Abstract
Purpose
The interest in using bioresidues produced by the agri-food industry is increasingly intrinsic to the world economy. As such, researchers started to look for new ways to enhance its use, developing innovations to transform these residues into high-value raw materials with industrial application, promoting the circular economy concept.
Methods
Therefore, pruning residues from the production of Vaccinium myrtillus L. could be an interesting exploitation field, given its position as a superfood due to its well-known antioxidant properties. The antioxidant, antibacterial, anti-inflammatory, anti-tyrosinase, and cytotoxicity potentials of aqueous and hydroethanolic extracts of V. myrtillus aerial parts were evaluated.
Results
From the obtained results, ultrasound-assisted and maceration extracts were found to be as effective as, if not more effective than, conventional antibiotics against methicillin-resistant Staphylococcus aureus. Aside from antibacterial activity, the extracts also showed antioxidant and anti-tyrosinase effects, which were found to be favourably related to the level of caffeoylquinic acid derivatives.
Conclusion
The obtained results highlight the bioactive potential and the importance of exploiting this bio residue as a novel candidate for industrial application, taking advantage of their biological properties.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
A novel opportunity for exploiting blueberry pruning leftovers by recovering valuable bioactive molecules was established. The proposed strategy follows a circular bioeconomy approach by valorising a discharged agri-food bio residue, namely pruning wastes from Vaccinium myrtillus L. cultivation, which, contrarily to the well-known and extensively studied fruit, blueberries, are still unexplored matrices. For the first time, blueberry pruning leftovers were characterized concerning the phenolic profile and bioactivity. Among the tested properties, the extracts evidenced antioxidant properties and anti-tyrosinase capacity. Moreover, all the extracts presented an antibacterial activity similar to ampicillin. Overall, the obtained promising results highlight that blueberry aerial parts might be transformed into high-value raw materials and used as natural bioactive preservatives in food and cosmetics.
Introduction
The Vaccinium genus consists of woody perennial shrubs belonging to the Ericaceae family and contains, among other species, the Vaccinium myrtillus L. This shrub varies in size from approximately 5 to 90 cm; the flowers are bell-shaped, white, pale pink, or red, sometimes tinged greenish, producing a blue-colored berry, known as blueberry [1]. This species is well known, and its fruits are highly consumed, not only for their organoleptic characteristics but also for their medicinal properties, given their richness in bioactive molecules such as phenolic compounds [2].
The demand for these fruits is increasing in various parts of the world, with major world producers such as the United States and South American countries, finding an opportunity to increase their exportation to European retail chains that are seeking more suppliers of V. myrtillus [3]. Therefore, the blueberries world production has been increasing since 2003, reaching about 750 kton of blueberries in 2018, being Europe responsible for 18.2% of its distribution [4].
Because of its extensive production, the processing of these fruits generates high amounts of bioresidues, mostly from pruning and weeding or from berry processing. In response to current environmental and economic concerns regarding the sustainable (re)use of natural resources (including bioresidues), the agri-food industry is often confronted with several solutions, including the animal feed, which means investments in technology to provide a healthy and nutritive by-product. Another solution is the spreading and composting for energy, and incineration for biogas production [5]. Beyond the bioresidues generated by the blueberries manufacture, the ones derived from berry crops are a new focus of study, since the industry is more and more interested in its valorization, namely through the sustainable obtaining of high added-value products that can find industrial application in several sectors, namely the food, cosmetic and also the pharmaceutical ones. In this context, the leaves, and other aerial parts of V. myrtillus, are a good example of waste biomass that can be exploited; due to their high content in antioxidants, such as phenolic compounds, and in fibers, these by-products could be a valuable raw material for the recovery of added value molecules [6].
While blueberry fruits have already been studied in detail, their bio residues have not been fully exploited, although some results described in the literature pointed out the leaf as a potential source of phenolic compounds [7], mainly flavonoids (quercetin derivatives), which contribute to the high antioxidant potential described for this biomass [8]. It is also well known that the regular consumption of vegetables and fruits rich in polyphenolic compounds provides protection against the harmful effects of UV radiation such as oxidative stress, inflammation, and cancer. Botanicals with a photo protective activity are gaining attention from researchers of cosmetic and pharmaceutical corporations due to the multiple health benefits and cost-effectiveness [9]. In skin applications, antioxidants are a promising tool to mitigate oxidative injury [10]. In fact, the use of natural extracts is a growing tendency since the beginning of the XXI century, mostly with those plants that have evolved a variety of photo adaptive mechanisms including the production of antioxidant and UV absorbing compounds by being exposed to intense radiation in their environmental conditions [9]. This evolutionary change in the society is reflected in a strong interest by the consumer in cosmetic care formulations having a ‘‘natural’’ benefit. The trend toward ‘‘nature’’ is paralleled by a fast-growing scientific knowledge about plant constituents, human molecular biology, and cell physiology [10]. In this sense, blueberries have already been successfully used in the cosmetic industry and were able to increase the photo protective capacity of sunscreen formulations, providing also extra benefits due to their antioxidant and anti-aging properties [11].
Bearing this in mind, the objective of the present work was to bring high added value to the pruning biowaste resulting from the production of blueberries. Plant extracts obtained from V. myrtillus aerial parts were characterized regarding their content in bioactive phenolic compounds. Furthermore, these extracts were also analyzed for their biological potential through antioxidant, antimicrobial, anti-inflammatory, anti-tyrosinase, and cytotoxicity, aiming at developing a promising extract for application in the cosmetic industry.
Material and Methods
Plant Material
Vaccinium myrtillus aerial parts resulting from the pruning were collected in September of 2019 in Baião, Portugal (geographic coordinates: Latitude: 41° 09′ 45″ N Longitude: 8°02′04″ W Elevation above sea level: 577 m = 1893 ft), by the company Hortitool Consulting Lda. After air drying at room temperature (around 25 °C) during 5 days, this raw material was grounded to a 20 mesh (~ 0.8 mm) particle size in a ZM200 Retsch model mill. Afterward, the dried material was kept protected from light and moisture until further analysis.
Preparation of the V. myrtillus Extracts
Aqueous Extracts
Infusion
Approximately 1 g of powdered sample was added to 200 mL of boiling distilled water. The mixture was maintained in these conditions for 5 min to allow the extraction of the bioactive compounds.
Decoction
In this case, after the addition of 200 mL of water to 1 g of powdered sample, the mixture was heated until boiling point and maintained in these conditions for 5 min.
For both the aqueous extracts, the samples were subjected to gravity filtration using a Whatman paper filter nº 4. Afterward, the aqueous extracts were frozen at − 24 °C, and lyophilized (Freeze Dryer Sacnvac, Coolsafe, 0.4 mbar, Lillerod, Denmark) at a final condensation temperature of − 100 °C and stored in a dry room.
Hydroethanolic Extracts
Maceration
Approximately 1 g of the powdered sample was added to 30 mL of ethanol–water solution (80:20, v/v) and maintained with stirring for 60 min at room temperature.
Ultrasound-Assisted Extraction (UAE)
This extraction was performed in a sonicator (Qsonica, Q500, ultrasonic processor sonicator, 20 kHz, Newtown, USA) following the extraction procedure described by Heleno et al. [12] with slight modifications to optimize the extraction in order to recover phenolic compounds. Briefly, about 1 g of powdered sample was mixed with 150 mL of an ethanol–water solution (80:20, v/v). The extraction was carried out for 10 min, with cycles of 30 s intercalated by pauses of 10 s, at 75% intensity of 500 W. An ice bath was also used to avoid sample overheating.
The mixtures were filtered by gravity, and evaporated under reduced pressure (Büchi R-114, rotary evaporator; Büchi B-480, waterbath, and Büchi B-721, vacuum controller system, Flawil, Switzerland) at 40 °C, 100 rpm, and ≈ 90 mbar until the ethanol was entirely removed. The aqueous residue was then frozen at − 24 °C, lyophilized, and stored in a dry room.
The extracts were subjected to UV decontamination for 15 min prior to each bioactive analysis, ensuring extracts sterility.
Chemical Characterization and Bioactive Properties
Profile in Phenolics Compounds
To identify and quantify the phenolic compounds, the followed protocol was the one of Bessada et al. [13]. Briefly, a portion of 10 mg of each different sample was dissolved in 1 mL of a methanol–water solution (20:80 v/v) and filtered through a 0.22 µm disposable filter. The phenolic compounds identification was done by ultra-performance liquid chromatography coupled to a diode array detector and an electrospray ionization mass spectrometry detector (UPLC-DAD-ESI/MS), a Dionex Ultimate 3000 (Dionex Ultimate 3000 UPLC and Linear Ion Trap LQT XL, Thermo Scientific, San Jose, CA, USA). A Waters Spherisorb S3 ODS-2C18, 3 μm (4.6 mm × 150 mm) column thermostatted at 25 °C was used. The solvents used were: (A) 2.5% acetic acid in water, (B) HPLC-grade acetonitrile. The elution gradient established was 0% B for 5 min, from 0 to 10% B for 35 min, from 10 to 14.5% B for 5 min, from 14.5 to 19% B for 10 min, from 19 to 55% B for 10 min, isocratic 80% B for 3 min, and re-equilibration of the column, using a flow rate of 0.5 mL/min. Double online detection was carried out in the DAD using 280, 330, and 370 nm as preferred wavelengths and in a mass spectrometer (MS) connected to HPLC system via the DAD cell outlet. MS detection was performed in an API 3200 Qtrap (Applied Biosystems, Darmstadt, Germany) equipped with an ESI source and a triple quadrupole-ion trap mass analyzer. Nitrogen served as the curtain (20 psi) and collision gas (medium). The ion spray voltage was set at − 4500 V in the negative mode. The MS detector was programmed for recording in two consecutive modes: Enhanced MS (EMS) and enhanced product ion (EPI) analysis. EMS was employed to show full scan spectra, so as to obtain an overview of all of the ions in the sample. Settings used were: declustering potential (DP) − 40 V, entrance potential (EP) − 7 V, and collision energy (CE) − 20 V. EPI mode was performed in order to obtain the fragmentation pattern of the parent ion(s) in the previous scan using the following parameters: DP − 40 V, EP − 10 V, CE − 25 V, and collision energy spread (CES) 0 V. Spectra were recorded in negative ion mode between m/z 100 and 1800 [14]. The phenolic compounds were identified by comparing their retention time, UV–vis, and mass spectra with those obtained from standard compounds, when available. Otherwise, compounds were tentatively identified by comparing the obtained information with available data reported in the literature. To quantify the detected molecules, calibration curves were prepared for each compound using authentic standards (Extrasynthése S.A., Genay, France). The referred standards, as also available literature data, were used to identify the phenolic compounds. The acquisition and data processing were carried out with the Xcalibur® data system (Thermo Scientific, San Jose, CA, USA). The obtained results were presented in mg/g extract.
Antibacterial Activity
To determine the antibacterial capacity of the obtained extracts, different bacterial strains isolated from hospitalized patients (Hospital Center of Trás-os-Montes e Alto Douro (Vila Real, Portugal)) were tested, namely five Gram-negative bacteria: Escherichia coli (isolated from urine), Proteus mirabilis (isolated from wound exudate), Klebsiella pneumoniae (isolated from urine), Pseudomonas aeruginosa (isolated from expectoration) and Morganella morganii (isolated from urine); and three Gram-positive bacteria: Enterococcus faecalis (isolated from urine), Listeria monocytogenes (isolated from cerebrospinal fluid), and methicillin-resistant Staphylococcus aureus (MRSA) (isolated from expectoration). The assay was performed through the microdilution method using p-iodonitrotetrazolium chloride (INT) (Panreac Applichem-Barcelona, Spain) as a viability marker, as described by Pires et al. [15]. Different controls were prepared as follows: negative controls (one with Mueller–Hinton Broth (MHB)/Tryptic Soy Broth (TSB), another one with the extract, and the third with medium and antibiotic. As positive controls, for the Gram-negative bacteria, antibiotics, such as amikacin, tobramycin, amoxicillin/clavulanic acid, and gentamicin were used; for the Gram-positive bacteria, ampicillin and vancomycin were selected. Also, controls to ensure the bacterial viability were also prepared with medium and each of the bacterial strains The extracts were analyzed in the range 0.015–20 mg/mL, being the minimal inhibitory/bactericidal concentrations (MIC/MBC) determined and expressed in mg/mL.
Antioxidant Activity
The antioxidant capacity was determined by oxidative haemolysis inhibition (OxHLIA) and thiobarbituric acid reactive substances (TBARS) formation inhibition assays. The OxHLIA was performed according to Lockowandt et al. [16] in which the extracts (30–1000 µg/mL in PBS) were analyzed for their capacity to protect sheep erythrocytes from oxidation induced by free radicals generated by the thermal decomposition of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH). Trolox (7.81–250 µg/mL) was used as a positive control. The results were expressed as IC50 values (µg/mL) at Δt of 60 and 120 min, which translates to the extract concentration required to keep 50% of the erythrocyte population intact for 60 and 120 min.
Regarding the TBARS assay, the procedure was carried out according to Gómez-Mejía et al. [17] in which the capacity of the extracts to inhibit the lipidic oxidation is evaluated through the inhibition of TBARS formation, which is monitored as malondialdehyde (MDA)- thiobarbituric acid (TBA) complexes at 532 nm (Specord 200 spectrophotometer, Analytik Jena, Jena, Germany). The results were given as IC50 values, meaning the extract concentration (mg/mL) providing 50% of antioxidant activity. Trolox was used as a positive control.
Cytotoxicity
In order to determine the cytotoxicity of the developed extracts in tumor and also in non-tumor cell lines, they were tested against four different human tumour cell lines (MCF-7: breast adenocarcinoma; NCI-H460: non-small-cell lung cancer; AGS gastric adenocarcinoma and CaCo-2 colorectal adenocarcinoma). The cytotoxicity for non tumor cells was evaluated in porcine liver cells (assigned as PLP2), and kidney cells of the African green monkey (assigned as VERO). These analyses were carried out through the sulforhodamine B method [18]. The extracts were tested in a concentration range of 400 to 1.56 µg/mL. The results were expressed in GI50 values (concentration that inhibits 50% of cell growth), in µg/mL. The positive control used was ellipticine.
Anti-inflammatory Activity
For this assay, mice macrophage cell lines (RAW264.7), were used, according to the procedure reported by Taofiq et al. [19]. The extracts were tested at different concentrations in the range of 400 to 1.56 µg/mL. The activity was based on the extract’s capacity to inhibit the production of NO, therefore obtaining an active concentration with anti-inflammatory capacity. NO production was determined by measuring the absorbance at 540 nm in a microplate reader (Bio-Tek Instruments, ELX800) against a calibration curve and using dexamethasone as the positive control.
Anti-tyrosinase Activity
The obtained extracts were evaluated for their capacity to inhibit the tyrosinase enzyme. The assay was carried out as described by Moonrungsee et al. [20]. This approach is based on the mushroom tyrosinase oxidation of L-DOPA to o-dopaquinone. The extracts were dissolved in EtOH (50%) in a concentration range from 250 to 62.5 µg/mL. The percentage of tyrosinase inhibition was calculated as follows: [(ABlank − ASample)/ABlank] × 100. The EC50 values, which represent the extract concentration responsible for 50% tyrosinase inhibition, were calculated based on a calibration curve (tyrosinase inhibition percentage versus extract concentration). Kojic acid was used as the positive control.
Statistical Tools
All the assays were performed in triplicate, and the results were expressed as mean ± standard deviation (except for antimicrobial activity). Data were analyzed using an analysis of variance (ANOVA) followed by a Tukey’s test for homoscedastic samples and a Tamhane T2 for non-homoscedastic samples. A significance of 0.05 was applied for all analyses throughout the whole manuscript.
Results and Discussion
Profile in Phenolic Compounds
The phenolic composition of the aerial parts extracts of V. myrtillus is presented in Table 1. The individual phenolic compounds were tentatively identified according to their retention time (Rt), maximum absorbance wavelength (λmax), pseudomolecular ion ([M−H]–), and respective fragmentation pattern (MS2). It was possible to identify fourteen phenolic compounds: six phenolic acids (caffeic acid derivatives), four flavonoids (flavones and flavonols), and four procyanidins.
Peaks 1 and 5 ([M − H]– at m/z 353), 2 and 4 ([M − H]– at m/z 707), and 12 ([M − H]– at m/z 515) were assigned as caffeoylquinic acids, caffeoylquinic acids dimers, and dicaffeoylquinic acid, respectively. These identifications were performed considering the hierarchical keys previously reported by Clifford et al. [21]. Caffeoylquinic acid derivatives were also identified in V. myrtillus leaves by Bujor et al. [22]. Peak 3 ([M−H]– at m/z 341) indicated that it corresponds to a caffeic acid derivative bearing one hexosyl residues (− 162 u), being tentatively assigned to a caffeic acid hexoside. Both compounds, caffeic acid, and caffeoylquinic acid derivatives were also previously identified in blackberry and raspberry leaves [23].
Peaks 6–9 revealed a [M−H]– at m/z 863 and 1153, with a λmax around 276–280 nm, which is characteristic of proanthocyanidins, being assigned as procyanidins trimers and tetramers, respectively. According to Bujor et al. [22], epicatechin or catechin-based oligomeric flavanols involve various A-type or B-type dimeric, trimeric, and tetrameric forms that are normally present in blueberries leaves and stems. Nevertheless, these compounds were identified as procyanidins trimers and tetramers.
Among the four remaining flavonoids, peaks 10–14, they were identified as quercetin-3-O-rutinoside, quercetin-3-O-glucoside, luteolin di-6,8-C-hexoside, and luteolin-6-C-glucoside, respectively, after testing the coelution with their corresponding standards. Their MS data also corroborate the positive identification. These compounds have been described in blueberry leaves by Hokkanen et al. [24].
Considering the 14 phenolic compounds detected in the different extracts, their quantities varied considerably with the applied technique. Still, the most abundant phenolic compound, a dimer of caffeoylquinic acid, was present at higher statistically significant amounts in the extracts produced by maceration and UAE. This is a quite interesting finding, due to the fact that the two techniques are very different in terms of extraction time and applied energy. Maceration implies long times to be completed but does not demand high energy input to the extractive system, apart from a gentle stirring, while the UAE requires short extraction periods, but demands significant energy input, namely in the form of ultrasonic waves. The extract similarity in this specific phenolic compound could be explained by its high amounts in the sample or also by its location in the sample, in tissues that do not need more energetic techniques to increase its extraction [25], and both long time and high energetic methods promote its extraction. Most phenolics, including caffeoylquinic acids, accumulate in vacuoles or apoplasts, suggesting that in these organs whose tissue architecture is not complete, these compounds are easier extracted. Considering maceration, it was the technique giving rise to the highest statistically significative amounts for five individual phenolic compounds: caffeoylquinic acid dimer; 3-O-caffeoylquinic acid; procyanidin tetramer; luteolin di-6,8-C-hexoside and procyanidin trimer, while the lowest amounts were found in exequo for the infusion, maceration and ultrasound extracts, with four individual phenolic compounds: 3,5-O-dicaffeoylquinic acid; procyanidin tetramer; caffeic acid hexoside and quercetin-3-O-rutinoside showing the lowest contents. Considering the groups of polyphenols, interestingly, the technique conducting to the highest amounts for total phenolic acids; total flavan-3-ol, and total phenolic compounds, was the infusion. This is explained by the moderate extractive capacity of the infusion (probably due to the solvent heating), which, due to the average extraction capacity for each molecule, concomitantly with the low destruction, adds up to obtain an overall high amount. The UAE might be penalized for the high amount of added energy to the system, destroying some polyphenols. On the other hand, maceration could be penalized by the long extraction time, which also promotes the destruction of some polyphenols. Decoction showed no statistical difference from infusion for the total phenolic compounds, while the highest amount of total “other” flavonoids was sought for maceration. To achieve a higher number of polyphenols, the recommended technique is infusion, while maceration is recommended to extract some of the most abundant phenols present in the sample.
Bioactive Potential
Antibacterial Activity
The antibacterial properties were studied through the microdilution method against pathogenic bacteria and the results are displayed in Table 2. All the extracts presented inhibition capacity (MIC) against most of the tested bacterial strains, but without the capacity to effectively kill these bacteria. In general, a MIC of 10 to 20 mg/mL for all the tested extracts was needed to inhibit the analyzed strains. Nevertheless, a very promising result was achieved against MRSA, a serious pathogenic bacterial strain resistant to ampicillin, for which a MIC of 1.25 mg/mL was obtained for the extracts produced by UAE and maceration, and a MIC of 5 mg/mL was achieved for the extracts of decoction and infusion methods. Therefore, maceration and UAE extracts were the most active against these microorganisms, exhibiting the lower MIC values. Compared to the positive controls, namely with ampicillin, all the extracts presented similar activity, especially against Gram-negative bacteria. For example, to inhibit K. pneumoniae a MIC of 10 mg/mL of ampicillin was needed, with the same MIC value presented by all the analyzed extracts. Also, a MIC of 20 mg/mL of ampicillin was obtained to inhibit M. morganii, while a MIC of 10 mg/mL for all the extracts was obtained, highlighting the potential of the blueberry leaf extracts in this action, given the exponential growth of bacterial resistance to common antibiotics.
The microbiological quality of cosmetic products is one of the most important parameters to guarantee their safe use, and, to control the microbial growth, artificial preservatives are usually added. Since the obtained extracts can act similarly to the antibiotics, which are overused giving rise to bacteria resistance, they offer the potential to act as substitutes. Moreover, antibiotics use is very restrictive because in certain amounts they can become toxic to human health, highlighting also the benefits of developed extracts. Therefore, new challenges arise for researchers, namely the development of new products that have anti-microbial properties and at the same time, present no toxicity to the consumer [26].
Methanolic extracts of Vaccinium myrtillus L. berries were analyzed regarding their antimicrobial effect, with the authors describing an activity weaker than the one obtained in the present work, by presenting higher MIC and MBC values for the Gram-positive bacteria. Concerning Gram-negative strains, a MIC of 126 mg/mL was needed against K. pneumoniae, and to inhibit E. coli, P. mirabillis, and P. aeruginosa a MIC of 31.5 mg/mL was obtained. P. mirabillis showed the lower MBC value of 63 mg/mL, while for E. coli, K. pneumoniae, and P. aeruginosa an MBC of 126 mg/mL was needed. Regarding the Gram-positive studied strains, both E. faecalis and MRSA presented a MIC of 63 mg/mL [27].
In other studies, [28], the berries’ aqueous extracts presented MICs for Gram-negative strains, of 25 mg/mL (expressed as 2.5% (w/v)) for E. coli and 50 mg/mL (expressed as 5% (w/v)) for P. aeruginosa. These authors reported that L. monocytogenes was inhibited with a concentration of 12.5 mg/mL (expressed as 1.25% (w/v)) while in this work, UAE extract presented a result of 10 mg/mL against the same bacteria. Regarding MBC results, all the studied extracts presented a bacteriostatic effect. The authors Khalifa et al. [28] obtained better results against E. coli and L. monocytogenes, with 25 mg/mL (presented as 2.5% (w/v)) for both bacterial strains.
Maceration acetone–water blueberry leaf extract´s anti-microbial activity were studied by the microdilution method. The authors observed a significant direct biocidal activity of the extracts from V. myrtillus leaves on the Gram-positive bacteria strain S. aureus [29]. studied. In another work, blueberry leaves were extracted by UAE with ethanol–water as a solvent and their antimicrobial activity was studied [30]. The microdilution method was applied to S. aureus, E. faecalis, P. aeruginosa, Klebsiella pneumonia, and E. coli. Extracts presented high antimicrobial activity and were mostly strain-dependent.
Antioxidant Activity
The antioxidant potential of the different blueberry aerial parts extracts was evaluated according to the OxHLIA and TBARS methods (Table 3). Regarding the OxHLIA assay, at 60 min, the decoction extract presented the highest capacity, with a lower IC50 value (20 μg/mL). Relatively to TBARS results, the most efficient extracts were the decoction and maceration ones, with the same IC50 result of 8 μg/mL. All the extracts presented low IC50 values, similar to the results obtained for Trolox, the tested positive control. Previously to this study, Schiavon et al. [11] reported that blueberry extract has an antioxidant capacity of 16.71 μg/mL, equivalent to vitamin C, through the DPPH assay. Overall, at 60 min, decoction and maceration extracts showed the best IC50 values, thus, the highest antioxidant activity, while the infusion and UAE ones showed statistically lower values. At 120 min, the infusion extract showed the best antioxidant capacity, followed by the decoction and maceration in exequo, and finally by the UAE extract, which presented the lower antioxidant activity. This antioxidant behavior over time could be explained by the protective action of the antioxidant compounds present in the extracts, including the short-term and long-term reaction kinetics and the rate at which antioxidants react with the radicals. So, while some antioxidants may react quickly and be depleted in the reaction system, others may offer prolonged antioxidant protection over time (such as what happened for the infusion). Therefore, this bioassay allowed a distinction between short-term and long-term antioxidant protection, hence the results were presented for two Δt. Once again, the high energy input of the UAE [31] seemed to not promote the extraction of the most bioactive molecules, or even decompose these compounds during the extraction process.
As observed in Table 3, the OxHLIA results were given at two Δt values since plant extracts, due to their complexity as mixtures of various antioxidant compounds, are capable of interacting with each other and offering protection during different periods of time. Interestingly, the infusion extract, although not showing the best result for 60 min, was the most efficient in protecting the erythrocyte membranes for longer periods of exposure to the free radicals generated in the system. For the TBARS assay, which comparatively with the OxHLIA test relies on different antioxidant mechanisms, only the infusion extract was statistically different from the extracts obtained by the other extraction techniques, showing a slightly lower ability to prevent the formation of TBARS in vitro.
DPPH Free-radical–scavenging activity was a method previously used by several authors to determine the antioxidant activity of blueberry leaf extracts. Stefănescu et al. [30], studied six cultivars UAE ethanol–water extracts, and described these extracts as good sources of antioxidants. Other authors using microwave citric acid aqueous solution leaf extracts observed that the antioxidant activity showed interdependency to TPC results [22]. In another report, that studied the maceration ethanol–water and acetone–water extractions, and evaluated the antioxidant activity, the authors concluded that crude extracts of blueberry leaves exhibited strong antioxidant properties regarding DPPH and TBARS methods [32]. Also, using UA acidified methanol extraction, other scientific study analised seventy-three different leaf cultivars that presented strong in vitro antioxidant capacities by DPPH, ABTS, FRAP and ORAC methods [8].
Cytotoxic and Anti-inflammatory Properties
Considering the anti-inflammatory assay, the UAE showed the lowest IC50 values, thus, the higher activity, followed by the infusion extract. The least effective technique to extract anti-inflammatory compounds was maceration extraction. All the extracts presented cytotoxic activity against the tumor cell lines, being the NCI-H460 cells, the most sensible ones to the tested samples, highlighting the activity of UAE with IC50 values of 118 ± 5 µg/mL.
In a scientific study that investigated the cytotoxicity of acetone–water blueberry leaf extract obtained by maceration, using an in vitro method with mouse fibroblasts line to estimate the IC50, the authors reported that plant-derived extracts exhibited lower toxicity than phytochemicals used as controls [29] (Table 4).
Appositively to the antioxidant assays, the UAE seemed to be the best technique to obtain compounds with antitumor and anti-inflammatory activity, exhibiting the best results in all assays, while decoction was the least effective technique. No cytotoxicity was sought for both the VERO and PLP2 cell lines, assuring the safety of the obtained extracts.
Anti-tyrosinase Activity
Tyrosinase is the rate-limiting enzyme in the pathway leading to melanin biosynthesis, and effective inhibitors of this enzyme are used in cosmeceutical formulations to suppress hyperpigmentation [33]. In the present work, the tyrosinase inhibitory effects of the different extracts from V. myrtillus are displayed in Fig. 1. The maceration extract presented the strongest tyrosinase inhibitory effect (more than 50% inhibition) with an IC50 value of 221 ± 3 µg/mL. It also appeared that the decoction, UAE, and infusion extracts presented tyrosinase inhibition up to 19%, 37%, and 30%, respectively, at the highest tested concentration (250 µg/mL) but could not be considered active enough since a 50% inhibition against the tyrosinase enzyme was not achieved.
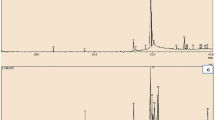
Phenolic profile of V. myrtillus recorded at 280 nm. (A) Decoction; (B) Infusion; (C) Maceration; (D) UAE. For peak, numbers refer to Table 1
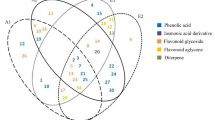
Kojic acid is a well-known tyrosinase inhibitor used in many topical formulations. Even though there are many challenges regarding its long-term clinical effectiveness, it is still commonly used as a reference compound in the tyrosinase inhibition assay [34]. In the present work, kojic acid presented a tyrosinase inhibition effect characterized by a very low IC50 value (5.84 ± 0.1 µg/mL). Nevertheless, these results showed that the type of used extraction method, besides significantly influencing the bioactive composition of the extracts, also impacted the bioactive properties. There is enormous evidence reporting a positive correlation between tyrosinase inhibition and the total phenolic content. There is a longstanding interest in naturally occurring caffeoylquinic acid derivatives due to their widely reported biological properties. In vitro studies have shown that caffeoylquinic acid derivatives, including 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, and 5-O-caffeoylquinic acid at lower concentrations, effectively inhibited tyrosinase activity and melanin biosynthesis [35]. Considering the tested extracts, which were abundant in phenolic acids and related compounds, the observed anti-tyrosinase effect might be attributed to the contribution of this class of compounds (Fig. 2).
Conclusions
The huge amounts of biowastes generated by the agri-food industries, which are constantly discarded in land fields, are considered a serious environmental threat. In line with well-established priorities by several governmental entities, this work intended to transform discarded biowastes from the production of blueberries, transforming them into valuable raw materials to be explored as sources of bio-based bioactive molecules, which are in constant demand by the industry. According to the obtained results crossing the different types of extractions, all the applied technologies allowed to obtain the target molecules, namely phenolic compounds, which revealed bioactive potential according to the performed assays. It should be highlighted that, e.g., the infusion technique can be used to obtain high levels of phenolics, but maceration allowed the highest yields in some of the major compounds. Regarding the bioactivity, all the extracts were able to exert biological function, highlighting the UAE and maceration extracts that revealed a strong capacity to inhibit MRSA, a multiresistant bacterial strain. Also notable is the performance of all the extracts that presented an antibacterial activity similar to the common antibiotic ampicillin. The extracts also presented antioxidant properties and anti-tyrosinase capacity, positively correlated with the presence of caffeoylquinic acid derivatives. This work resulted in important scientific evidence about the potential of blueberry aerial parts as raw materials for bioactive molecules obtainment, which can provide the industry sectors with natural agents, compliant with the constant demand of, e.g., natural preservatives, by the cosmetic and food industries.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Nestby, R., Percival, D., Martinussen, I., Opstad, N., Rohloff, J.: The European blueberry (Vaccinium myrtillus L.) and the potential for cultivation. Eur. J. Plant Sci. Biotechnol. 5, 5–16 (2011)
Neamtu, A.A., Szoke-kovacs, R., Mihok, E., Georgescu, C., Turcus, V., Olah, N.K., Frum, A., Tita, O., Neamtu, C., Szoke-kovacs, Z., Cziaky, Z., Mathe, E.: Bilberry (Vaccinium myrtillus L.) extracts comparative analysis regarding their phytonutrient profiles, antioxidant capacity along with the in vivo rescue effects tested on a drosophila melanogaster high-sugar diet model. Antioxidants 9, 1–33 (2020). https://doi.org/10.3390/antiox9111067
Park, K., Cook, R.: Blueberries: is supply developing more rapidly than demand? (2018)
FAOSTAT: Production share of Blueberries by region, www.fao.org
Ispiryan, A., Viškelis, J.: Advances in agriculture, horticulture and entomology valorisation of raspberries by-products for food and pharmaceutical industries. Adv. Agric. Hortic. Entomol. 2019, 1–6 (2019)
Mirabella, N., Castellani, V., Sala, S.: Current options for the valorization of food manufacturing waste: a review. J. Clean. Prod. 65, 28–41 (2014). https://doi.org/10.1016/j.jclepro.2013.10.051
Ferlemi, A.V., Lamari, F.N.: Berry leaves: an alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants (2016). https://doi.org/10.3390/antiox5020017
Wu, H., Chai, Z., Hutabarat, R.P., Zeng, Q., Niu, L., Li, D., Yu, H., Huang, W.: Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 122, 548–560 (2019). https://doi.org/10.1016/j.foodres.2019.05.015
Rojas, J., Londoño, C., Ciro, Y.: The health benefits of natural skin uva photoprotective compounds found in botanical sources. Int. J. Pharm. Pharm. Sci. 8, 13–23 (2016)
Weber, S., Saliou, C., Packer, L.: Handbook of Cosmetic Science and Technology. Marcel Dekker Inc, New York (2001)
Schiavon, D., et al.: Multifunctional cosmetic containing blueberry and tinosorb M@-loaded microparticles improves sunscreen performance. Adv Pharm. Bull. 9(2), 241–248 (2019). https://doi.org/10.15171/apb.2019.027
Heleno, S.A., Diz, P., Prieto, M.A., Barros, L., Rodrigues, A., Barreiro, M.F., Ferreira, I.C.F.R.: Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 197, 1054–1063 (2016). https://doi.org/10.1016/j.foodchem.2015.11.108
Bessada, S.M.F., Barreira, J.C.M., Barros, L., Ferreira, I.C.F.R., Oliveira, M.B.P.P.: Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: an underexploited and highly disseminated species. Ind. Crops Prod. 89, 45–51 (2016). https://doi.org/10.1016/j.indcrop.2016.04.065
Guimarães, R., Barros, L., Dueñas, M., Carvalho, A.M., Queiroz, M.J.R.P., Santos-Buelga, C., Ferreira, I.C.F.R.: Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 141, 3721–3730 (2013). https://doi.org/10.1016/j.foodchem.2013.06.071
Pires, T.C.S.P., Dias, M.I., Barros, L., Calhelha, R.C., Alves, M.J., Oliveira, M.B.P.P., Santos-Buelga, C., Ferreira, I.C.F.R.: Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 105, 580–588 (2018). https://doi.org/10.1016/j.foodres.2017.11.014
Lockowandt, L., Pinela, J., Roriz, C.L., Pereira, C., Abreu, R.M.V., Calhelha, R.C., Alves, M.J., Barros, L., Bredol, M., Ferreira, I.C.F.R.: Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: the blue flowers and the unexplored non-edible part. Ind. Crops Prod. 128, 496–503 (2019). https://doi.org/10.1016/j.indcrop.2018.11.059
Gómez-Mejía, E., Roriz, C.L., Heleno, S.A., Calhelha, R., Dias, M.I., Pinela, J., Rosales-Conrado, N., León-González, M.E., Ferreira, I.C.F.R., Barros, L.: Valorisation of black mulberry and grape seeds: chemical characterization and bioactive potential. Food Chem. 337, 127998 (2021). https://doi.org/10.1016/j.foodchem.2020.127998
Abreu, R.M.V., Ferreira, I.C.F.R., Calhelha, R.C., Lima, R.T., Vasconcelos, M.H., Adega, F., Chaves, R., Queiroz, M.J.R.P.: Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2- carboxylate derivatives: in vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 46, 5800–5806 (2011). https://doi.org/10.1016/j.ejmech.2011.09.029
Taofiq, O., Calhelha, R.C., Heleno, S., Barros, L., Martins, A., Santos-Buelga, C., Queiroz, M.J.R.P., Ferreira, I.C.F.R.: The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 76, 821–827 (2015). https://doi.org/10.1016/j.foodres.2015.07.044
Moonrungsee, N., Shimamura, T., Kashiwagi, T., Jakmunee, J., Higuchi, K., Ukeda, H.: Sequential injection spectrophotometric system for evaluation of mushroom tyrosinase-inhibitory activity. Talanta 101, 233–239 (2012). https://doi.org/10.1016/j.talanta.2012.09.015
Clifford, M.N., Johnston, K.L., Knight, S., Kuhnert, N.: Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 51, 2900–2911 (2003). https://doi.org/10.1021/jf026187q
Bujor, O.C., le Bourvellec, C., Volf, I., Popa, V.I., Dufour, C.: Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 213, 58–68 (2016). https://doi.org/10.1016/j.foodchem.2016.06.042
Pavlović, A., Papetti, A., Zagorac, D.ČD., Gašić, U.M., Mišić, D.M., Tešić, ŽL., Natić, M.M.: Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 87, 304–314 (2016). https://doi.org/10.1016/j.indcrop.2016.04.052
Hokkanen, J., Mattila, S., Jaakola, L., Pirttilä, A.M., Tolonen, A.: Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), Bilberry (Vaccinium myrtillus L.) and Hybrid Bilberry (Vaccinium x intermedium Ruthe L.) Leaves. J. Agric. Food Chem. 57, 9437–9447 (2009). https://doi.org/10.1021/jf9022542
Mondolot, L., la Fisca, P., Buatois, B., Talansier, E., de Kochko, A., Campa, C.: Evolution in caffeoylquinic acid content and histolocalization during Coffea canephora leaf development. Ann. Bot. 98, 33–40 (2006). https://doi.org/10.1093/aob/mcl080
Orus, P., Leranoz, S.: Current trends in cosmetic microbiology. Int. Microbiol. 8, 77–79 (2005). https://doi.org/10.2436/im.v8i2.9506
Miljković, V.M., Nikolić, G.S., Zvezdanović, J., Mihajlov-Krstev, T., Arsić, B.B., Miljković, M.N.: Phenolic profile, mineral content and antibacterial activity of the methanol extract of Vaccinium myrtillus L. Not. Bot. Horti Agrobot. Cluj Napoca. 46, 122–127 (2018). https://doi.org/10.15835/nbha46110966
Khalifa, H.O., Kamimoto, M., Shimamoto, T., Shimamoto, T.: Antimicrobial effects of blueberry, raspberry, and strawberry aqueous extracts and their effects on virulence gene expression in Vibrio cholerae. Phytother. Res. 29, 1791–1797 (2015). https://doi.org/10.1002/ptr.5436
Sadowska, B., Paszkiewicz, M., Podsȩdek, A., Redzynia, M., Rózalska, B.: Vaccinium myrtillus leaves and Frangula alnus bark derived extracts as potential antistaphylococcal agents. Acta Biochim. Pol. 61, 163–169 (2014). https://doi.org/10.18388/abp.2014_1939
Stefanescu, B.-E., Calinoiu, L., Ranga, F., Fetea, F., Vodnar, D.C., Cris, G., Mocan, A.: The chemical and biological profiles of leaves from commercial blueberry varieties. Plants 9, 1–19 (2020)
Antolovich, M., Prenzler, P.D., Patsalides, E., McDonald, S., Robards, K.: Methods for testing antioxidant activity. Analyst 127, 183–198 (2002). https://doi.org/10.1039/b009171p
Naczk, M., Amarowicz, R., Zadernowski, R., Pegg, R., Shahidi, F.: Antioxidant activity of crude phenolic extracts from wild blueberry leaves. Pol. J. Food Nutr. Sci. 12, 166–169 (2003)
Taofiq, O., González-Paramás, A.M., Martins, A., Barreiro, M.F., Ferreira, I.C.F.R.: Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics-a review. Ind. Crops Prod. 90, 38–48 (2016). https://doi.org/10.1016/j.indcrop.2016.06.012
Mann, T., Gerwat, W., Batzer, J., Eggers, K., Scherner, C., Wenck, H., Stäb, F., Hearing, V.J., Röhm, K.H., Kolbe, L.: Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 138, 1601–1608 (2018). https://doi.org/10.1016/j.jid.2018.01.019
Kim, H.H., Kim, J.K., Kim, J., Jung, S.H., Lee, K.: Characterization of caffeoylquinic acids from Lepisorus thunbergianus and their melanogenesis inhibitory activity. ACS Omega 5, 30946–30955 (2020). https://doi.org/10.1021/acsomega.0c03752
Acknowledgements
Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to CIMO (UIDB/00690/2020); the contract of L. Barros through the institutional scientific employment program-contract, the contracts of M. Carocho and S.A. Heleno (CEEC-IND/00831/2018 and CEECIND/03040/2017) through the individual scientific employment program-contract, and the PhD studentship granted to M. Añibarro-Ortega (2020.06297.BD). To the “La Caixa” Foundation and to FCT for the financial support through Project “Aquae Vitae — Thermal water as a source of life and health”, Promove Mobilizer programme. This work is also supported by MICINN for the Juan de la Cierva Formación contract for T. Oludemi (FJC2019-042549-I).
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plasencia, P., Heleno, S.A., Finimundy, T. et al. Recovery of High Valuable Bioactive Molecules from Vaccinium myrtillus L. Bioresidues. Waste Biomass Valor 14, 2873–2884 (2023). https://doi.org/10.1007/s12649-023-02042-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02042-6