Abstract
The aim of the study was to investigate the biological activity and chemical composition of Satureja kitaibelii Wierzb. ex Heuff. LC–PDA/MS analyses for the aqueous extracts (A1-stem, leaves and flowers, A2-leaves and flowers) and ethyl-acetate extracts (E1-stem, leaves and flowers, E2-leaves and flowers) obtained by ultrasound-assisted extraction enabled the identification of thirty-four compounds. Quantitative analysis revealed that the aqueous extract obtained from leaves and flowers was the richest in total phenolic acids (65.36 mg/g) and flavonoids (21.17 mg/g). The total polyphenol content was the highest in the aqueous extract obtained from leaves and flowers (274 ± 2.4 mg Gallic Acid equivalents/g). The best antioxidant activity was observed for the same extract using the DPPH (SC50 20 ± 10 µg/mL), ABTS (2.834 ± 0.02 mg Ascorbic Acid/g), FRAP (1.922 ± 0.03 mmol Fe2+/mg), and total reducing power tests (16.4 ± 1.0 mg Ascorbic Acid/g). Both ethyl acetate extracts were the most active against strains of Bacillus cereus and Micrococcus flavus (MIC 1.70–1.99 mg/mL and 1.99–3.41 mg/mL, respectively). They were more efficient against Aspergillus ochraceus (MFC 0.86 mg/mL) and towards HeLa cell lines. All the obtained results implied the good potential of the investigated extracts to be used as effective preservatives and functional ingredients in food products and dietary supplements.
Similar content being viewed by others
Introduction
Satureja kitaibelii Wiersb. ex Heuff. is an annual, aromatic species that grows in eastern Serbia on warm limestone rocks. It is also referred to as S. montana subsp. kitaibelii (Wierzb ex Heuff.) P.W. Ball or S. montana var. kitaibelii (Wierzb. ex Heuff.) Nyman1. This is one of the most popular species in Serbian traditional herbal medicine, usually consumed as winter savory ("rtanj tea")2 for treatment of respiratory diseases, urinary problems and digestive disorders, externally for skin and mucous inflammation, and as an aphrodisiac3,4,5. Essential oil analysis of S. kitaibelii and its antimicrobial activity was well documented6,7,8,9,10,11,12, while data on the composition and bioactivity of S. kitaibelii extracts are scarce. It was confirmed that aqueous-alcohol and ethanol extracts were rich in phenolic compounds and exhibited good antioxidant activity13,14,15. Methanol extract exhibited modest antimicrobial activity and antitumor activity against malignant cells3,16. The antimicrobial activity of aqueous extract obtained using various extraction techniques was also proven16,17. The examination of time-kill kinetics of S. kitaibelii subcritical aqueous extract showed its effects on the viability of the tested sensitive microorganisms in the initial contact phase, while prolonged contact time was needed for bacteriostatic effects17. However, there are still insufficient data on the chemical composition and biological activities of aqueous and ethyl acetate extracts of S. kitaibelii obtained using ultrasound-assisted extraction, as well as on the distribution of biactive molecules in their different aerial parts. A different distribution of bioactive components and antioxidants in different parts of plants is well documented18,19,20,21. In the current study, we analyzed different aerial parts of the plant in order to gain insight into the content and distribution of bioactive ingredients. The aim of the present study was to evaluate these extracts as a potential source of valuable phytochemicals and beneficial activity, which might promote S. kitaibelii as a functional food ingredient or food additive. In that regard, the examination carried out within the study encompassed the chemical composition, antioxidative, antimicrobial and cytotoxic activities of the ethyl acetate and aqueous extracts of the different aerial plant parts of S. kitaibelii obtained using ultrasound-assisted procedure.
Results
Phytochemical analysis
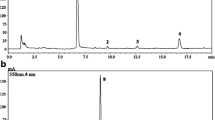
LC–PDA/MS analysis of the examined extracts of S. kitaibelii revealed the presence of phenolic acids and flavonoids, as well as of jasmonic acid derivatives and a diterpene. The obtained chromatographic and spectral data, i.e. retention times (Rt) on UV (350 nm) and MS chromatograms, λmax (nm), ESI–MS data (m/z), peak assignments, as well as the occurrence and content of the compounds in the extracts, are presented in Table 1. Chromatograms of the extracts are shown on Supplementary Fig. 1.
Qualitative composition of the extracts
Fourteen out of 34 identified compounds belonged to phenolic acids, all being derivatives of hydroxycinnamic acid (HCA), including simple acids, depsides of quinic acid, and especially HCA oligomers. Among the latter, the most abundant were dimeric rosmarinic acid (21) and hexameric clinopodic acid O (25) (Supplementary Fig. 1). Moreover, two trimers (7 and 19), two tetramers (22 and 24), and two additional hexamers of HCA (27 and 30) were detected in the extracts. The majority (14) of eighteen identified flavonoids were flavones, i.e. aglycons luteolin (29), apigenin (32), and genkwanin (34), as well as glycosides of luteolin (5, 8, 10, 11, 13, and 16) and apigenin (6 and 14) and their methylated derivatives (17, 23, and 26) (Table 1). The MS data indicated that the sugar moieties of glycosides were either monosaccharide (hexose or hexuronic acid) or disaccharide units (composed of two hexuronic acids or deoxyhexose and hexose). In addition, among luteolin glycosides, three were identified as those acylated with HCAs (10, 11, 16) based on their UV and MS spectral characteristics31,32,33. In the current study, we also detected the presence of one flavonol glycoside (9), two flavanone aglycons (28, 31), two hexosyl jasmonic acid derivatives (2, 20) and one diterpene (33) in the S. kitaibelii extracts.
Figure 1 shows the distribution of the identified constituents between the extracts. Nine compounds were common to all extracts: 12-hydroxyjasmonic acid 12-O-hexoside (2), caffeic acid (3), luteolin 7-O-diglucuronide (5), salvianolic acid K/isomer (7), luteolin 7-O-glucuronide (13), rosmarinic acid (21), Me-apigenin deoxyhexosyl-hexoside (23), clinopodic acid O (25), and genkwanin (34). By sharing 18 common compounds, ethyl acetate extracts E1 and E2 were the most similar in terms of qualitative composition compared to other extracts, emphasizing the selectivity of the extracting solvent, while the aqueous extracts shared 13 compounds.
Quantitative composition of the extracts
Quantitative chromatographic analysis revealed that extract A2 was the richest among the assayed extracts regarding the content of two major groups of metabolites, i.e. phenolic acids and flavonoids (Table 1, Supplementary Fig. 1), while other extracts contained substantially lower amounts of these specialized metabolites. The lowest amount of phenolic acids was observed in the extract A1, with HCA oligomers present only in trace amounts, while E2 was the poorest regarding flavonoids.
In the extract A2, the most abundant compounds were clinopodic acid O, rosmarinic acid, and luteolin 7-O-diglucuronide (35.627 mg/g, 15.046 mg/g and 7.955 mg/g, respectively). Rosmarinic acid and clinopodic acid O, together with Me-apigenin deoxyhexosyl-hexoside, were the dominant constituents of the extract E2 (5.719 mg/g, 1.164 mg/g, and 1.258 mg/g, respectively). In the extract A1, luteolin 7-O-diglucuronide, p-coumaric acid, and luteolin 7-O-glucuronide dominated over other compounds (2.569 mg/g, 1.223 mg/g, and 0.778 mg/g, respectively), while E1 contained 3,5-dicaffeoylquinic acid, rosmarinic acid, and chlorogenic acid as the major constituents (1.822 mg/g, 1.755 mg/g, and 1.317 mg/g, respectively). Moreover, the abundance of every single caffeoylquinic acid was the highest in the extract E1.
Total phenolics content
Differences between extracts in their total phenol content that were noticed due to different polarity of extracting solvents and using different plant parts and results are summarized in Table 2.
The antioxidant activity
The antioxidant activity of an extract was determined as free radical scavenging capacity and reducing power and measured by DPPH, ABTS, FRAP, and TRP assays. The results are presented in Table 3.
Antimicrobial activity of extracts of S. kitaibelii
Results of the antimicrobial activity of the investigated extracts, presented as a minimal inhibitory concentration for both bacteria and fungi are shown in Fig. 2a–d.
Antibacterial (a, b) and antifungal activity (c, d) of aqueous and ethyl acetate extracts of stems, leaves and flowers (A1 and E1, respectively) and leaves and flowers (A2 and E2, respectively) of S. kitaibelli. MIC—minimal inhibitory concentration, (mg/mL); STR—streptomycin; AMP—ampicillin; BIF—bifonazole; KET—ketoconazole. Detailed data on all microorganisms are given in the Supplementary Table 2.
MIC values for bacteria varied from 1.70 to 22.73 mg/mL, while MBC ranged from 3.41 to 45.45 mg/mL (data not shown). Ethyl-acetate and aqueous extract obtained from stem, leaves, and flowers (A1 and E1) were more effective against the majority of the tested bacteria than extracts made of leaves and flowers (A2 and E2). The ethyl acetate extracts (E1 and E2) exhibited lower MICs compared to the aqueous ones, indicating that the antibacterial properties of extracts also depended on solvent polarity which was also confirmed by13. Particularly sensitive to ethyl acetate extracts E1 and E2 were B. cereus (MIC 1.70 and 1.99 mg/mL, respectively) and M. flavus (MIC 3.41 and 1.99 mg/mL, respectively) among gram (+) bacteria. The most affected among tested gram (−) bacteria were E. cloacae by ethyl acetate extract of aerial parts (E1) and E. coli by ethyl acetate extract of leaves and flowers (E2). However, E. coli was the most resistant to the remaining examined extracts (A1, A2, and E1).
Cytotoxic activity of S. kitaibelii extracts
The in vitro cytotoxic activity of the investigated extracts is presented as IC50 values in Table 4.
The obtained data suggest that extracts of S. kitaibelii showed tumor-selective cytotoxic activity depending on the cell line type and extracting agent. Both ethyl acetate extracts showed stronger cytotoxic effects towards HeLa, PC-3, and MCF-7 cancer cell lines, as well as towards non-malignant cell lines, compared to the aqueous extracts. The HeLa cell line was the most sensitive to the presence of ethyl acetate extracts, PC-3 and healthy MRC-5 cells were moderately sensitive, whereas quite a poor effect was observed towards the MCF-7 cell line. Aqueous extracts A1 and A2 exhibited low cytotoxic activity only towards the MCF-7 cell line (Table 4).
Discussion
In the current study, phytochemical analysis by LC–PDA/MS was performed to investigate the bioactive compounds present in an aqueous and ethyl acetate extract of Satureja kitaibelii obtained from different aerial parts by ultrasound assisted extraction. LC/PDA-MS analysis revealed the presence of numerous compounds, some of which were identified for the first time. Derivatives of HCA are common for plants belonging to the family Lamiaceae. Rosmarinic acid is often one of the major polyphenol constituents in aerial parts of polar extracts of these plants, including plants of the genus Satureja34,35,36,37,38. Besides rosmarinic acid, Lamiaceae species produce higher HCA oligomers which contain up to 8 monomer units33,39. In several studies, trimeric33,40 and tetrameric oligomers41,42,43 were detected in the leaf or herb extracts of Satureja species, including previously analyzed ethanol extracts of S. kitaibelii15. On the other hand, to the best of our knowledge, clinopodic acids O and K, i.e. HCA hexamers, were reported only in the extracts of S. biflora, S. hortensis L., and previously analyzed ethanol extracts of S. kitaibelii15,33,44, in addition to a few other Lamiaceae species45,46. In the previously analyzed polar extracts of savory species, in addition to various flavone glycosides, a limited number of flavonol and flavanone glycosides were found, which is consistent with the currently analyzed aqueous extracts of S. kitaibelii. The presence of mono- and diglucuronides, glucosides, diglucosides, and rutinosides of luteolin, apigenin, and their hydroxylated and methylated derivatives was confirmed in several studies40,43,47,48. On the other hand, as far as we are aware, the presence of acylated glycosides, such as those detected in the present work, was rarely reported in plants of the genus Satureja33. The extraction conditions, as well as species of specific composition, might influence such behavior.
Several more observations could be made on the distribution of compounds concerning the starting plant material and solvent used for the preparation of the extracts. With the exception of luteolin glucuronides (5 and 13), which were detected in all extracts (although in low amounts in ethyl acetate extracts), other detected flavonoid hexuronides (6, 10, 11, 16) were found exclusively in aqueous extracts, which corresponds to their high hydrophilicity. In that regard, rosmanol/isomer (33) and all flavonoid aglycons (28, 29, 31, 32, and, 34), i.e. compounds of lower hydrophilicity, were common for both ethyl acetate extracts regardless of the starting plant material. It is also worth noting that, except for clinopodic acid O which was present in all extracts, all four other HCA tetramers and hexamers were detected only in extracts A2 and E2. On the other hand, caffeoylquinic acids were detected in A1, E1, or both. The presented results indicate that HCA oligomers are predominantly located in the leaves and flowers of S. kitaibelii, whereas caffeoylquinic acids are distributed mainly in the stems. However, an especially high content of rosmarinic acid was previously determined in flowers of Glechoma hederacea L. (Lamiaceae) in comparison to leaves and stems. At the same time, the highest amount of chlorogenic acid was found in the leaves of this herb (even though its distribution varied very slightly)49,50. All this suggests that the proportion of leaves, flowers, and stems in the starting material played an important role in the process of extraction in the current study. The obtained results indicate that the composition of extracts is highly influenced by both the starting plant material and the extracting agent. This is to some extent in contrast with the previous findings, that the contents of rosmarinic acid and clinopodic acid O in the ethanol extracts of leaves + flowers or stems + leaves + flowers of S. kitaibelii were much more dependent on the application of ultrasound during the extraction than on the starting plant material15. However, the solubility and extractability of metabolites present in S. kitaibelii in ethanol, which was used in the previous study, and water and ethyl acetate are much different, and thus substantially control the process of extraction.
The TPC values measured in this work (from both aqueous and ethyl acetate extracts) were comparable with some among those that were previously obtained14,15,51 (Table 2) but higher than the values for EtOAc and n-ButOH extracts of aerial parts in13.
The results of both assays for free radical scavenging ability of the investigated extracts of S. kitaibelii indicate the influence of the solvents used in this activity. Namely, aqueous extracts showed higher antioxidant activity than ethyl acetate ones, which is well correlated with the determined TPC. The difference in the activity of the aqueous and ethyl acetate extracts is a consequence of different compositions of obtained extracts in terms of the components that are extracted depending on the different properties of the extractants (Table 1). The aqueous extracts, especially from leaves and flowers (A2), were the most powerful in the neutralization of DPPH free radicals (IC50 20 µg/mL), which follows the most favorable composition of this extract in comparison to other investigated extracts concerning radical scavenging features of the present compounds. Ethyl acetate extracts of S. kitaibelii expressed lower values as compared to the aqueous ones (IC50 280–320 µg/mL). Those data are in agreement with the reported results of52, where S. kitabelii hot and cold aqueous extracts showed IC50 between 9.75–27.92 µg/mL.
Lopez-Cobo et al.14 presented results of DPPH activity of 116.36 µg/mL for 70% methanol extract. Ethanol extracts of S. kitaibelii aerial parts showed higher SC50 values—102.24 and 106.94 µg/mL15. Aqueous extracts obtained with ultrasonic extraction with a gradual increase in temperature show IC50 value of 0.087 mg/mL51. The scavenging effect of both aqueous extracts of S. kitaibelii on the ABTS was also more pronounced compared to the ethyl acetate. Aqueous extracts from leaves and flowers showed the strongest free ABTS radicals scavenging ability (2.834 mg AA/g), with values that are comparable to those obtained by51. To our knowledge, there is no comparable data on this activity of S. kitaibelii extracts.
Likewise, aqueous extracts showed better results on Fe3+ reducing activity, measured by the FRAP assay, but they are significantly lower than the values obtained in52: (221.74 and 271.88 µmol Fe/g). The highest reducing capacity was showed by an aqueous extract of leaf and flower (16.4 mg AA/g). TRP assay confirmed the results obtained from FRAP. There are no literature data to compare these values.
HCA oligomers, especially rosmarinic acid, were identified in considerable amounts in the examined extracts. Rosmarinic acid shows good antioxidant activity: the value obtained from FRAP was 16.54 mmol Fe2+/g and from DPPH assay SC50 3.90 μg/mL16.
In a limited number of studies, the activity of HCA oligomers was examined mainly in vitro, and less frequently in vivo. Good antioxidant activity was found for several higher oligomers53,54, as along with the ability to inhibit hyaluronidase55,56, the ability to inhibit metalloproteinase51, the ability to inhibit lipoxygenase54, antimicrobial activity57, anticancer activity58 etc. Antioxidant activity of another phenolic acid present especially in A2, the caffeic acid (CA), could be related to its iron-chelating property through the formation of iron-CA complexes inhibiting Fenton-induced oxidative damage by preventing the formation of free hydroxyl radicals59. Furthermore, good antioxidant activity was confirmed for the majority of other constituents. For example, chlorogenic acid (CGA), the most abundant isomer among caffeoylquinic acid in the E1 extract of S. kitaibelii, is an important and biologically active polyphenol, playing several important and therapeutic roles such as free radicals scavenger, antioxidant activity, antimicrobial, and many others60,61,62,63. Flavonoid luteolin and its glycosides possess a variety of pharmacological activities, including antioxidant, anti-inflammatory, antimicrobial, and anticancer activities17,64.
Although the antimicrobial activity of the essential oil of different Satureja species is well-known, the reports about the antimicrobial activity of extracts are limited. For the evaluation of antibacterial activity, this study used the bacteria causing foodborne diseases and human infections, which are particularly serious in hospitals. Some of them, such as P. aeruginosa, S. aureus, and E. coli are pan-drug-resistant and thus often associated with nosocomial infections. The need for finding new alternative antibacterial agents is therefore quite urgent65. The antibacterial effects of S. kitaibelii petroleum ether, chloroform, ethyl acetate, and n-butanol extracts were examined and the obtained MIC values ranged from 10 to > 100 mg/mL13.
The effects of S. kitaibelii methanol extract were examined in3 and the found MICs (0.32 to 1.25 mg/mL) and MBCs (2.50 to 5.00 mg/mL) were significantly lower than in the current study. The antibacterial activity of the subcritical water extract of S. kitaibeli was estimated in17 and the obtained MIC values ranged from 1.04 to > 33.3 mg/mL. Unlike in17, the results presented herein indicated no selectivity of S. kitaibelii extracts among gram (−) and gram (+) bacteria, which is congruent with the results of other researchers3.
The microfungi chosen for the evaluation of antifungal effects of S. kitaibelii extracts cause plant infections and deterioration of different materials and stored foods. However, the tested microfungi could be potentially hazardous for human health due to the production of mycotoxins, causing infections that especially affect immunosuppressed patients. The MICs for fungi were from 0.86 to 16.06 mg/mL, while MFCs were between 1.72 and 32.12 mg/mL (data not shown). Similar to antibacterial activity, E1 was more efficient in preventing fungal growth, especially against A. ochraceus (MIC/MFC 0.86/1.72 mg/mL). Unlike antibacterial activity, the aqueous extracts were more efficient in inhibiting fungal growth, particularly A.versicolor, A.niger, and P. verrucosum var cyclopium. The least potent antifungal agent was E2, especially on T.viride (MFC/MIC 32.12/16.06 mg/mL). There is very limited research on the antifungal properties of those S. kitaibelii extracts. However, S. kitaibelii methanol extract inhibited the growth of 6 micromycetes (MIC varied from 0.16 to 1.25 mg/mL) and two Candida species (MIC varied from 0.32 to 0.62 mg/mL)3. The anticandidial activity of S. kitaibelii aqueous extract was later confirmed in66. Moreover, S.cerevisiae and Aspergillus brasiliensis were also sensitive to S. kitaibelii aqueous extract66.
The antimicrobial properties of S.kitaibelii methanol extract were associated with the presence of rosmarinic acid in3, which is contradictory not only to results presented herein since its content was the highest in the low active aqueous extract of aerial parts, but also to the previous report on modest antimicrobial activity of this compound16. On the other hand, it was suggested in13 that other non-phenolic compounds could contribute to the antimicrobial activity of S. kitaibelii extracts. In this study, as positive control weused ampicillin, streptomycin, ketoconazole, and fluconazole, antibiotics/antimycotics that are commonly prescribed for the treatment of diseases caused by tested pathogens. The results presented herein showed lower antibacterial/antifungal activity of S.kitaibelii extracts than reference standards, which is in agreement with prior published results3. The antimicrobial activities of S. kitaibelii extracts were insufficiently studied so far, hence the presented study aimed to screen it using the microdilution method and to enable the basis for further examination using various methods for evaluation of antimicrobial properties to obtain more relevant results. However, the presented results indicate that examined S.kitaibelii extracts are rich in phenolics, which suggests that particularly influence on the antimicrobial response of extracts, as well as synergistic effects among them, require further study.
In the current study, both types of extracts contained phenolic compounds which previously showed cytotoxic activity in different assays. Several studies have shown that extracts rich in certain phenolic carboxylic acids and flavonoids exhibit cytotoxic activity against the different cell lines, both malignant and healthy, by various mechanisms43,59,67,68,69,70. The study70 has revealed the potential molecular mechanisms of phenolic acids and flavonoids and suggested great promising effects of phenolic acids and flavonoids against breast, colon, lung, and prostate cancers. On the other hand, extracts investigated in the present study were all abundant with phenolic compounds, but the sensitivity of the tested cell lines (except MCF-7) differed markedly depending on the extracting agent used for extract preparation. Additionally, the extract which was the most abundant with phenolic compounds (A2) was not the most active. The reason for this might lie in some differences in the composition which could not be detected through the applied methods, and in the fact that non-phenolic constituents are responsible for the good cytotoxic activity of ethyl acetate extracts. Furthermore, the polarity of compounds present in ethyl acetate extracts might be beneficial for better availability for the tested cell lines. In congruence with the presented results, less polar extracts, especially ethyl acetate extract of Origanum majorana showed more pronounced cytotoxicity towards MDA-MB-231 and HT-29 cell lines (IC50 30.90 ± 1.39 and 50.11 ± 1.44 µg/ml) in comparison to aqueous extract (IC50 69.18 ± 3.10 and 177.82 ± 4.07 µg/ml)71.
Following the obtained results on the cytotoxicity of ethyl acetate extracts, in a previous study, methanol extract of S. kitaibellii exhibited moderate activity against estrogen-dependent (MDA-MB-361) and estrogen-non-dependent (MDA-MB-453) breast cancer, colon cancer (LS174), and HeLa cell lines, as well as strong cytotoxic activity against human malignant melanoma cells (Fem-x), which was partly correlated with the tested activity of rosmarinic acid3. On the other hand, the low cytotoxic potential of aqueous extracts of related species S.subspicata and S.horvatii was previously noted in human lymphocytes in vitro37, as well as the low potential of S. Montana aqueous and aqueous ethanol extracts with IC50 values above 600 μg/ml against Caco-2, TR146 and HeLa cell lines42. Moreover, aqueous and DMSO extracts of S. subspicata and S. horvatii downregulated pro-apoptotic and upregulated anti-apoptotic genes of the Bcl-2, showing anti-apoptotic activity, which authors linked to the previously shown similar effects of rosmarinic acid. Similar anti-apoptotic effects, i.e. increased Bcl-2/Bax ratio, were observed for the methanol extract of S.hortensis, which was characterized by a high amount of rosmarinic acis71. These data, together with the previously confirmed low genotoxic potential of aqueous extracts of Satureja plants and rosmarinic acid as their dominant compound37, concur with safe usage of S. kitaibelii aqueous extracts, but this certainly needs to be better justified in the future.
Materials and methods
Plant material, extraction and LC–PDA/MS analysis
Aerial parts of Satureja kitaibelii were collected in August 2015, on Mt. Rtanj N 43.752697 E 21.87479, in eastern Serbia. The herb was collected by the botanist Slavica Grujić, in full compliance with all the rules for collecting herbs (at that time, the Ministry of Environmental Protection did not issue permits for collecting this plant, and the species was placed under protection only a few years ago). The plant was identified by Slavica Grujić, and a voucher specimen was deposited in the Herbarium of the Institute of Botany and Botanical Garden "Jevremovac", Faculty of Biology, University of Belgrade, Serbia (No. 17141, BEOU). To avoid destruction of the species population, only individual specimens were collected. A total of 1 kg of fresh plant material was collected (300 g of dried materials). The plant material was air-dried at room temperature in the shadow, deprived of wooden parts.
The air-dried material (10 g), containing stem, leaves, and flowers (sample 1), or leaves and flowers (sample 2) was extracted with 100 mL distilled water or ethyl acetate (polarity of water and ethyl acetate are 1.000 and 0.228, respectively). The extraction procedure was described previously15 and resulted in extracts E1 and E2 (obtained from samples 1 and 2, respectively, ethyl acetate), A1, and A2 (obtained from samples 1 and 2, respectively, aqueous). The obtained dried extracts were refrigerated at 4 °C before use.
Qualitative and quantitative chromatographic analysis of the extracts was carried out exactly as described in Gopcevic et al.15, on an Agilent 1260 Liquid Chromatograph equipped with an autosampler, a PDA detector, and coupled with a mass detector Agilent MSD 6100 with an electrospray ionization (ESI) source and a single quadrupole analyzer. Chromatographic separation was performed using a Zorbax SB-aq column (150 × 2.1 mm; particle size 3.5 μm) maintained at 27 °C. Extract solutions (3 μL of 5 mg/mL of ethanol) were injected while the pump was operating at a flow rate of 0.3 mL/min. Gradient elution was performed using mobile phase A—0.1% formic acid, and mobile phase B—acetonitrile, being at 0 min 10% B, 25 min 60% B, 30 min 95% B, and returning to the initial conditions during the following 2 min. Chromatograms were recorded at 210, 280, 320, 350, and 370 nm, whereas ESI MS spectra were obtained in negative mode, at the range 150–1150 m/z. The ion chamber was maintained at 350 °C, with nebulization under a nitrogen flow of 10 L/min, pressure of 30 psi, and capillary voltage of 3500 V. Signals were recorded by applying the fragmentor voltage of 100 V, as well as 250 V to obtain additional fragment ions. The identification of constituents was performed by comparison of ultraviolet (UV), mass spectrometry (MS), and retention time (Rt) data with those obtained for the standard compounds, as well as tentatively, i.e. by comparison of the obtained UV and MS spectra of constituents with the literature reports.
The quantification of phenolic acids and flavonoids was performed by external calibration after regression and correlation analysis, based on the PDA chromatographic data. Rosmarinic acid and chlorogenic acid were used as standard compounds for the determination of phenolic acids, luteolin 7-O-glucoside for flavone glycosides, while flavone aglycons were quantified using luteolin as the standard compound. All standard compounds used in LC–PDA/MS analysis of extracts were purchased from Carl Roth, except luteolin 7-O-diglucuronide, which was previously isolated from the aerial parts of Thymus pannonicus22.
The results of quantitative analysis are expressed as a mean from three independent analysis ± standard deviation. Data on the measuring conditions and obtained calibration curves, as well as on the limits of detection (LOD) and quantitation (LOQ), are presented in Supplementary Table S1.
Total Phenolic Content. Total phenolic content (TPC) was determined by the method of Singleton and Rossi23 with some modifications. To 0.2 mL of suitably pre-diluted extract, 1.8 mL of dH2O and 0.2 mL of Folin-Ciocalteu reagent were added sequentially. After five minutes 2 mL of 7% Na2CO3 were added, and then the mixture was diluted to up to 5 mL with dH2O. The reaction continued in the dark for 90 min. The absorbance was measured at 765 nm against dH2O. The standard calibration curve was plotted using gallic acid (1–200 µg/mL). The data were calculated according to gallic acid that was used for preparation of calibration curve.
The total phenolic content was expressed as gallic acid equivalents per gram of dry extract (mg GA/g).
Antioxidant activity
Ferric reducing antioxidant power (FRAP) assay
The reducing power of extracts was determined using the ferric reducing ability of FRAP assay by Benzie and Strain24. This assay was based on the reducing power of a compound (antioxidant). A potential antioxidant will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+); the latter forms a blue complex (Fe2+/TPTZ), which increases the absorption at 593 nm. In brief, the FRAP reagent was prepared by mixing acetate buffer (200 µL, pH 3.6), a solution of 20 µL TPTZ and 20 µL FeCl3 at 10:1:1 volume ratio. The sample solution (200 µL) and the reagent (2.95 mL H2O and 1 mL FRAP) were mixed thoroughly and incubated at 37 °C. Absorbance was taken at 593 nm after 10 min. Standard calibration curve was prepared using different concentrations of FeSO4⋅7H2O. All solutions were freshly prepared. The results were expressed in µmol Fe2+/mg dried extract (µmol Fe2+/mg d.e.).
Scavenging effect on 2,2-diphenyl-1-picrylhydrazyl radical (DPPH)
Antioxidant activity of S. kitaibelii extracts was evaluated by the 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging method by Blois25. Extracts were diluted in the appropriate solvent, from which further dilutions were made in methanol and DPPH solution (0.04 mg/mL) was added. Samples were vigorously shaken and left for 30 min at room temperature in the dark. Absorbance was measured at 517 nm. All measurements were carried out in triplicate. Absorbance of the remaining DPPH radical in the sample (A1) was measured on 517 nm. Every sample and positive controls (ascorbic acid and BHA) concentration were done in triplicate and the same was done with blank probes, which were prepared to contain methanol instead of the investigated sample (blank absorbance A0).
The percentage of scavenging (RSC %) was calculated, as follows:
The extract concentration providing 50% of free radical scavenging activity (IC50) was calculated from the graph of radical scavenging activity (RSA) percentage against the extract concentration.
2-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) radical-scavenging activity (ABTS)
The ABTS method, based on the reduction of ABTS•+ radical in alcohol solution by antioxidants, was performed according to26. The ABTS radical cation was generated by the oxidation of ABTS with potassium persulfate (K2S2O8) and its reduction in the presence of hydrogen donating antioxidants was measured spectrophotometrically at 734 nm. The reaction mixture of 19.2 mg of ABTS was dissolved in K2S2O8 before the experiment. This solution was dissolved in distilled water to adjust working solution absorbance to 0.700 at 734 nm. The sample concentration was 1 mg/mL. The reaction mixture was prepared by mixing 100 µL of test sample and 4 mL ABTS. After 30 min’ incubation at 30 °C in a water bath, absorbance of the mixture was measured at 734 nm. The radical scavenging activity for each extract was determined on the basis of the linear calibration curve of ascorbic acid:
and expressed as mg ascorbic acid/g of dry extract (mg AA/g d.e.).
The total reducing power (TRP)- It was determined according to the method of Oyaizu27. The total reducing power assay measures the electron donating capacity of an antioxidant. The presence of reducers causes the conversion of the Fe3+/ferricyanide complex to the ferrous form, which serves as a significant indicator of its antioxidant capacity. The reducing capacity of different extracts was compared with ascorbic acid for the reduction of Fe3+ to Fe2+. In this assay, the color of the test solution changes to various shades of blue, depending on the reducing power of each compound. Therefore, measuring of the formation of Perls’ Prussian blue at 700 nm can monitor the Fe2+ concentration. One mL of the extracts was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide K3[Fe(CN)6] (2.5 mL, 1%). The mixtures were incubated at 50 °C for 20 min, after which trichloroacetic acid (10%, 2.5 mL) was added and the mixture was centrifuged. Finally, the upper layer (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 ml; 0.1%). The absorbance of the solution was measured at 700 nm. The blank was prepared with all the reaction agents without extract. Higher absorbance of the reaction mixture indicated that the reducing power is increased. Ascorbic acid was used as standard. The percent of increase in reduction power was calculated by using the following formula:
where Atest is the optical density of the test solution and Ablank is the optical density of the blank solution. The assays were carried out in triplicate and the results were expressed as mean values ± standard deviations as mg AA/g.
All absorbance readings were performed on UV-2600 UV–VIS spectrophotometer (Shimadzu, Japan). All analyses were done in triplicate.
Antimicrobial activity
The antibacterial activity was evaluated using a microdilution method as previously described in28 towards eight bacteria (in Supplementary Table S2): Bacillus cereus (clinical isolate), Micrococcus flavus ATCC10240 and Salmonella Typhimurium ATCC13311, Listeria monocytogenes NCTC7973, Enterobacter cloacae ATCC35030, Pseudomonas aeruginosa IBRSP001, Staphylococcus aureus ATCC6538, and Escherichia coli ATCC35210. The following microfungi were used for testing antifungal activity according to29 (in Supplementary Table S2): Aspergillus fumigatus (human isolate), A. versicolor ATCC11730, A. ochraceus ATCC12066, A. niger ATCC6275, Trichoderma viride IAM5061, Penicillium funiculosum ATCC36839, P. ochrochloron ATCC9112, and P. verrucosum var. cyclopium (food isolate). The examined extracts of S. kitaibelii were dissolved in 15% ethanol. Then, serial dilutions were made using Tryptic soy broth (TSB) (bacteria) and Malt extract broth (MEB) (microfungi) in 96-well microplates with a flat bottom. That was followed by incubation for 24 h at 37 °C with inoculated bacteria and for 72 h at 28 °C with inoculated microfungi. Next, the lowest concentrations that caused visible inhibition of bacterial/microfungi growth observed under binocular microscope were determined and defined as minimal inhibitory concentrations (MICs). MBCs/MFCs were determined by re-inoculation of 10 µL and 2 µL of samples, respectively, into the sterile broth. After incubation for another 24 h at 37 °C (bacteria) and for 72 h at 28 °C (microfungi), the lowest concentrations without visible growth that indicated 99.5% killing of the original inoculums were defined as MBCs/MFCs. Negative control in both assays was 15% ethanol. Streptomycin and ampicillin were used as positive controls for antibacterial activity, while bifonazole and ketoconazole were used for antifungal activity since they are commercially available and commonly used standard reference drugs.
Cytotoxic activity
Cell lines. For the in vitro evaluation of the cytotoxic activity of the tested extracts, the following was used: normal human embryonic lung fibroblast (MRC-5), human cervical adenocarcinoma (HeLa), human breast adenocarcinoma (MCF-7) and human prostate adenocarcinoma from bone metastasis (PC-3) cell lines obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were grown in RPMI-1640 medium supplemented with 3 mM L-glutamine, 100 μg/mL streptomycin, 100 IU/mL penicillin, 10% heat-inactivated (56 °C) fetal bovine serum and 25 mM Hepes adjusted to pH 7.2 with a bicarbonate solution. The cells were grown at 37 °C in an atmosphere of 5% CO2 and humidified air. Cells were obtained from the American Type Culture Collection (Manassas, VA, USA), while RPMI 1640, L-glutamine and Hepes were obtained from PAA (Pasching, Austria).
MTT assay for cell viability. For the evaluation of cytotoxicity of the tested extracts, the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide—MTT) was used, described previously in30. With this colorimetric assay, reductive activity of the cells is determined through conversion of the yellow tetrazolium dye (MTT) to purple formazan crystals by NAD(P)H-dependent oxidoreductase enzymes, present in viable cells. The degree of formazan concentration in the cells correlates to the degree of light absorption. Cells were seeded into 96-well plates by adding 7,000 cells per well for MCF-7; 5,000 cells per well for MRC-5 and PC-3; and 3,000 cells per well for HeLa. On the next day, after the adhesion, cells were treated with five different concentrations of the tested extracts (final concentration ranged from 12.5 μg/ml to 400 μg/ml). Stock solutions, prepared in dimethyl sulfoxide (DMSO), were diluted with a complete nutrient medium and applied to target cells. The treated cells were incubated for 72 h. After the addition of 20 µL of MTT solution (5 mg/mL in phosphate-buffered saline, PBS), samples were incubated for an additional 4 h at 37 °C in a humidified atmosphere of 5% CO2 (v/v). Subsequently, 100 mL of 100 g/L sodium dodecyl sulfate (SDS) were added. The absorbance (A) was measured at 570 nm 24 h later.
The obtained value, IC50, refers to the concentration of the compound that inhibits cell survival by 50% compared to the control. Each experiment with every cell line was performed in triplicate.
Statistical analysis
All the results are presented as mean ± standard deviations of three determinations. Statistical analyses were performed using the Student’s t test and one-way analysis of variance. Correlation between tested activities of various extracts was established using regression analysis at a 95% significance level (p ≤ 0.05). IC50 values were calculated by nonlinear regression analysis from the sigmoid dose–response inhibition curves. Statistica 6.0 software (2001, Stat Soft, Tulsa, OK, USA) was used to evaluate the p < 0.05 level (regarded as significant) and p < 0.01 (highly significant).
All methods were performed in accordance with the relevant guidelines and regulations.
Conclusion
The results of the current study highlighted the aqueous and ethyl acetate extracts of different aerial parts of Satureja kitaibelii Wierzb. ex Heuff. endemic plant as a reach source of active compounds, some of which are now extracted and detected for the first time. The aqueous extract of leaves and flowers was the richest source of two major groups of metabolities, i.e. phenolic acids and flavonoids. Dominant constituents, which could be considered as chemical markers, were hydroxycinnamic acid oligomers, rosmarinic acid and clinopodic acid O. All the examined extracts possessed considerable antioxidant activity, which was confirmed by four different assays. Regarding antibacterial activity, both ethyl acetate extracts showed the best results towards Bacillus cereus and Micrococcus flavus, whereas in case of other tested bacteria all the investigated extracts exhibited moderate to weak activity. In the presence of ethyl acetate extract of all aerial parts, the most sensitive fungus was Aspergillus ochraceus, while all other fungal strains were moderately susceptible to all tested extracts. Ethyl acetate extracts were cytotoxic against all tested cell lines, including healthy ones. On the other hand, aqueous extracts exhibited weak activity only towards MCF-7 cells. All the obtained results suggest a good potential of the investigated, especially aqueous, extracts of S. kitaibelii to be used as a source of new pharmaceuticals and healthcare products, as well as effective preservatives and functional ingredients in food products, dietary supplements, and other pharmaceutical products.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Šilić, Č. Monografija rodova Satureja L., Calamintha Miller, Micromeria Bentham, Acinos Miller i Clinopodium L. u flori Jugoslavije. (Zemaljski Muzej BiH, Sarajevo, 1979)
Zlatković, B., Bogosavljev, S., Radivojević, A. & Pavlović, M. Traditional use of the native medicinal plant resource of Mt. Rtanj (Eastern Serbia): Ethnobotanical evaluation and comparison. J. Ethnopharmacol. 151, 704–713 (2014).
Stanojkovic, T. et al. Cytotoxicity and antimicrobial activity of Satureja kitaibelii wierzb. Ex heuff (Lamiaceae). Digest J. Nanomat. Biostr. 8, 845–854 (2013).
Dodoš, T., Rajčević, N., Janaćković, P., Vujisić, L. J. & Marin, P. Essential oil profile in relation to geographic origin and plant organ of Satureja kitaibelii Wierzb. ex Heuff. Ind. Crop. Prod. 139, 111549 (2019).
Baraldi, M. Google Patents. Available online: 18, 1167–1178 https://patents.google.com/patent/US7976878B2/en. (Accessed on 30 May 2022) (2015).
Slavkovska, V., Jančić, R., Bojović, S., Milosavljević, S. & Djoković, D. Variability of essential oils of Satureja montana L. and Satureja kitaibelii Wierzb. ex Heuf from the central part of Balkan peninsula. Phytochemistry 57, 71–76 (2001).
Živković, M. et al. The antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff., Lamiaceae. Planta Med. 75, 1049–1049 (2009).
Kundaković, T. et al. Cytotoxicity and antimicrobial activity of the essential oil from Satureja montana subsp pisidica (Lamiceae). Nat. Prod. Commun. 9, 1934578X1400900437 (2014).
Mihajilov-Krstev, T. et al. Chemical composition and antimicrobial activity of Satureja kitaibelii essential oil against pathogenic microbial strains. Nat. Prod. Commun. 6, 1167–1172 (2011).
Djordjević, A., Palić, I., Stojanović, G., Ristić, N. & Palić, R. Chemical profile of Satureja kitaibelii Wierzb. ex Heuff. Essential oil: Composition of Satureja kitaibelii essential oil. Intern. J. Food Prop. 17, 10 (2014).
Aćimović, M., Pezo, L., Tešević, V., Čabarkapa, I. & Todosijević, M. QSRR Model for predicting retention indices of Satureja kitaibelii Wierzb. ex Heuff. essential oil composition. Ind. Crops Prod. 154, 112752 (2020).
Zheljazkov, V. D., Semerdjieva, I. B., Cantrell, C. L., Astatkie, T. & Aćimović, M. Phytochemical variability of essential oils of two Balkan endemic species: Satureja pilosa Velen. and S. kitaibelii Wierzb. ex Heuff. (Lamiaceae). Molecules 27, 3153 (2022).
Ćetković, G. S. et al. Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L. subsp. kitaibelii extracts. Int. J. Mol. Sci. 8(10), 1013–1027 (2007).
López-Cobo, A., Gómez-Caravaca, A. M., Švarc-Gajić, J., Segura-Carretero, A. & Fernández-Gutiérrez, A. Determination of phenolic compounds and antioxidant activity of a Mediterranean plant: The case of Satureja montana subsp. kitaibelii. J. Funct. Foods 18, 1167–1178 (2015).
Gopčević, K. et al. Phytochemical properties of Satureja Kitaibelii, potential natural antioxidants: A new insight. Plant Foods Hum. Nutr. 74, 179–184 (2019).
Šeregelj, V. et al. Modern green approaches for obtaining Satureja kitaibelii Wierzb. ex Heuff extracts with enhanced biological activity. J. Serb. Chem. Soc. 87, 1–7 (2022).
Aćimović, M. et al. In vitro antihyperglicemic, anti-inflammatory, and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. subcritical water extract. Ind. Crops Prod. 169, 113672 (2021).
Feduraev, P., Chupakhina, G., Maslennikov, P., Tacenko, N. & Skrypnik, L. Variation in phenolic compounds content and antioxidant activity of different plant organs from Rumex crispus L. and Rumex obtusifolius L. at different growth stages. Antioxidants (Basel) 8(7), 237–252 (2019).
Nantongo, J. S. et al. Variability of phenolic and alkaloid content in different plant parts of Carissa edulis Vahl and Zanthoxylum chalybeum Engl. BMC Res Notes 11, 125–132 (2018).
Chepel, V., Lisun, V. & Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants (Basel) 9(8), 926–945 (2020).
Ingle, K. P. et al. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 6(1), 32–36 (2017).
Arsenijević, J. et al. Bioactivity of herbal tea of Hungarian thyme based on the composition of volatiles and polyphenolics. Ind. Crops Prod. 89, 14–20 (2016).
Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 16, 144–158 (1965).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Blois, M. S. Antioxidant determination by the use of stabile free radical. Nature 181, 1199–1200 (1958).
Rice-Evans, C. & Miller, N.J. Total antioxidant status in plasma and body fluids. In Methods in Enzymology, Vol. 234, 279–293 (Academic Press, 1994).
Oyaizu, M. Studies on product of browning reaction prepared from glucoseamine. Jpn. J. Nutr. 44, 307–315 (1986).
Soković, M., Glamočlija, J., Marin, P. D., Brkić, D. & Van Griensven, L. J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 15, 7532–7546 (2010).
Kostić, M. et al. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 8, 3239–3249 (2017).
Mosmann, T. Rapid colorimetric assays for cellular growth and survival: Application to proliferation and cytotoxic assays. J. Immunol. Methods 65, 55–63 (1983).
Marczak, Ł, Stobiecki, M., Jasiński, M., Oleszek, W. & Kachlicki, P. Fragmentation pathways of acylated flavonoid diglucuronides from leaves of Medicago truncatula. Phytochem. Anal. 21, 224–233 (2009).
Stochmal, A. & Oleszek, W. Effect of acylation of flavones with hydroxycinnamic acids on their spectral characteristics. Nat. Prod. Commun. 2, 571–574 (2007).
Moghadam, S. E. et al. Metabolite profiling for caffeic acid oligomers in Satureja biflora. Ind. Crops Prod. 76, 892–899 (2015).
Janicsák, G., Máthé, I., Miklóssy-Vári, V. & Blunden, G. Comparative studies of the rosmarinic and caffeic acid contents of Lamiaceae species. Biochem. Syst. Ecol. 27, 733–738 (1999).
Chkhikvishvili, I. et al. Rosmarinic acid-rich extracts of summer savory (Satureja hortensis L.) protect Jurkat T cells against oxidative stress. Oxid. Med. Cell. Long. 2013, 456253 (2013).
Cabana, R., Silva, L., Valentão, P., Viturro, C. & Andrade, P. Effect of different extraction methodologies on the recovery of bioactive metabolites from Satureja parvifolia (Phil.) Epling (Lamiaceae). Ind. Crops Prod. 48, 49–56 (2013).
Čakar, J. et al. Satureja subspicata and S. horvatii extracts induce overexpression of the BCl-2 family of anti-apoptotic genes and reduce micronuclei frequency in mice. Nat. Prod. Comm. 13, 723–726 (2018).
Casoni, D., Olah, N., Soran, M.-L. & Codruta, C. Comparison of different extraction techniques for the evaluation of polyphenols content in Summer savory extracts. Studia Universitatis Babeș-Bolyai Chemia 62, 45–56 (2017).
Pereira, O. & Cardoso, S. Overview on Mentha and Thymus polyphenols. Curr. Anal. Chem. 9, 382 (2013).
Šimunović, K. et al. In vitro effect of the common culinary herb winter savory (Satureja montana) against the infamous food pathogen Campylobacter jejuni. Foods 9, 537 (2020).
Damašius, J., Venskutonis, P. R., Kaškonienė, V. & Maruška, A. Fast screening of the main phenolic acids with antioxidant properties in common spices using on-line HPLC/UV/DPPH radical scavenging assay. Anal. Meth. 6, 2774–2779 (2014).
Moreira, S. A. et al. Effect of high hydrostatic pressure extraction on biological activities and phenolics composition of winter savory leaf extracts. Antioxidants 9, 1–21 (2020).
Gomes, F. et al. Satureja montana L. and Origanum majorana L. decoctions: Antimicrobial activity, mode of action and phenolic characterization. Antibiotics 9, 294 (2020).
Mudrić, J. et al. Tablet and capsule formulations incorporating high doses of a dry optimized herbal extract: The case of Satureja kitaibelii. J. Drug Del. Sci. Technol. 66, 102776 (2021).
Aoshima, H., Miyase, T. & Warashina, T. Caffeic acid oligomers with hyaluronidase inhibitory activity from Clinopodium gracile. Chem. Pharm. Bull. 60, 499–507 (2012).
Celano, R. et al. Oil distillation wastewaters from aromatic herbs as new natural source of antioxidant compounds. Food Res. Int. 99, 298–307 (2017).
Pacifico, P. et al. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 69, 121–132 (2015).
Tsimogiannis, D. et al. Exploitation of the biological potential of Satureja Thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 4, 12–20 (2017).
El-Hagrassy, A. M., Osman, A. F., Mhafouz Eskander, D. & Nassar, M. I. Chemical constituents and cytotoxic evaluation of Abelmoschus esculentus L. leaves grown in Egypt. J. Chem. Pharm. 11, 1–13 (2019).
Döring, A.S. & Petersen, M. Production of caffeic, chlorogenic and rosmarinic acids in plants and suspension cultures of Glechoma hederacea, Phytochemistry Letters, 10, cxi-cxvii (2014).
Trifonova, D. et al. Preliminary in vitro study of anti-oxidant activity and anti-diabetic potential of plant extracts from 4 herbal substances not traditionally used for treatment of diabetes mellitus. Pharmacia 68, 755–762 (2021).
Serrano, C. et al. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 91, 1554–1560 (2011).
Lu, Y. & Foo, L. Y. Polyphenolics of Salvia—a review. Phytochemistry 59, 117–140 (2002).
Jiang, R. W. et al. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 12, 237–246 (2005).
Murata, T., Watahiki, M., Tanaka, Y., Miyase, T. & Yoshizaki, F. Hyaluronidase inhibitors from Takuran, Lycopus lucidus. Chem. Pharm. Bull. 58, 394–397 (2010).
Murata, T. et al. Matrix Metalloproteinase-2 Inhibitors from Clinopodium chinense var. parviflorum. J. Nat. Prod. 72, 1379–1384 (2009).
Khan, F., Bamunuarachchi, N. I., Tabassum, N. & Kim, Y. M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agricult. Food Chem. 69, 2979–3004 (2021).
Abotaleb, M., Liskova, A., Kubatka, P. & Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 10, 221 (2020).
Naveed, M. et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed Pharmacother 97, 67–74 (2018).
Maalik, A., Bukhari, S. M., Zaidi, A., Shah, K. H. & Khan, F. A. Chlorogenic acid: A pharmacologically potent molecule. Acta Pol. Pharm. 73, 851–854 (2016).
Sova, M. & Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 12, 2190 (2020).
Lu, H., Tian, Z., Cui, Y., Liu, Z. & Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 19, 3130–3158 (2020).
Lopez-Lazaro, M. Distribution and biological activity of the flavonoid luteolin. Mini-Rev. Med. Chem. 9, 31–59 (2009).
Rehfledt, S. C. H. et al. Neuroprotective effect of luteolin-7-O-glucoside against 6-OHDA-induced damage in undifferentiated and RA-differentiated SH-SY5Y Cells. Intern. J. Mol. Sci. 23, 2914–2932 (2022).
Chursi, S. et al. Evaluation of antibacterial activity, phytochemical constituents, and cytotoxicity effects of thai household ancient remedies. J. Altern. Complem. Med. 20(12), 909–918 (2014).
Morry, J., Ngamcherdtrakul, W. & Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 11, 240–253 (2017).
Yedjou, C. G. & Tchounwou, P. B. In vitro assessment of oxidative stress and apoptotic mechanisms of garlic extract in the treatment of acute promyelocytic leukemia. J. Cancer Sci. Ther. 2012, 6 (2012).
Hazra, B., Ghosh, S., Kumar, A. & Pandey, B. N. The prospective role of plant products in radiotherapy of cancer: A current overview. Front. Pharmacol. 2, 94 (2012).
Bonta, R.K. Dietary phenolic acids and flavonoids as potential anti-cancer agents: Current state of the art and future perspectives 20:29–48. In: Anti-Cancer Agents in Medicinal Chemistry, Editor-in- Chief Simone Carradori, ISSN (Print), 1871–5206, ISSN (Online): 1875–5992 (2020).
Makrane, H. et al. Cytotoxicity of the aqueous extract and organic fractions from Origanum majorana on human breast cell line MDA-MB-231 and human colon cell line HT-29. Adv. Pharmacol. Pharm. Sci. Article ID 3297193 (2018).
Boroja, T. et al. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. 118, 252–263 (2018).
Acknowledgements
This study was supported by Ministry of Education, Science and Technological Development of the Republic of Serbia Grants No.173029, 175056, 451-03-9/2021-14/200161, 451-03-68/2022-14/200161.
Author information
Authors and Affiliations
Contributions
K.G.: conceptualization, designed research work, interpreted and analyzed the data, reviewed, and edited the manuscript draw the figures/tables, and approved the final version of the manuscript. S.G.: conducted experiments on antioxidant activity, wrote the paper, reviewed, and edited the manuscript, and approved the final version of the manuscript. J.A.: conducted experiments on qualitative and quantitative chromatographic analysis using liquid chromatography with UV/VIS and MS detection, wrote the paper, draw the tables/figures, reviewed the manuscript, and approved the final version of the manuscript. A.Dž.: conducted experiments on antioxidant activity and approved the final version of the manuscript. I.V.: conducted experiments on antimicrobial activity and approved the final version of the manuscript. L.I.Ž: prepared extracts, wrote the paper, and approved the final version of the manuscript. A.M.: prepared extracts, wrote the paper, draw the figures/tables, and approved the final version of the manuscript. J.M.: prepared extracts, conducted experiments on antioxidant activity, wrote the paper, draw the figures/tables, and approved the final version of the manuscript. M.S.: conducted experiments on antimicrobial activity, and approved the final version of the manuscript. A.Đ.: conducted experiments on cytotoxic activity, draw the tables, and approved the final version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gopčević, K., Grujić, S., Arsenijević, J. et al. Bioactivity and phenolics profile of aqueous and ethyl acetate extracts of Satureja kitaibelii Wierzb. ex Heuff. obtained by ultrasound-assisted extraction. Sci Rep 12, 21221 (2022). https://doi.org/10.1038/s41598-022-25668-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25668-3
- Springer Nature Limited
This article is cited by
-
The hidden treasures in endophytic fungi: a comprehensive review on the diversity of fungal bioactive metabolites, usual analytical methodologies, and applications
Archives of Microbiology (2024)
-
Anti-microbial efficacy and notable biocompatibility of Rosa damascene and Citrus sinensis biomass-derived metabolites
Biomass Conversion and Biorefinery (2023)