact that T. onnurineus NA1 showed enhanced growth on glucose through adaptive evolution, the ability of WG-100T to grow on various saccharides composed of glucose units, maltose, maltodextrin and starch, was investigated. No growth enhancement was detected in the presence of maltose in WG-100T compared to the parent strain (Fig. 3). On the other hand, WG-100T exhibited 1.2- and 2.3-fold higher cell densities in the presence of starch and maltodextrin, respectively, compared to the parent strain (Fig. 3a). The H2 production increased in proportion to the increase in cell density (Fig. 3b). These results indicate that glucose-adapted cells can use oligosaccharides for H2 production more effectively than the parent strain.
Growth of WG-100T cells in the presence of carbohydrate as a substrate. Effect of maltose, starch and maltodextrin on cell growth (a) and H2 production (b) in the parent strain (black bars) and WG-100T (gray bars). Error bars indicate standard deviation of two biological replicates. OD600, optical density at 600 nm
H2 Production Using Food Waste
Potato peel waste was tested as a substrate for H2 production using T. onnurineus NA1 parent strain and WG-100T. Potato peels were dried and ground to be added to serum vials, leading to H2 production by the parent strain and WG-100T. As the amount of dried potato peels (DPP) increased from 3.3 to 6.7 g/L, H2 production rates decreased in both cells. Toxic compounds of potato peel might affect microbial growth as reported with DPPs [36, 37]. Meanwhile, H2 production rates of WG-100T were 1.3 to 1.5-fold higher than those of the parent strain (Fig. 4a). In order to overcome the growth limit due to pH drop, culture was attempted in a pH-stat bioreactor and H2 production rate was found to be increased compared to the serum vial (Fig. 4b).
H2 production of WG-100T using potato peel waste. a H2 production of the parent strain (open circles) and WG-100T (closed circles) in a serum vial containing different amounts of potato peel waste. b H2 production of the parent strain (open circles) and WG-100T (closed circles) in a pH-stat bioreactor containing 3.3 g/L of potato peel waste. Error bars indicate standard deviation of two biological replicates
WG-100T showed much better performance than the parent strain in H2 production rate (maximum value, 2.83 vs. 0.97 mmol/L/h) and H2 productivity (1.29 vs. 0.37 mmol/L/h) (Table 1). These results show that adaptation to glucose enhanced the strain’s ability to consume carbohydrate-containing food waste. It is noteworthy that the hyperthermophilic archaeon T. onnurineus NA1 produced H2 using potato peel waste pretreated only by low-temperature drying and grinding. Compared with the H2 production rates of Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana using potato steam peels generated by the potato processing industry [7], the H2 yield per substrate supplied was twice lower in WG-100T. At present, it is difficult to say whether this difference is attributed to the pretreatment of the potato peels or the ability of the strains.
Conclusion
Thermococcus onnurineus NA1 was confirmed to be able to grow on glucose, but its performance on glucose was not comparable to glucose-utilizing hyperthermophiles. Through adaptive evolution, the microbe developed ways to utilize glucose more efficiently. Genome sequencing revealed genomic changes in the adapted cells. Further studies are necessary to identify molecular mechanisms of action that could explain the physiological changes of the adapted cells. The hyperthermophilic archaeon was determined as a potential utilizer for the potato peel waste by simultaneous saccharification and fermentation to produce H2. Further applications of genetic engineering will help increase H2 productivity.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Dahiya, S., Chatterjee, S., Sarkar, O., Mohan, S.V.: Renewable hydrogen production by dark-fermentation: current status, challenges and perspectives. Bioresour Technol. 321, 124354 (2021)
Rittmann, S.K.R., Lee, H.S., Lim, J.K., Kim, T.W., Lee, J.-H., Kang, S.G.: One-carbon substrate-based biohydrogen production: microbes, mechanism, and productivity. Biotechnol. Adv. 33, 165–177 (2015)
Liang, S.B., McDonald, A.G.: Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J. Agric. Food Chem. 62, 8421–8429 (2014)
Chang, K.C.: Polyphenol antioxidants from potato peels: extraction, optimization and application to stabilizing lipid oxidation in foods. Proc. Natl. Conf. Undergrad. Res. (NCUR), Ithaca College, New York, USA, pp. 1–8 (2011)
Awogbemi, O., Kallon, D.V.V., Owoputi, A.O.: Biofuel generation from potato peel waste: current state and prospects. Recycling. 7, 23 (2022)
Javed, A., Ahmad, A., Tahir, A., Shabbir, U., Nouman, M., Hameed, A.: Potato peel waste-its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food. 4, 807–823 (2019)
Mars, A.E., Veuskens, T., Budde, M.A.W., van Doeveren, P.F.N.M., Lips, S.J., Bakker, R.R., de Vrije, T., Claassen, P.A.M.: Biohydrogen production from untreated and hydrolyzed potato steam peels by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy. 35, 7730–7737 (2010)
Guo, X.M., Trably, E., Latrille, E., Carrère, H., Steyer, J.P.: Hydrogen production from agricultural waste by dark fermentation: a review. Int. J. Hydrogen Energy. 35, 10660–10673 (2010)
Bhurat, K.S., Banerjee, T., Pandey, J.K., Bhurat, S.S.: A lab fermenter level study on anaerobic hydrogen fermentation using potato peel waste: effect of pH, temperature, and substrate pre-treatment. J. Mater. Cycles Waste Manag. 23, 1617–1625 (2021)
Walmsley, A.R., Barrett, M.P., Bringaud, F., Gould, G.W.: Sugar transporters from bacteria, parasites and mammals: structure-activity relationships. Trends Biochem. Sci. 23, 476–481 (1998)
Schroder, C., Selig, M., Schonheit, P.: Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: involvement of the Embden-Meyerhof pathway. Arch. Microbiol. 161, 460–470 (1994)
Van Ooteghem, S.A., Beer, S.K., Yue, P.C.: Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Appl. Biochem. Biotechnol. 98, 177–189 (2002)
Derosa, M., Gambacorta, A., Nicolaus, B., Giardina, P., Poerio, E., Buonocore, V.: Glucose metabolism in the extreme thermoacidophilic archaebacterium Sulfolobus solfataricus. Biochem. J. 224, 407–414 (1984)
Grogan, D.W.: Phenotypic characterization of the archaebacterial genus sulfolobus: comparison of five wild-type strains. J. Bacteriol. 171, 6710–6719 (1989)
Chhabra, S.R., Shockley, K.R., Conners, S.B., Scott, K.L., Wolfinger, R.D., Kelly, R.M.: Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thermotoga maritima. J. Biol. Chem. 278, 7540–7552 (2003)
Rodionov, D.A., Rodionova, I.A., Li, X.Q., Ravcheev, D.A., Tarasova, Y., Portnoy, V.A., Zengler, K., Osterman, A.L.: Transcriptional regulation of the carbohydrate utilization network in Thermotoga maritima. Front. Microbiol. 4, 244 (2013)
Kim, K.W., Lee, S.B.: Inhibitory effect of maillard reaction products on growth of the aerobic marine hyperthermophilic archaeon Aeropyrum pernix. Appl. Environ. Microbiol. 69, 4325–4328 (2003)
Driskill, L.E., Kusy, K., Bauer, M.W., Kelly, R.M.: Relationship between glycosyl hydrolase inventory and growth physiology of the hyperthermophile Pyrococcus furiosus on carbohydrate-based media. Appl. Environ. Microbiol. 65, 893–897 (1999)
Bae, S.S., Kim, T.W., Lee, H.S., Kwon, K.K., Kim, Y.J., Kim, M.S., Lee, J.-H., Kang, S.G.: H2 production from CO, formate or starch using the hyperthermophilic archaeon, Thermococcus onnurineus. Biotechnol. Lett. 34, 75–79 (2012)
Kim, Y.J., Lee, H.S., Kim, E.S., Bae, S.S., Lim, J.K., Matsumi, R., Lebedinsky, A.V., Sokolova, T.G., Kozhevnikova, D.A., Cha, S.S., Kim, S.J., Kwon, K.K., Imanaka, T., Atomi, H., Bonch-Osmolovskaya, E.A., Lee, J.-H., Kang, S.G.: Formate-driven growth coupled with H2 production. Nature. 467, 352–355 (2010)
Lee, H.S., Kang, S.G., Bae, S.S., Lim, J.K., Cho, Y., Kim, Y.J., Jeon, J.H., Kwon, K.K., Kim, H.-T., Park, C.-J., Lee, H.-W., Kim, S.I., Chun, J., Colwell, R.R., Kim, S.-J., Lee, J.-H.: The complete genome sequence of Thermococcus onnurineus NA1 reveals a mixed heterotrophic and carboxydotrophic metabolism. J. Bacteriol. 190, 7491–7499 (2008)
Lee, S.H., Kim, M.-S., Lee, J.-H., Kim, T.W., Bae, S.S., Lee, S.-M., Jung, H.C., Yang, T.-J., Choi, A.R., Cho, Y.-J., Lee, J.-H., Kwon, K.K., Lee, H.S., Kang, S.G.: Adaptive engineering of a hyperthermophilic archaeon on CO and discovering the underlying mechanism by multi-omics analysis. Sci. Rep. 6, 22896 (2016)
Jung, H.-C., Lee, S.H., Lee, S.-M., An, Y.J., Lee, J.-H., Lee, H.S., Kang, S.G.: Adaptive evolution of a hyperthermophilic archaeon pinpoints a formate transporter as a critical factor for the growth enhancement on formate. Sci. Rep. 7, 6124 (2017)
Lee, S.H., Lee, S.-M., Kang, S.G., Lee, H.S.: Enhanced H2 production by deletion of the tfx family DNA-binding protein in the hyperthermophilic archaeon Thermococcus onnurineus NA1. Int. J. Hydrogen Energy. 46, 35189–35197 (2021)
Kim, M.-S., Bae, S.S., Kim, Y.J., Kim, T.W., Lim, J.K., Lee, S.H., Choi, A.R., Jeon, J.H., Lee, J.-H., Lee, H.S., Kang, S.G.: CO-dependent H2 production by genetically engineered Thermococcus onnurineus NA1. Appl. Environ. Microbiol. 79, 2048–2053 (2013)
Bae, S.S., Kim, Y.J., Yang, S.H., Lim, J.K., Jeon, J.H., Lee, H.S., Kang, S.G., Kim, S.J., Lee, J.-H.: Thermococcus onnurineus sp. nov., a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent area at the PACMANUS field. J. Microbiol. Biotechnol. 16, 1826–1831 (2006)
Eid, J., Fehr, A., Gray, J., Luong, K., Lyle, J., Otto, G., Peluso, P., Rank, D., Baybayan, P., Bettman, B., Bibillo, A., Bjornson, K., Chaudhuri, B., Christians, F., Cicero, R., Clark, S., Dalal, R., Dewinter, A., Dixon, J., Foquet, M., Gaertner, A., Hardenbol, P., Heiner, C., Hester, K., Holden, D., Kearns, G., Kong, X., Kuse, R., Lacroix, Y., Lin, S., Lundquist, P., Ma, C., Marks, P., Maxham, M., Murphy, D., Park, I., Pham, T., Phillips, M., Roy, J., Sebra, R., Shen, G., Sorenson, J., Tomaney, A., Travers, K., Trulson, M., Vieceli, J., Wegener, J., Wu, D., Yang, A., Zaccarin, D., Zhao, P., Zhong, F., Korlach, J., Turner, S.: Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138 (2009)
Chin, C.S., Alexander, D.H., Marks, P., Klammer, A.A., Drake, J., Heiner, C., Clum, A., Copeland, A., Huddleston, J., Eichler, E.E., Turner, S.W., Korlach, J.: Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods. 10, 563–569 (2013)
Latif, H., Sahin, M., Tarasova, J., Tarasova, Y., Portnoy, V.A., Nogales, J., Zengler, K.: Adaptive evolution of Thermotoga maritima reveals plasticity of the ABC transporter network. Appl. Environ. Microbiol. 81, 5477–5485 (2015)
Park, C.B., Lee, S.B.: Constant-volume fed-batch operation for high density cultivation of hyperthermophilic aerobes. Biotechnol. Tech. 11, 277–281 (1997)
Schiraldi, C., Marulli, F., Di Lernia, I., Martino, A., De Rosa, M.: A microfiltration bioreactor to achieve high cell density in Sulfolobus solfataricus fermentation. Extremophiles. 3, 199–204 (1999)
Yernool, D., Boudker, O., Jin, Y., Gouaux, E.: Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 431, 811–818 (2004)
Jensen, S., Guskov, A., Rempel, S., Hanelt, I., Slotboom, D.J.: Crystal structure of a substrate-free aspartate transporter. Nat. Struct. Mol. Biol. 20, 1224–1226 (2013)
Ryan, R.M., Compton, E.L.R., Mindell, J.A.: Functional characterization of a Na+-dependent aspartate transporter from Pyrococcus horikoshii. J. Biol. Chem. 284, 17540–17548 (2009)
Joshua, C.J., Dahl, R., Benke, P.I., Keasling, J.D.: Absence of diauxie during simultaneous utilization of glucose and xylose by Sulfolobus acidocaldarius. J. Bacteriol. 193, 1293–1301 (2011)
De Sotillo, D.R., Hadley, M., Wolf-Hall, C.: Potato peel extract a nonmutagenic antioxidant with potential antimicrobial activity. J. Food Sci. 63, 907–910 (1998)
Silva-BeltrAn, N.P., Chaidez-Quiroz, C., Lopez-Cuevas, O., Ruiz-Cruz, S., Lopez-Mata, M.A., Del-Toro, S.C.L., Marquez-Rios, E., Ornelas-Paz, J.J.: Phenolic compounds of potato peel extracts: their antioxidant activity and protection against human enteric viruses. J. Microbiol. Biotechnol. 27, 234–241 (2017)
Funding
This work is supported by the Korea Institute of Ocean Science and Technology (KIOST) in-house program (PEA0122) and the study on operational stability and risk assessment of CO-converting hyperthermophilic archaeon program of the Ministry of Oceans and Fisheries in the Republic of Korea.
Author information
Authors and Affiliations
Contributions
SHL: Conceptualization, Formal analysis, Investigation, Data curation, Writing - original draft. SL: Formal analysis, Data curation, Writing - original draft. S-ML: Investigation, Formal analysis. JHC: Conceptualization. HSL: Conceptualization, Investigation, Data curation, Writing - review & editing. SGK: Conceptualization, Investigation, Data curation, Writing - review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.H., Lee, S., Lee, SM. et al. Biohydrogen Production from Food Waste Using Glucose-Adapted Hyperthermophilic Archaeon. Waste Biomass Valor 14, 2923–2930 (2023). https://doi.org/10.1007/s12649-023-02049-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02049-z